* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download If this leaflet is difficult to see or read please contact the following
Pharmacognosy wikipedia , lookup
Plateau principle wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Oral rehydration therapy wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Ciprofloxacin wikipedia , lookup
Intravenous therapy wikipedia , lookup
Dydrogesterone wikipedia , lookup
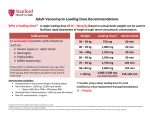
PACKAGE LEAFLET: INFORMATION FOR THE USER Vancomycin 500 mg & 1000 mg Powder for concentrate for solution for infusion and oral solution Vancomycin Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. Keep this leaflet. You may need to read it again. • If you have any further questions, ask your doctor or nurse. • This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. • If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4. The name of your medicine is one of the following: Vancomycin 500 mg Powder for concentrate for solution for infusion and oral solution Vancomycin 1000 mg Powder for concentrate for solution for infusion and oral solution In the rest of this leaflet your medicine is called Vancomycin. _____________________________________________________________________ What is in this leaflet: 1. What Vancomycin is and what it is used for 2. What you need to know before you are given Vancomycin 3. How you are given Vancomycin 4. Possible side effects 5. How to store Vancomycin 6. Contents of the pack and other information ___________________________________________________________________ 1. What Vancomycin is and what it is used for Vancomycin contains the active ingredient vancomycin hydrochloride, which is an antibiotic. Vancomycin powder is made into a solution for infusion or oral solution. Vancomycin infusion is used to treat: • very serious infections that cannot be treated with other, better tolerated, antibiotics such as penicillins and cephalosporins. • severe staphylococcal (a type of bacteria) infections in patients who cannot be given or do not respond to penicillins and cephalosporins, or who have staphylococcal infections that are resistant to other antibiotics. • endocarditis (infection of the inside lining of the heart) and to prevent endocarditis in patients at risk from dental or surgical procedures. • other infections caused by the staphylococcal bacteria such as bone infection (osteomyelitis), lung tissue infection (pneumonia), blood poisoning (septicaemia) and skin and muscle (soft tissue) infections. Vancomycin can be given orally for the treatment of two specific bacterial gut infections, namely, staphylococcal enterocolitis and pseudomembranous colitis (caused by Clostridium difficile). 2. What you need to know before you are given Vancomycin Do not take Vancomycin • If you are allergic to vancomycin or any of the other ingredients of this medicine (listed in section 6). Warnings and precautions Talk to your doctor or nurse before taking Vancomycin if you: • have an inflammatory disorder of the digestive tract (you may be at risk of side effects, especially if you also have a kidney disorder). • have a kidney disorder (you will need to have your blood and kidneys tested during treatment). • are elderly (you may need your blood and hearing monitored). • have a hearing disorder, especially if you are elderly (you may need hearing tests during treatment). • are receiving Vancomycin for a long time (you may develop another type of infection). Other medicines and Vancomycin Tell your doctor or nurse if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription. This is especially important of the following, as they may interact with your Vancomycin: • anaesthetics – these may cause redness, flushing, fainting, collapse or even heart attacks. You should, therefore, tell your doctor that you are taking Vancomycin if you are going to have an operation. • any drug that affects your nerves or kidneys such as amphotericin B (treats fungal infections), aminoglycosides, bacitracin, polymixin B, colistin, viomycin (antibiotics) or cisplatin (a chemotherapy drug). It may still be all right for you to be given Vancomycin and your doctor will be able to decide what is suitable for you. Pregnancy and breast-feeding If you are pregnant, or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or nurse for advice before taking this medicine. Ask your doctor or nurse for advice before taking any medicines. Driving and using machines Vancomycin should not affect your ability to drive or use machines. 