* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CYP2D6 - PGXL Laboratories
Prenatal testing wikipedia , lookup
Public health genomics wikipedia , lookup
Pharmacognosy wikipedia , lookup
Nanomedicine wikipedia , lookup
Harm reduction wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Multiple sclerosis research wikipedia , lookup
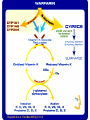
Brad Esarey VP Business Operations A need for Personalized Medicine PGx Integration: Role of the Clinical Laboratory • • • • • • Catalyze the delivery of biotechnology products to healthcare consumers Overcome barriers to genotyping Develop genetic profiling strategies to maximize sensitivity and specificity of predicting phenotype Provide availability of testing Develop methods to reduce technical difficulty, cost Improve interpretations Pharmacogenetics Links differences in gene structure (inherited polymorphism) to drug metabolism and response Genetic Drug metabolism polymorphism & response (Genotype) (Phenotype) Why is this important ? ~60% of meds in top 20 list causing ADRs are linked to a genetic variation 122 drugs have FDA box warnings related to genetics Explanatory Use Predictive Use Decision for Drug Treatment Decision for Drug Treatment Standard Drug and Dose CYP Genotyping Dose Adjustment According to Efficacy and Adverse Events Drug Selection and Dosage Based on Genotype Therapy Refractoriness or Adverse Events CYP Genotyping Further Adjustment According to Efficacy and Adverse Events PGXL Enables Transformation Intuitive Medicine Precision Medicine Indicators Suggest: Diagnostic services will trump therapeutics (Clayton M Christenson in: The Innovator’s Prescription, MacGraw Hill, 2009) Applications of pharmacogenomics • Individualizing drug therapy selection • Predicting adverse reactions, dosing, response • Identify increased sensitivity to drug interactions 8 Clinical Applications of Pharmacogenetic Information • Anti-coagulation – Warfarin – Plavix (clopidogrel) • Psychiatry – Anti-depressants • Oncology 9 – KRAS – Tamoxifen – EGFR’s • Pain management – Codeine – Hydrocodone – Oxycodone Medications and metabolic pathways CYP2C9 CYP2C19 CELECOXIB IBUPROFEN NAPROXEN GLYBURIDE GLIPIZIDE TOLBUTAMIDE GLIMEPIRIDE PHENYTOIN FLUVASTATIN LOSARTAN CELEBREX ADVIL, MOTRIN ALEVE DIABETA GLUCOTROL ORINASE AMARYL DILANTIN LESCOL COZAAR CYP2C9/VKORC1 WARFARIN COUMADIN CLOPIDOGREL CITALOPRAM ESCITALOPRAM IMIPRAMINE SERTRALINE OMEPRAZOLE ESOMEPRAZOLE PANTOPRAZOLE RABEPRAZOLE LANSOPRAZOLE DIAZEPAM NELFINAVIR PLAVIX CELEXA VARIOUS BRANDS TOFRANIL ZOLOFT PRILOSEC NEXIUM PROTONIX ACIPHEX PREVACID VALIUM VIRACEPT Medications and CYP2D6 metabolic pathway ANTIDEPRESSANTS AMITRIPTYLINE CLOMIPRAMINE DESIPRAMINE DOXEPIN IMIPRAMINE NORTRYPTYLINE VARIOUS BRAND NAMES ANAFRANIL NORPRAMIN SINEQUAN TOFRANIL PAMELOR, AVENTYL ANTI-PSYCHOTICS SSRI’S ARIPIPRAZOLE FLUOXETINE MAPROTOLINE PAROXETINE RISPERIDONE ABILIFY PROZAC LUDIOMIL PAXIL RISPERIDOL BETA-BLOCKERS CARVEDILOL METOPROLOL PROPANOLOL COREG TOPROL-XL VARIOUS BRAND NAMES OPIOIDS CODEINE OXYCODONEOXYCONTIN HYDROCODONE DESTROMETHORPHAN TRAMADOL NAMES CODEINE PHOSPHATE VARIOUS BRAND NAMES VARIOUS BRAND NAMES ULTRAM, VARIOUS BRAND SNRI’S ATOMOXETINE VENLAFAXINE STRATTERA EFFEXOR OTHERS DONEPEZIL FLECANIDE FLUVASTATIN LORATIDINE PROPAFENONE TAMOXIFEN ARICEPT TAMBOCOR LESCOL CLARITIN RYTHMOL VARIOUS BRAND NAMES Vision: enable transition of pharmacogenetic diagnostics into actionable healthcare results Mission and Core Business Strategies: • Provide Clinical Diagnostic Services (healthcare providers for direct patient care) • Support Development of Diagnostics (discovery & translation of biological markers) Product Lines Cardiovascular: Pain Management: - Opiods - Anticoagulation - HTN/CHF Oncology: - Cancer therapy - Prevention Respiratory: - Asthma - COPD PGXL is uniquely positioned Behavioral Health: - ADHD - Schizophrenia - Depression Current Test Menu MOLECULAR ONCOLOGY - CYP2D6 tamoxifen - KRAS - BRAF -BCR/ABL (quantitative) - BCR/ABL Kinase Domain mutations JAK2 (quantitative) BCL1 BCL2 PML-RARα c-KIT EGFR EGFR (FISH) HER2/neu (FISH) ALK (FISH) DPD/TYMS TPMT PHARMCOGENETICS - CYP1A2 CYP2C9 CYP2C9/VKORC1 CYP2C19 CYP2D6 NAT2 HLA-B*1502 Carbamazepine sensitivity HLA-B*5701 Abacavir sensitivity 5-HTTLPR Serotonin Transporter MTHFR Factor II (Prothrombin 20210 G>A) Factor V Leiden Hepatitis-B Virus DNA Quantitative THERAPEUTIC DRUG MONITORING - MPA,serum - MPA + MPA-G, serum PGx of Warfarin Overview • Quick review of the problem • Review of Pharmacokinetic Basics • Application of knowledge FDA Updates Coumadin Label Genetic Information may improve initial dosing estimate for individual patients The U.S. Food and Drug Administration announced today the approval of updated labeling for the widely used blood-thinning drug, Coumadin, to explain that people's genetic makeup may influence how they respond to the drug. The labeling change highlights the opportunity for healthcare providers to use genetic tests to improve their initial estimate of what is a reasonable warfarin dose for individual patients. Testing may help optimize the use of warfarin and lower the risk of bleeding complications from the drug. U.S. Food and Drug Administration August 16, 2007 Reynolds K et al. Pers Med 2007;4(1):11-31. • Pharmacogenetics of Warfarin – Inactivated and eliminated via Cytochrome P4502C9 metabolism • 40% of populations have deficient CYP2C9 – Inhibits Vitamin K epoxide reductase complex • > 70% of population have decreased VKOR and are more sensitive to warfarin • Dose effectiveness should be assessed at steady-state 3.00 S-Wa rfa rin (m g/L) • CYP2C9 *2 and *3 – delay time to reach steady-state – Increase blood concentration 2.40 CYP2C9*1/*3 1.80 CYP2C9*1/*2 1.20 0.60 CYP2C9*1/*1 0.00 0 3 6 9 12 15 18 Time (days) Linder et al. J Thrombosis & Thrombolysis 2002;14:227-232 Confidential 21 24 27 30 Variables influencing maintenance dose. Optimal WARFARIN dose can be calculated based on AGE, GENDER, WEIGHT and PHARMACOGENETICS Zhu Y et al. Clin Chem 2007;53(7):1199-1205. Impact of Testing • Estimate Maintenance Dose Requirement Guides dose selection • Estimate of Time to Reach Steady-state Avoid Mis-interpretation of INR’s Avoid Premature Dosage Changes © Copyright 2007 Pharmacogenetics Diagnostic Laboratory, LLC. All rights reserved. Duplication of material prohibited. Application of Pharmacogenomics to Anti-platelet therapy Antiplatelet Response • Clopidogrel (Plavix) is a pro-drug which is converted to an active metabolite by hepatic cytochrome P4502C19 (CYP2C19). • ~ 30% of patients have deficiency in CYP2C19 25 Influence of CYP2C19 on Clopidogrel Response Incidence of Adverse events in patients prescribed standard dosages of Clopidogrel by CYP2C19 Phenotype. PHENOTYPE Stent Thrombosis EM CV death, MI, Ischemic Stroke 8% (BASELINE) 8.9% 1.4% IM 10% 2.4% PM 12.7% 5.7% Mega et.al., JAMA. 2010;304(16);1821-1830. 