* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download mineralogy - West Virginia University
Survey
Document related concepts
Transcript
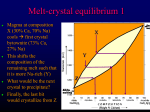
Dr. Helen Lang Dept. of Geology & Geography West Virginia University FALL 2015 GEOLOGY 284: MINERALOGY Igneous Rocks and their Minerals: Perkins, Chapter 5 What are IGNEOUS rocks? • Rocks that are formed by solidification of a MAGMA • Magma is “a naturally occurring molten (melted, liquid) rock material” (see glossary in textbook) Some General Terms about Igneous Rocks • intrusive • plutonic • magma • extrusive • volcanic • lava • usually phaneritic • usually aphanitic or porphyritic Most Magmas are silicate magmas (containing 40 to 75 wt.% SiO2) • Felsic (silicic or sialic) magmas are high in SiO2 and Al2O3 and low in MgO and FeO • Intermediate magmas are between Felsic and Mafic magmas • Mafic magmas contain less than 50 wt.% SiO2 and higher MgO, FeO and Fe2O3 • Ultramafic magmas are even more SiO2 poor and MgO/FeO rich Sample Chemical Analyses Wt. % Oxide SiO2 TiO2 Al2O3 Fe2O3 FeO MnO MgO CaO Na2O K2O H2O P2O5 Granite (felsic) 72.08 0.37 13.86 0.86 1.67 0.06 0.52 1.33 3.08 5.46 0.53 0.18 Gabbro (mafic) 50.78 1.13 15.68 2.26 7.41 0.18 8.35 10.85 2.14 0.56 0.48 0.18 Magmas contain Volatiles • Elements or compounds that “prefer” to be in gaseous form • Mostly H2O and CO2 in magmas • Also S, Cl and F • Contribute to formation of hydrous minerals – like Biotite K(Fe,Mg)3(AlSi3O10)(OH)2 • May separate and form bubbles that are preserved as vesicles Mount St. Helens 2004 Escape of gases from the magma causes explosive eruptions like this October 1, 2004, eruption of Mount St. Helens Magmas may crystallize in stages with Crystals separating from the remaining Liquid • Minerals are generally more dense than liquid, fall to bottom of magma chamber • Called fractional crystallization • The last liquid to crystallize will contain more volatiles and incompatible elements (K, Rb, Li, Be, B, and REEs) • Pegmatites form from these residual liquids, large crystals because H2O acts as a flux Bowen’s Reaction Series • Idealized model for crystallization of magmas • Shows order in which minerals crystallize from a typical mafic or basaltic magma • We will use it to organize igneous minerals • Left side is called Discontinuous Side – Mafic minerals change abruptly (discontinuously) • Right side is called Continuous Side – Plagioclase changes composition gradually Bowen’s Reaction Series high T olivine Ca plagioclase orthopyroxene temperature clinopyroxene NaCa plagioclase amphibole (Hb) biotite Na plagioclase cooling alkali feldspar muscovite low T quartz residual phases Most of the Minerals in Igneous Rocks are Silicate Minerals • Felsic (high in SiO2 and alkalis) Silicate Minerals – Quartz and Feldspars (framework silicates) • Mafic (high in Mg and Fe) Silicate Minerals – Pyroxenes, Amphiboles and Micas (chain and sheet silicates) We’ll start with Felsic Minerals (framework silicates) • Quartz SiO2 • Alkali Feldspars (K,Na)AlSi3O8 • Plagioclase Feldspars (Ca,Na)(Al,Si)4O8 • Right-hand side of Bowen’s Reaction Series • Important in all igneous rocks • Especially in Granites Quartz and Feldspars in Polished Granite Quartz and Feldspar are • Framework silicates (tectosilicates) – #corners shared 4/tetrahedron – Si,O formula (SiO2)0 or ((Al,Si)4O8)1-to2– Si (+Al):O ratio 1:2 – Example quartz, K-feldspar – Formula SiO2, KAlSi3O8 SiO2 like many other compounds comes in several different structures • Called Polymorphs (many forms) – Minerals with the same formula, but different structures • Quartz - stable form of SiO2 at most conditions found on Earth • High Temperature (low P) forms: tridymite, cristobalite – occur in hot volcanic rocks • Very high Pressure forms: coesite, stishovite – occur in meteorite impacts and deep subduction SiO2 P-T Phase Diagram Each Polymorph has a completely different 3D framework of linked SiO4 tetrahedra 3d Spirals Quartz Structure Tridymite Structure Low Qtz Low and High Quartz Structures Same name Change from one to the other is displacive High Qtz Same framework Tridymite High Quartz and