3. How you are given Vancomycin Always take this medicine exactly as your doctor or nurse has told you. Check with your doctor or nurse if you are not sure. Dosage The dose of Vancomycin will depend on your age, the type of infection you have, and other conditions. Infusions should be given over at least 60 minutes and the solution you are given should not contain more than 5 mg/ml of Vancomycin. The usual adult infusion dose is 500mg every six hours or 1000 mg every twelve hours. Before it is given to you, this medicine will initially be dissolved in water and then added to an infusion bag containing sodium chloride (salt) or dextrose (a sugar) solution. It will then be given as a slow infusion though a drip into a vein. The usual infusion dose for children is 10 mg/kg (body weight) given every 6 hours (total daily dose 40 mg/kg of body weight). The elderly, pregnant mothers, premature infants, and patients with a kidney disorder may need a different dose. The usual adult oral dose is 500 mg per day in divided doses for 7 to 10 days. The maximum daily dose is 2 g/ day for severe infections. Each dose can be added to 30 ml water and either given to the patient to drink, or given by nasogastric tube (a tube passing through the nose into the stomach). The usual oral dose for children is 40 mg/kg (body weight) in three or four doses for 7 to 10 days. The maximum daily dose is 2 g. If you take more Vancomycin than you should As Vancomycin will be given to you whilst you are in hospital it is unlikely that you will be given too little or too much; however, tell your doctor or nurse if you have any concerns. If you have any further questions on the use of your medicine, ask your doctor or nurse. 4. Possible side effects Like all medicines this medicine can cause side effects, although not everybody gets them. Stop taking the medicine immediately and seek medical attention if signs of an allergic reaction occur: • hives; swelling of your face, lips, tongue or throat; difficulty breathing or swallowing or dizziness. If you think you have any of the following side effects or symptoms, tell your doctor as soon as possible: Common side effects (affect 1 to 10 users in 100): • decrease in blood pressure; swelling, redness and pain along a vein; • breathlessness, a high pitched sound resulting from turbulent air flow in the upper airway; • generalised rash and mucosal inflammation, itching, itchy rash; • redness of the upper body and the face, pain and spasm of the chest and back muscles; • kidney problems which may be detected primarily by increased creatinine or urea concentrations in your blood. Uncommon side effects (affect 1 to 10 users in 1,000): • temporary or permanent loss of hearing. Rare side effects (affect 1 to 10 users in 10,000): • anaphylactic reactions, allergic reactions; • drug fever, chills; • increased or reduced (sometimes severely decreased) urine output, or traces of blood in urine; • increase or decrease in some of the cells in the blood; • noises (e.g.) hissing in ears; • feeling faint; • red or purple skin (possible signs of blood vessel inflammation); • nausea. Very rare side effects (affect less than 1 user in 10,000): Skin disorders resulting from an allergic reaction (multiple skin lesions, joint aches), cardiac arrest, or inflammation of the bowel which causes abdominal pain or bloody diarrhoea. Reporting of side effects If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via: For UK: Yellow Card Scheme, Website: www.mhra.gov.uk/yellowcard. For IE: HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517, Website: www.hpra.ie, E-mail: [email protected]. By reporting side effects you can help provide more information on the safety of this medicine. 5. How to store Vancomycin Your doctor or nurse knows how to store Vancomycin. Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the carton and the label on the glass container (vial) after (EXP). The expiry date refers to the last day of that month. Store below 25°C. Keep the vial in the outer carton in order to protect from light. Once reconstituted, Vancomycin solution for infusion should be used immediately. Chemical and physical in-use stability has been demonstrated for 24 hours at 2-8 oC. Your doctor will ensure that the solution is not discoloured or contains particles. Solutions intended for oral dosing may be stored in a refrigerator (2°-8°C) for 96 hours. Do not throw away any medicines via wastewater or household waste. Ask your doctor how to throw away medicines you no longer use. 6. Contents of the pack and other information What Vancomycin contains The active substance is vancomycin hydrochloride 500 mg or 1000 mg. The other ingredient is hydrochloric acid. Each glass container will contain either 500 mg (equivalent to 525,000 IU) or 1000mg (equivalent to 1,050,000 IU) of vancomycin (as hydrochloride). What Vancomycin looks like and contents of pack Vancomycin is a powder for concentrate for solution for infusion. This means liquid must be added to make a solution and more liquid must then be added to dilute the solution before it can be given to you intravenously (by infusion). Normally your doctor or nurse will prepare your medicine before it is given to you. Each pack contains 1, 5, 10 or 20 vials (glass containers). Not all pack sizes may be available. Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder: Noridem Enterprises Ltd., Evagorou & Makariou, Mitsi Building 3, Office 115, 1065 Nicosia, Cyprus. Manufacturer: DEMO S.A., 21st km National Road Athens - Lamia, 14568 Krioneri, Athens, Greece. This medicinal product is authorised in the Member States of the EEA under the following names: UK: Vancomycin 500 mg / 1 g Powder for Concentrate for Solution for Infusion Ireland: Vancomycin 500 mg / 1 g Powder for Concentrate for Solution for Infusion Germany: Vancomycin Noridem 500 mg / 1000 mg Pulver zur Herstellung einer Infusionslösung Austria: Vancomycin Noridem 500 mg / 1000 mg Pulver zur Herstellung einer Infusionslösung Greece: Vancomycin Noridem 500 mg / 1 g Κόνις για πυκνό σκεύασμα για Παρασκευή Διαλύματος προς Έγχυση Spain: Vancomycin KERN PHARMA 500 mg / 1 g Polvo para Concentrado para Solución para Infusión This leaflet was last approved in January 2016. If this leaflet is difficult to see or read please contact the following address for help: Fannin Limited, Fannin House, South County Business Park, Leopardstown, Dublin 18, Ireland Tel: +353-1-2907000. -----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------The following information is intended for healthcare professionals only: 1. NAME OF THE MEDICINAL PRODUCT Vancomycin 500 mg Powder for concentrate for solution for infusion and oral solution. Vancomycin 1000 mg Powder for concentrate for solution for infusion and oral solution. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains 500 mg vancomycin (equivalent to 525,000 IU) (as vancomycin hydrochloride). When reconstituted with 10 ml of water for injections, the resulting concentrate for solution for infusion contains 50 mg/ml vancomycin. Each vial contains 1000 mg vancomycin (equivalent to 1,050,000 IU) (as vancomycin hydrochloride). When reconstituted with 20 ml of water for injections, the resulting concentrate for solution for infusion contains 50 mg/ml vancomycin. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Powder for concentrate for solution for infusion and oral solution. A white crystalline powder. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Vancomycin is indicated in potentially life-threatening infections which cannot be treated with other effective, less toxic antimicrobial drugs, including the penicillins and cephalosporins. Vancomycin is useful in the therapy of severe staphylococcal infections in patients who cannot receive or who have failed to respond to the penicillins and cephalosporins, or who have infections with staphylococci resistant to other antibiotics. Vancomycin is used in the treatment of endocarditis and as prophylaxis against endocarditis in patients at risk from dental or surgical procedures. Its effectiveness has been documented in other infections due to staphylococci, including osteomyelitis, pneumonia, septicaemia and soft tissue infections. Vancomycin may be used orally for the treatment of staphylococcal enterocolitis and pseudomembranous colitis due to Clostridium difficile. Parenteral administration of vancomycin is not effective for these indications. Intravenous administration may be used concomitantly if required. Consideration should be given to official guidance on the appropriate use of antibacterial agents. 4.2 Posology and method of administration For intravenous infusion and oral use only and not for intramuscular administration. Infusion-related adverse events are related to both concentration and rate of administration of vancomycin. Concentrations of no more than 5 mg/ml are recommended. In selected patients in need of fluid restriction, a concentration up to 10 mg/ml may be used; use of such higher concentrations may increase the risk of infusion-related events. Infusions should be given over at least 60 minutes. In adults, if doses exceeding 500 mg are used, a rate of infusion of no more than 10 mg/min is recommended. Infusionrelated events may occur, however, at any rate or concentration. Intravenous Infusion in Patients with Normal Renal Function Adults: The usual intravenous dose is 500 mg every six hours or 1 g every 12 hours, in Sodium Chloride Intravenous Infusion BP or 5% Dextrose Intravenous Infusion BP. Each dose should be administered at no more than 10 mg/min. Other patient factors, such as age, obesity or pregnancy, may call for modification of the usual daily dose. The majority of patients with infections caused by organisms sensitive to the antibiotic show a therapeutic response within 48-72 hours. The total duration of therapy is determined by the type and severity of the infection and the clinical response of the patient. In