0.9% Applications of PGx in Pain Management • Pharmacokinetics: genetic variation in cytochrome P450’s – Decreased drug clearance • Mis-interpretation of over compliance – Ultra-rapid drug clearance • Mis-interpretation of drug diversion • Mis-interpretation of poor compliance Cytochrome P4502D6 Substrates • Pain management – – – – – – Hydrocodone Oxycodone Methadone Propoxyphene Codeine Others …. • In addition many drugs commonly co-prescribed with pain management medications – Antidepressants – Anti-anxiety Genetic polymorphism of CYP2D6 • Ultra-rapid metabolizers (UM) – 3 – 7 % of population • Extensive metabolizers (EM) – 55 – 60 % of population • Intermediate metabolizers (IM) – 25 – 30% of population • Poor Metabolizers (PM) – 5 – 10 % of population Example: Codeine • Principal metabolism by CYP2D6 • Pro-drug to morphine • CYP2D6 PM: in adequate morphine • CYP2D6 UM: morphine poisoning Case Report: Neonatal Morphine Overdose • Full-term healthy male infant showed intermittent periods of difficulty in breastfeeding and lethargy starting on day 7 • Day 11 pediatric visit baby regained birth weight • Day 12 gray skin and milk intake had fallen • Baby found dead on day 13 • Postmortem analysis showed no anatomical anomalies Koren et al. Lancet 2006;368:704. Toxicology Results • Blood concentration of morphine = 70 ng/mL – neonates breastfed by mothers receiving codeine typically have morphine serum concentrations of 0–2.2 ng/mL • Day 10 breast milk morphine concentration = 87 ng/mL – Typical range after repeated maternal codeine doses of 60 mg every 6 h is 1.9–20.5 ng/mL • The mother was prescribed 2 weeks codeine 30 mg + acetaminophen 500 mg (Tylenol #3) after birth for episiotomy pain – 4 tabs first day, but only 2 tabs daily thereafter because of maternal somnolence and constipation Koren et al. Lancet 2006;368:704. CYP2D6 Genotype Analysis CYP2D6 *1/*2xN (gene duplication) = Ultra-rapid Metabolizer (UM) • Leads to increased formation of morphine from codeine • Maternal grandfather, the father, and the infant had 2 functional CYP2D6 alleles (Extensive Metabolizer, EM) • The maternal grandmother was a UM Koren et al. Lancet 2006;368:704. Clinical Case Conclusions • The clinical and laboratory picture was consistent with neonatal death from opioid toxicity • Most of the analgesic and CNS depressant effects of codeine are secondary to its metabolism to morphine by CYP2D6 • Neonates invariably have impaired capacity to metabolize and eliminate morphine Koren et al. Lancet 2006;368:704. Case Conclusions, cont’d • Codeine is a commonly used analgesic after labor – Episiotomy – Caesarean section • American Academy of Pediatrics lists codeine as compatible with breastfeeding, despite lack of sufficient published data to support this recommendation • Inherited differences in CYP2D6 can be life-threatening for some breastfed babies “Codeine cannot be considered as a safe drug for all infants during breastfeeding” • CYP2D6 UM frequency – 1.5 - 2% in Caucasians and Asians – 14% in African Americans – 29% in Ethiopians Koren et al. Lancet 2006;368:704. Strategies to manage breastfeeding while on codeine Action Avoid codeine; use alternative Advantages Avoids potential neonatal toxicity Disadvantages Potential uncontrolled pain Minimizes potential Avoid high-dose codeine (240mg/d) for neonatal toxicity more than a few days Uncontrolled pain; dose still too high for UMs Avoid breastfeeding Avoids potential neonatal toxicity while on codeine Inform and monitor Early Intervention and prevention of serious for signs of opioid toxicity toxicity Loss of breastfeeding benefits Genotype mothers for CYP2D6 Parental anxiety and false-positive toxicity Predicts risk of excess Expensive, not morphine production presently routine Adapted from Koren et al. Lancet 2006;368:704. CYP2D6 Genotyping Routine CYP2D6 testing IS available Metabolizer status (phenotype) can be predicted Predicts risk of toxicity, therapeutic failure, and promotes optimal