* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Electrons in Atoms
Survey
Document related concepts
Transcript
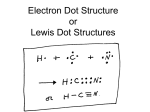
Electrons in Atoms Chapter 2 Section 2 Objectives How does the electron relate to the modern atomic theory? How do electron energy levels in an atom differ from one another? How are Lewis electron dot diagrams used to illustrate valence electrons? Important Vocabulary Electromagnetic spectrum Emission spectrum Energy level Electron cloud Valence electrons Lewis dot diagrams Wavelength Frequency Electrons Motion & Energy Electrons have enough energy to keep them in constant motion around the nucleus This enables them to overcome the attraction of the positive nucleus Electrons occupy orbitals of only certain amounts of energy For them to move up a level of energy, energy must be added For them to move down a level of energy, energy must be released in the form of light or heat Waves Transfer Energy Energy is the ability to exert a force over a certain distance It is also the ability to do work Waves carry energy because they can do work For example: Water waves can transfer energy to a leaf, to a boat, or onto a beach Sound waves can transfer energy to your eardrum Light waves can transfer energy to your eye The bigger the wave the _______ energy it carries Electromagnetic Radiation Electromagnetic radiation travels in the form of waves that have both electric and magnetic properties Electromagnetic waves travel through a vacuum at the speed of light (300 million m/s) Two properties of waves are frequency and wavelength Wavelength & Frequency • Wavelength is the distance from one crest to the next • Frequency is the number of waves per second • A low frequency results in a long wavelength and a high frequency results in a shorter wavelength Electromagnetic Spectrum The electromagnetic spectrum consists of electromagnetic radiation waves at all possible energies, frequencies, and wavelengths The spectrum ranges from 103 m to 10-12 meters Each part of the electromagnetic spectrum has unique properties Radio Waves Heinrich Hertz proved the existence of radio waves in the late 1880s Radio waves have wavelengths that range from 200 to 600 m Have the longest wavelengths and the lowest energy They are used as TV signals, AM and FM radio signals, and for radar equipment Radio telescopes view planets, comets, giant clouds of gas and dust, stars, and galaxies Microwaves Are low energy, low frequency radiation waves They are used in Doppler radar for weather forecasting and to cook your food Microwaves are also used to carry telecommunication signals Most mobile phones use microwaves to transmit information, and space probes transmit signals back to Earth with microwaves Different wavelengths of microwaves (grouped into "sub-bands") provide different information to scientists. Infrared Waves In 1800, William Herschel discovered them Have less energy than visible light Are given off by the human body and other warm objects We experience infrared rays as heat from fires and electric heaters Through night-vision goggles and infrared thermal cameras we can see infrared waves Visible Spectrum Cone-shaped cells in our eyes act as receivers tuned to the wavelengths in this narrow band of the spectrum A typical human eye will respond to wavelengths from about 380 to 750 nm The spectrum does not contain all the colors that the human eye and brain can distinguish Unsaturated colors such as pink, and purple colors such as magenta, are absent because they can only be made by a mix of multiple wavelengths When white light shines through a prism, the white light is broken apart into the colors of the visible light spectrum Water vapor in the atmosphere can also break apart wavelengths creating a rainbow Ultraviolet Waves In 1801, Johann Ritter discovered them Have shorter wavelengths than visible light UV waves are invisible to the human eye, but some insects, such as bumblebees, can see them. The Sun is a source of the full spectrum of ultraviolet radiation, which is commonly subdivided into UV-A, UV-B, and UV-C X-rays X-rays were first observed and documented in 1895 by German scientist Wilhelm Conrad Roentgen X-rays have very small wavelengths, between 0.03 and 3 nanometers X-rays are used by doctors to see the internal structures of the body X rays have very high energies, so they may kill living cells or turn them into cancer cells when exposed to too much of this type of radiation Gamma Rays Have the highest energy and shortest wavelengths They are produced by the hottest and most energetic objects in the universe, such as neutron stars and pulsars, supernova explosions, and regions around black holes On Earth, gamma waves are generated by nuclear explosions, lightning, and the less dramatic activity of radioactive decay Gamma rays can be used to treat cancer by killing the diseased cells Electrons and Light When electrons become excited they give off light The spectrum of light released is called the emission spectrum Each element has a different emission spectrum So it serves as evidence of energy levels within atoms Energy Levels There are seven levels of energy available for electrons to occupy Electrons can move between energy levels like the rungs of a ladder absorbing or releasing energy The number of filled energy levels depends on the number of electrons Lower levels are filled first Energy Levels 1st energy level = 2 electrons 2nd energy level = 8 electrons 3rd energy level = 8 electrons 4th energy level = 18 electrons 5th energy level = 18 electrons 6th energy level = 32 electrons 7th energy level = 32 electrons For example: Na has 11 electrons 2 in level 1 8 in level 2 1 in level 3 Valence Electrons Every atom has between 1 and 8 valence electrons Valence electrons are electrons in the outermost energy level of an atom They determine an atom’s chemical properties and its ability to form bonds For example: Neon Has 10 electrons 2 electrons in the lowest level 8 electrons in the 2nd level Thus, it has 8 valence electrons! Lewis Dot Diagrams Valence electrons are usually the only electrons used in chemical reactions We represent valence electrons as Lewis dot diagrams, which illustrate the valence electrons of an element as dots Normally, all the elements within a group have the same Lewis dot structure with the exception of Helium Lewis Dot Diagrams