Tridymite Structures Different names Change from one to the other is reconstructive High Qtz Different frameworks View Crystal Structure Movies http://socrates.berkeley.edu/~eps2/wisc/geo360 low-Quartz http://socrates.berkeley.edu/~eps2/wisc/geo360/Quartz.mov Cristobalite http://socrates.berkeley.edu/~eps2/wisc/geo360/cristobalite.mov Tridymite Coesite http://socrates.berkeley.edu/~eps2/wisc/geo360/tridymite.mov http://socrates.berkeley.edu/~eps2/wisc/geo360/Coesite.mov SiO2 P-T Phase Diagram G=4.30 G=2.93 G=2.65 G=2.65 G=2.33 G=2.28 Higher Density Minerals are Stable at Higher Pressure Coesite has been found in Crustal Rocks! • Before 1984 Coesite and Stishovite had been found only in impact craters (very high pressures for very short times) • In 1984 Coesite was found for the first time in rocks that were once at the surface • This means that in continent-continent collision zones (like the Himalayas and Alps), rocks somehow got from the surface down to >100km (>60mi) and back fast enough to preserve coesite Quartz Properties • H=7, G=2.65 • Generally clear and glassy, may have a variety of colors (clear, smoky, brown, rose; it’s allochromatic) • Conchoidal fracture, no cleavage • Habit: hexagonal (6-sided prisms) or massive • Optical: low relief and low birefringence Quartz Crystals Quartz Crystals Amethyst Rutilated Quartz Quartz and Feldspars in Granite Quartz in Granite Thin Section PPL XPL From Atlas of Rocks & Minerals in Thin Section Feldspars • Also framework silicates • Most abundant minerals in the Earth’s crust • Also common in igneous rocks • Almost all igneous rocks have feldspars (not true for quartz) How do we get framework silicates with formulas different from SiO2? • When all SiO44- tetrahedra share all corners with other tetrahedra, formula is (SiO2)0, no need for other cations to balance charge • If Al3+ substitutes for Si4+ in some of the tetrahedra, there is a net negative charge on the framework and other cations are needed to balance charge • That’s how we get Feldspars! Feldspars • If Al3+ substitutes for 1/4 of the Si4+ in the framework • Formula changes from (Si4O8)0 to (AlSi3O8)1• Alkali Feldspars (K+, Na+)AlSi3O8 • Orthoclase, KAlSi3O8, and Albite, NaAlSi3O8 • If Al3+ substitutes for 1/2 of the Si4+ in the framework • Formula changes from (Si4O8)0 to (Al2Si2O8)2• Anorthite Ca(2+)Al2Si2O8 Feldspars all have similar 3D Frameworks that contain linked Double Crankshafts View Crystal Structure Movies K-feldspar (Sanidine) http://socrates.berkeley.edu/~eps2/wisc/geo360/Sanidine.mov Na-plagioclase (Albite) http://socrates.berkeley.edu/~eps2/wisc/geo360/Albitem.mov first frame shows Feldspar structure best Ca-plagioclase (Anorthite) http://socrates.berkeley.edu/~eps2/wisc/geo360/Anorthite.mov Note the big open spaces available for the cations, Na+, K+ and Ca2+, that balance the charge Three Feldspar End-members • Albite (Ab) NaAlSi3O8 • Anorthite (An) CaAl2Si2O8 • Orthoclase (Or) KAlSi3O8 Relationships shown on Triangular (Ternary) Diagram, one end-member at each corner The Feldspar Ternary CaAl2Si2O8 An Anorthite Ab Or Albite Orthoclase NaAlSi3O8 alkali feldspars KAlSi3O8 Suppose we have a Feldspar with the formula: Ca.05Na.25K.70Al1.05Si2.95O8 • To plot that feldspar on a triangular diagram, we need % of each of the three feldspar end members • Easiest way is to calculate the mole % of Ca, Na, and K: – Ca/(Ca+Na+K)*100 = (.05/1.00)*100 = 5 % An – Na/(Ca+Na+K) * 100 = (.25/1.00)*100 = 25 % Ab – K/(Ca+Na+K) * 100 = (.70/1.00)*100 = 70 % Or How do Triangular Diagrams Work? CaAl2Si2O8 100% CaAl Si O 2 2 8 Box 5.3 Shows CaO, Al2O3, SiO2; we’ll use feldspars An Count up from the opposite side Plot a Feldspar: 5% CaAl2Si2O8 25% NaAlSi3O8 70% KAlSi3O8 100% Ab NaAlSi3O8 5% CaAl2Si2O8 NaAlSi3O8 0% CaAl2Si2O8 100% KAlSi3O8 Or KAlSi3O8 The Feldspar Ternary CaAl2Si2O8 Anorthite solid solutions All natural feldspars No feldspars Miscibility Gap Albite NaAlSi3O8 alkali feldspars Orthoclase KAlSi3O8 Alkali Feldspar (esp. Orthoclase) Properties • H=6, G=2.56 • Generally turbid (cloudy); color white, pink or flesh-colored • 2 Perfect to good perpendicular cleavages • Habit: stubby