staphylococcal endocarditis, treatment for three weeks or longer is recommended. Pregnancy: It has been reported that significantly increased doses may be required to achieve therapeutic serum concentrations in pregnant patients, but see 'Warnings'. The elderly: Dosage reduction may be necessary to a greater extent than expected because of decreasing renal function (see below). Monitor auditory function - see 'Warnings' and 'Precautions'. Paediatric population: The usual intravenous dosage is 10 mg/kg per dose given every 6 hours (total daily dosage 40 mg/kg of body weight). Each dose should be administered over a period of at least 60 minutes. Neonates have a larger volume of distribution and incompletely developed renal function; therefore dosing guidelines differ from those recommended for children and adults. In neonates and young infants, the total daily dosage may be lower. An initial dose of 15 mg/kg is suggested, followed by 10 mg/kg every 12 hours in the first week of life and every 8 hours thereafter until one month of age. Each dose should be administered over 60 minutes. Close monitoring of serum vancomycin concentrations may be warranted in these patients. One dosing nomogram for dosing vancomycin in neonates is illustrated in the following table. PCA (Weeks) a <30 30-36 >36 Vancomycin Dosage Guideline for Neonates Chronological Age (Days) Serum ≤7 >7 ≤14 >14 ≤7 >7 Dosage (mg/kg) Creatinine Concentration (mg/dl)b ……c <12 15 every 24 hr 10 every 12 hr ---≤.6 0.7 - 1.2 ----≤0.6 0.7 - 1.2 10 every 12 hr 10 every 8 hr 10 every 12 hr 10 every 12 hr 10 every 8 hr 10 every 12 hr PCA = post conceptual age (gestational age at birth plus chronological age) If the serum creatinine concentration is >1.2 mg/dl, use an initial dosage of 15 mg/kg every 24 hours Serum creatinine concentration is not used to determine the dosage for this type of patient because of its lack of reliability or because of the lack of information. a b c Patients with impaired renal function: Dosage adjustments must be made to avoid toxic serum levels. In premature infants and the elderly, greater dosage reductions than expected may be necessary because of decreased renal function. Regular monitoring of serum levels is advised in such patients, as accumulation has been reported, especially after prolonged therapy. Vancomycin serum concentrations may be determined by use of a microbiological assay, radioimmunoassay, fluorescence polarisation immunoassay, fluorescence immunoassay or high-pressure liquid chromatography. The following nomogram, based on creatinine clearance values, is provided: The nomogram is not valid for functionally anephric patients on dialysis. For such patients, a loading dose of 15 mg/kg body weight should be given to achieve therapeutic serum levels promptly, and the dose required to maintain stable levels is 1.9 mg/kg/24 hours. Since individual maintenance doses of 250 mg to 1 g are convenient, in patients with marked renal impairment a dose may be given every several days rather than on a daily basis. In anuria a dose of 1 g every 7 to 10 days has been recommended. Preparation of solutions: see ‘Instructions for use and handling’. Measurement of serum concentrations: Following multiple intravenous doses, peak serum concentrations, measured 2 hours after infusion is complete, range from 18-26 mg/l. Trough levels measured immediately prior to the next dose should be 5-10 mg/l. Ototoxicity has been associated with serum drug levels of 80-100 mg/l, but this is rarely seen when serum levels are kept at or below 30 mg/l. Oral Administration The contents of vials for parenteral administration may be used. Adults and the elderly: The usual daily dose given is 500 mg in divided doses for 7 to 10 days, although up to 2 g/day have been used in severe cases. The total daily dosage should not exceed 2 g. Each dose may be reconstituted in 30 ml water and either given to the patient to drink, or administered by nasogastric tube. Children: The usual daily dose is 40 mg/kg in three or four divided doses for 7 to 10 days. The total daily dosage should not exceed 2 g. Common flavouring syrups may be added to the solution at the time of administration to improve the taste. 4.3 Contraindications Hypersensitivity to vancomycin or to any of the excipients listed in section 6.1. 4.4 Special warnings and precautions for use Oral Administration The contents of vials for parenteral administration may be used. Adults and the elderly: The usual daily dose given is 500 mg in divided doses for 7 to 10 days, although up to 2 g/day have been used in severe cases. The total daily dosage should not exceed 2 g. Each dose may be reconstituted in 30 ml water and either given to the patient to drink, or administered by nasogastric tube. Children: The usual daily dose is 40 mg/kg in three or four divided doses for 7 to 10 days. The total daily dosage should not exceed 2 g. Common flavouring syrups may be added to the solution at the time of administration to improve the taste. 