* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Bilateral corneal perforation secondary to Pseudomonas keratitis in
Survey
Document related concepts
Transcript
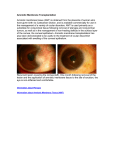
CASE REPORT Bilateral corneal perforation secondary to Pseudomonas keratitis in a patient with multiple injuries Miriam García-Fernández, PhD1; T. Parra-Rodríguez, MD1; B. Baamonde-Arbaiza, PhD1 ABSTRACT: Microbial keratitis, especially that caused by Pseudomonas aeruginosa, is associated with intense inflammation and proteolytic activity, which can lead to corneal perforation. We report a case of bilateral P. aeruginosa keratitis complicated with corneal perforation in a patient with multiple injuries, managed with tissue adhesive and amniotic membrane (AM) transplant, followed in a second step by penetrating keratoplasty. J Emmetropia 2014; 5: 95-97 Microbial keratitis is an unusual yet severe complication of corneal exposure in the intensive care patient1,2. Keratitis can lead to corneal ulceration, endophthalmitis, corneal perforation and loss of vision. Staphylococcus, Streptococcus, Pseudomonas and the Enterobacteriaceae (Citrobacter, Enterobacter, Klebsiella, Proteus and Serratia) are the species most commonly involved in the development of a nosocomial corneal infection2,3. Pseudomonas aeruginosa keratitis, triggered by contact lens wear or corneal trauma, can progress rapidly and lead to corneal scarring or melting with a profound impairment of vision. To minimize the damage to corneal tissue, an appropriate diagnosis and prompt initiation of appropriate antibacterial therapy are critical4. The aim of this case report is to describe a case of bilateral corneal perforation secondary to Pseudomonas aeruginosa infection in a patient with multiple injuries. CASE REPORT A 42-year-old Caucasian male was involved in a motorcycle accident, suffering multiples injuries that necessitated intensive care unit (ICU) stay with Submitted: 11/9/2013 Revised: 02/15/2014 Accepted: 02/27/2014 Department of Ophthalmology, Hospital Universitario Central de Asturias, Oviedo, Spain. 1 Financial disclosure: The authors have no financial interests Corresponding Author: Miriam García-Fernández. Department of Ophthalmology Hospital Universitario Central de Asturias C/ Pérez de Ayala, nº 6, 2.º B; 33007 Oviedo (Spain) Email: [email protected] © 2014 SECOIR Sociedad Española de Cirugía Ocular Implanto-Refractiva prolonged mechanical ventilation. During his stay in the ICU, routine eye care included ocular lubrication with artificial tear drops every six hours and ointments twice a day. On the second day in ICU, he developed a febrile syndrome with leukocytosis, so empirical treatment with intravenous cefepime 1 g every 12 hours was started. The patient went on to develop Pseudomonas aeruginosa in blood and nasopharyngeal exudates, so systemic treatment with intravenous colistin 240 mg every eight hours was initiated. Three days later, ocular symptoms such as loss of visual acuity and discomfort began in both eyes. A full ocular examination revealed bilateral keratoconjunctivitis, so topical antibiotic treatment was started with ciprofloxacin 0.3% drops (Oftacilox®, Alcon Cusi S.A., El Masnou, Barcelona), one drop every eight hours, and fortified antibiotic amikacin eyedrops, one drop every two hours, and ceftazidime, one drop every two hours. After one week of topical antibiotics, patient discomfort increased, and he described a subjective loss of visual acuity, decreasing from “count fingers” to “hand motion”. During the ocular examination, we could observe the presence of corneal melting and imminent corneal perforation in both eyes. For this reason, we decided to use 2-octyl-cyanoacrylate adhesive and Tachosil (Tachosil®, Nycomed Pharma AS, Zürich, Switzerland) as tissue adhesive for repair of the impending corneal perforation followed by amniotic membrane (AM) transplantation in both eyes. The AM was sutured with its basement membrane side up to the edge of the lesion by interrupted 10-0 nylon suturing. Postoperatively, topical antibiotics were continued. Total reepithelization was achieved 10 days later. Two months after surgical repair, severe corneal scarring could be observed in both eyes with anterior synechiae but no epithelial defect (Figure 1). Intraocular ISSN: 2171-4703 95 96 CORNEAL PERFORATION SECONDARY TO PSEUDOMONAS KERATITIS Figure 1. Biomicroscopic view of right eye two months after surgical repair of impending corneal perforation with cyanoacrylate and Tachosil as tissue adhesives followed by amniotic membrane (AM) transplantation. Figure 2. Biomicroscopic view of left eye two months after penetrating keratoplasty. pressure was normal. B-Scan Ultrasound revealed neither retinal detachment nor other alterations. Six months after surgery, once ocular and systemic stability was achieved, the patient underwent penetrating keratoplasty, with removal of anterior synechiae from the left eye. Postoperative treatment consisted of slow tapering of the systemic high-dose corticosteroid therapy (prednisone 60 mg) and topical treatment with tobramycin and dexamethasone ophthalmic drops (Tobradex®, Alcon Cusi S.A., El Masnou, Barcelona) and ciprofloxacin 0.3% (Oftacilox®) four times a day, and atropine twice a day during the first week, which was tapered slowly over the following two months. Postoperative progress was good and the patient’s visual acuity improved to 0.8 (in decimal notation) two months after surgery (Figure 2). Six months later, penetrating keratoplasty in right eye was also performed, with excellent functional and anatomical outcomes. The severity of bacterial keratitis is dependent on the host response and the integrity of the underlying cornea. The virulence of the bacteria is crucial. P. aeruginosa, Staphylococcus aureus, and Streptococcus pneumonia all have the ability