prisms, simple twins common • Optical: low relief and low birefringence • Commonly Perthitic (micro and macro) Typical Orthoclase Crystals (alkali feldspar) Orthoclase – Carlsbad Twin Alkali Feldspars have Perthites What do Perthites look like? Alkali Feldspars have Perthites What do Perthites look like? Thin section in XPL Alkali Feldspars have Perthites What do Perthites look like? 1mm What causes Perthites? • Caused by un-mixing, exsolution or separation of Na+ (diameter~1.1Å) and K+ (diameter~1.6Å) as the feldspar cools • At low temperatures, there is a miscibility gap between NaAlSi3O8 and KAlSi3O8 Miscibility gap in Alkali Fsp. causes Perthites 1000o 600o 400o Albite Temperature 800o X’ albite NaAlSi3O8 K-feldspar One Homogeneous X Alkali Feldspar Two Feldspars Perthite alkali feldspars X’’ orthoclase KAlSi3O8 Why Alkali Feldspars CAN have perthites? • Remember Al3+ substitutes for 1/4 of Si4+ in framework (AlSi3O8)1• Al3+ is locked tightly in the feldspar framework and can’t move • K+ (1.46Å) and Na+ (1.08Å) with the same charge can trade places freely in the structure and still balance Al3+ charge • Allows unmixing or exsolution (separation of Na+ and K+) in solid feldspar • Which allows Perthites to form as the rock cools Microcline (a polymorph of KAlSi3O8 different from orthoclase) is sometimes bluish green Microcline has plaid twinning 1mm The Feldspar Ternary CaAl2Si2O8 Anorthite No feldspars, Miscibility Gap Albite NaAlSi3O8 alkali feldspars Orthoclase KAlSi3O8 Plagioclase Properties • • • • • • • • H=6-6.5, G=2.62-2.76 Luster pearly, vitreous/translucent Color white to gray One perfect, one good cleavage Optical: low relief and low birefringence Polysynthetic albite twinning usually present Not Perthitic! Commonly zoned Plagioclase hand specimen - note polysynthetic twinning (striations) May be visible in handspecimen, usually visible in thin section (in XPL) Plagioclase Feldspars have (polysynthetic, lamellar) Albite Twins Zoning shows up with crossed polars under the microscope (diff. biref.) Plagioclase Feldspars are commonly zoned Zoning in Plagioclase Especially in volcanic rocks, conditions may change around a growing plagioclase, causing changes in plagioclase composition (variable Na and Ca) This is zoning Review Feldspars CaAl2Si2O8 Anorthite (An) No feldspars, Miscibility Gap Albite (Ab) NaAlSi3O8 alkali feldspars Orthoclase KAlSi3O8 (Or) Plagioclase Feldspars CAN NOT have perthites. Why? • In some parts of the plagioclase, Al3+ substitutes for half of the Si4+; formula (Al2Si2O8)2- Ca2+ or other divalent cation must balance charge! • In some parts of the plagioclase structure, Al3+ substitutes for 1/4 of the Si4+; formula (AlSi3O8)1Na1+ or other monovalent cation must balance charge! Why do Plagioclase Feldspars NOT have perthites? • Al3+ is locked tightly in feldspar framework • Therefore, Al3+ can’t move • Na+ and Ca2+ can’t move without Al3+ (That would destroy the charge balance!) • Therefore, exsolution or Perthites can’t happen in Plagioclase Feldspar • And alkali feldspars (except microcline) don’t have albite twins! Review Feldspars CaAl2Si2O8 Anorthite (An) No feldspars, Miscibility Gap Albite (Ab) NaAlSi3O8 alkali feldspars Orthoclase KAlSi3O8 (Or) Bowen’s Reaction Series high T olivine Ca plagioclase orthopyroxene temperature clinopyroxene NaCa plagioclase amphibole (Hb) biotite Na plagioclase cooling alkali feldspar muscovite low T quartz residual phases SiO2 P-T Phase Diagram Low Quartz, High Quartz and Tridymite Structures Low Qtz Tridymite High Qtz How do Triangular Diagrams Work? CaAl2Si2O8 100% CaAl Si O 2 2 8 Box 5.3 Shows CaO, Al2O3, SiO2; we’ll use feldspars An Plot a Feldspar: 5% CaAl2Si2O8 25% NaAlSi3O8 70% KAlSi3O8 Count up from the opposite side 100% Ab NaAlSi3O8 5% CaAl2Si2O8 NaAlSi3O8 0% CaAl2Si2O8 100% KAlSi3O8 Or KAlSi3O8 CaAl2Si2O8 Anorthite (An) No feldspars, Miscibility Gap Albite (Ab) NaAlSi3O8 alkali feldspars Orthoclase KAlSi3O8 (Or) Miscibility gap in Alkali Fsp. causes Perthites 1000o 600o 400o Albite Temperature 800o X’ K-feldspar One Homogeneous X Alkali Feldspar Two Feldspars Perthite X’’ albite orthoclase NaAlSi3O8 KAlSi3O8