outcomes for patients Other CYP2D6 Genotyping Considerations • CYP2D6 metabolizes 20-25% of all current medications • CYP2D6 inactivates medications – antidepressants, antipsychotics, beta- blockers, antiarrhythmics • CYP2D6 activates medications – opioids, tamoxifen • CYP2D6 is inhibited by medications – antidepressants (SSRIs in particular), antipsychotics, certain antimicrobials, quinidine, amiodarone • CYP2D6 is induced by medications – rifampin, carbamazepine, phenobarbital, ritonavir Consultation Services • PGXL provides state-of-the-art interpretive reports with actionable guidance • PGXL medical directors are available for clinical consultation with ordering practitioners before and after testing • Ongoing VIP review consultation after 50-100 patients tested • Educational lecture series and/or CME events as needed Diagnostic Testing • Clinical testing is available • Must be performed by high-complexity CLIA-certified laboratory • 5-7 business day TAT • Whole blood or cheek swab samples • Genotype result with phenotype interpretation Patient Information: Genetic Drug Sensitivity Testing Why test my genes before prescribing a drug? Just as gene variation controls hair or eye color, it also controls how the body reacts to certain drugs. Before prescribing one of those drugs, your physician may give you a genotype test to make sure the drug will be safe and effective for you. A genotype is an analysis of some aspect of your genetic make-up. Knowing your genotype will help you and your doctor choose the most effective treatment path. What is CYP450? CytochromeP450, abbreviated CYP450, is a complex of genes that controls liver enzymes that digest certain drugs. Those drugs include Plavix®, Coumadin®, warfarin, beta blockers, common pain medications and antidepressants, among many others. Different genes within the CYP450 complex control the metabolization of different drugs. CYP2C19, for example, indicates Plavix sensitivity, and CYP2C9 is commonly analyzed before prescribing Coumadin/warfarin. What will the test tell me? Specifically, how quickly your body filters a given drug out of your bloodstream. A high metabolizer flushes drugs out of the body quickly, and might never realize any benefit from taking a “normal” dose. A poor metabolizer is just the opposite, with a “normal” dose building to potentially dangerous levels. Understanding how quickly you will metabolize a drug helps your doctor calculate the safest, most effective dose for you. What is required to perform this test? You will need to provide a DNA sample using a buccal swab. A buccal swab is a sterile cotton swab made for DNA collection. You gather DNA simply and painlessly by rubbing the buccal swabs on the insides of your cheeks. The swabs are then sealed and shipped to the lab for testing. What happens to my DNA once the test is complete? Your privacy is assured. Samples and genetic information are stored securely in accord with HIPAA and other government regulations. Is there a cost for this test? This is a routine clinical laboratory test covered by Medicare and most private insurance plans. There may be a copay, depending on the specifics of your policy. How do I get my test results? Results will be sent to your doctor 3-5 business days after your sample is collected. All tests are performed by PGXL Laboratories, Louisville, KY – 877-564-2199 – CLIA License 18D0983143. The Actual In-service DON’T DO’S • Four swabs per test • One swab at a time • Two swabs per cheek • Thirty seconds per swab • One minute dry • • • • Blood is ok Swabs in envelope Envelopes in Ziploc Forms in Pouch QUESTIONS? THANK YOU!