to adhere to the edge or base of an epithelial defect. This has been attributed to the presence of appendages called fibrillae (Gram-positive) or fimbriae (Gram-negative) that allow for interaction between specific receptors on the corneal epithelium and the surface of these appendages. Moreover, Pseudomonas and Serratia have proteoglycanase activity, which can liquefy the corneal stroma and contribute to the virulence of these microorganisms3. Therefore, microbial keratitis caused by P. aeruginosa is associated with intense inflammation and excessive proteolytic activity. If proper management is not initiated, the cornea tends to become perforated, resulting in severe ocular consequences, such as development of cataract, glaucoma and even endophthalmitis. Corneal perforation is caused by stromal necrosis and may occur DISCUSSION JOURNAL OF EMMETROPIA - VOL 5, APRIL-JUNE CORNEAL PERFORATION SECONDARY TO PSEUDOMONAS KERATITIS earlier in fulminating infection or later, if treatment is unsuccessful5. The aims of treatment are to prevent the infection spreading, eliminate the pathogen, control inflammation, promote wound healing, and prevent perforation. If a patient develops a perforation, the size must be taken into consideration. If the perforation is less than 1-2 mm in diameter, a cyanocrylate adhesive with or without a patch graft should be attempted6. If this fails or in the case of larger perforations, therapeutic penetrating keratoplasty will be needed after intensive topical, and sometimes systemic antibiotic therapy, for days in order to eradicate bacteria from the cornea as best as possible5. On the other hand, preserved human AM, which has been reported to have anti-inflammatory, antiproteolitic and antimicrobial activities, has been used to promote wound healing and to prevent perforation in infectious keratitis, including P. aeruginosa keratitis, with good results, as occurred in our case5,7,8. The antibacterial properties of human AM have been previously described9. Possible explanations include intimate adherence of the AM to the wound surface, or the presence of antimicrobial peptides (defensins)10. Moreover, AM can act as a drug reservoir system as it does not interfere with the ocular penetration of topical antibiotics in corneas with epithelial defects5,8. Moreover, AM can also protect the ocular surface from being exposed to unwanted proteolytic damage caused by proteases released by bacteria and inflammatory cells. Moreover, it has been reported that AM contains various protease inhibitors which suppress the matrix-degrading activity of collagenase, plasmin and trypsin11. Although AM did not directly inhibit pseudomonal proteases, it may work indirectly by inhibiting host proteases which are activated by microbial proteases. The possibility of treatment of P. aeruginosa keratitis with combined corneal cross-linking and human amniotic membrane transplantation has even been described12. Recently, a place for immune intervention in P. aeruginosa keratitis has been proposed. Some studies have been published that may identify potential targets for immune intervention that could regulate the severity of the host response, and subsequently, its effect on blindness and visual impairment. For example, it has been shown that patients infected with P. aeruginosa expressing exotoxin exoS had a better outcome in terms of visual acuity following treatment compared with P. aeruginosa expressing exoU, possibly because it causes less severe disease13. 97 However, and as has been shown in our case report, the last step for recovery of visual acuity depends in most cases on achieving acceptable anatomical and functional outcomes with penetrating keratoplasty. REFERENCES 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Kirwan J, Potamitis T. Microbial keratitis in intensive care. Br Med J. 1997; 314:433-4. Johnson JL, Sagraves SG, Feild CJ, Block EF, Cheatham ML. An unusual case of corneal perforation secondary to Pseudomonas keratitis complicating a patient’s surgical/trauma intensive care unit stay. Am Surg. 2000; 66:972-4. Liesegang TJ. Bacterial keratitis. In: Infectious Disease Clinics of North America. Philadelphia: WB Saunders, 1992, pp. 815-31. Ishikawa E, Suzuki T, Yamaguchi S, Inoe T, Uno T, Ohashi Y. Serrated margins in Pseudomonas aeruginosa keratitis. Case Rep Ophthalmol. 2013; 4:12-5. Chen JH, Ma DH, Tsai RJ. Amniotic membrane transplantation for pseudomonal keratitis with impending perforation. Chang Gung Med J. 2002; 25:144-52. Hyndiuk RA, Hull DS, Kinyoun JL. Free tissue patch and cyanocrylate in corneal perforation. Ophthalmic Surg. 1974; 5:50-5. Na BK, Huang JH, Shin EJ. Analysis of human amniotic membrane components as protease inhibitors for development of therapeutic agent of recalcitrant keratitis. Invest Ophthalmol Vis Sci. 1998; 39:S90. Kim JS, Kim JC, Hahn TW, Park WC. Amniotic membrane transplantation in infectious corneal ulcer. Cornea. 2001; 20:720-726. Talmi YP, Sigler L, Inge E. Antibacterial properties of human amniotic membranes. Placenta. 1991; 12:285-8. Ganz T, Lehrer RI. Defensins. Curr Opin Immunol. 1994; 6:584-9. Kim JS, Kim JC, Na BK, Jeong JM, Song CY. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res. 2000; 70:329-37. Mattila JS, Korsbäck A, Krootila K, Holopainen JM. Treatment of Pseudomonas aeruginosa keratitis with combined corneal cross-linking and human amniotic membrane transplantation. Acta Ophthalmol. 2013; 91:e410-1. Karthikeyan RS, Priya JL, Leal SM Jr, Toska J, Rietsch A, Prajna V, Pearlman E, Lalitha P. Host response and bacterial virulence factor expression in Pseudomonas aeruginosa and Streptococcus pneumoniae corneal ulcers. PLoS One. 2013; 8:e64867. JOURNAL OF EMMETROPIA - VOL 5, APRIL-JUNE First author: Miriam García-Fernández, PhD Department of Ophthalmology. Hospital Universitario Central de Asturias Oviedo, Spain