4.3 Contraindications Hypersensitivity to vancomycin or to any of the excipients listed in section 6.1. 4.4 Special warnings and precautions for use Warnings Rapid bolus administration (e.g., over several minutes) may be associated with exaggerated hypotension, including shock, and, rarely, cardiac arrest. Vancomycin should be infused in a dilute solution over a period of not less than 60 minutes to avoid rapid infusion-related reactions. Stopping the infusion usually results in a prompt cessation of these reactions (see 'Posology and method of administration' and 'Undesirable effects' sections). Some patients with inflammatory disorders of the intestinal mucosa may have significant systemic absorption of oral vancomycin and, therefore, may be at risk for the development of adverse reactions associated with the parenteral administration of vancomycin. The risk is greater in patients with renal impairment. It should be noted that the total systemic and renal clearances of vancomycin are reduced in the elderly. Due to its potential ototoxicity and nephrotoxicity, vancomycin should be used with care in patients with renal insufficiency and the dose should be reduced according to the degree of renal impairment. The risk of toxicity is appreciably increased by high blood concentrations or prolonged therapy. Blood levels should be monitored and renal function tests should be performed regularly. Vancomycin should also be avoided in patients with previous hearing loss. If it is used in such patients, the dose should be regulated, if possible, by periodic determination of the drug level in the blood. Deafness may be preceded by tinnitus. The elderly are more susceptible to auditory damage. Experience with other antibiotics suggests that deafness may be progressive despite cessation of treatment. Vancomycin should be administered with caution in patients allergic to teicoplanin, since allergic cross reactions between vancomycin and teicoplanin have been reported. Paediatric population: In premature neonates and young infants, it may be appropriate to confirm desired vancomycin serum concentrations. Concomitant administration of vancomycin and anaesthetic agents has been associated with erythema and histamine-like flushing in children. Use in elderly: The natural decrement of glomerular filtration with increasing age may lead to elevated vancomycin serum concentrations if dosage is not adjusted (see 'Posology and method of administration'). Precautions Clinically significant serum concentrations have been reported in some patients being treated for active C. difficile-induced pseudomembranous colitis after multiple oral doses of vancomycin. Therefore, monitoring of serum concentrations may be appropriate in these patients. Patients with borderline renal function and individuals over the age of 60 should be given serial tests of auditory function and of vancomycin blood levels. All patients receiving the drug should have periodic haematological studies, urine analysis and renal function tests. Vancomycin is very irritating to tissue, and causes injection site necrosis when injected intramuscularly; it must be infused intravenously. Injection site pain and thrombophlebitis occur in many patients receiving vancomycin and are occasionally severe. The frequency and severity of thrombophlebitis can be minimised by administering the drug slowly as a dilute solution (2.5 to 5.0 g/l) and by rotating the sites of infusion. Prolonged use of vancomycin may result in the overgrowth of non-susceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken. In rare instances, there have been reports of pseudomembranous colitis, due to C. difficile, developing in patients who received intravenous vancomycin. 4.5 Interaction with other Medicinal Products and other Forms of Interaction Concomitant administration of vancomycin and anaesthetic agents has been associated with erythema, histamine-like flushing and anaphylactoid reactions. There have been reports that the frequency of infusion-related events increases with the concomitant administration of anaesthetic agents. Infusion-related events may be minimised by the administration of vancomycin as a 60-minute infusion prior to anaesthetic induction. Concurrent or sequential systemic or topical use of other potentially neurotoxic or nephrotoxic drugs, such as amphotericin B, aminoglycosides, bacitracin, polymixin B, colistin, viomycin or cisplatin, when indicated, requires careful monitoring. 4.6 Pregnancy and lactation Pregnancy: Teratology studies have been performed at 5 times the human dose in rats and 3 times the human dose in rabbits, and have revealed no evidence of harm to the foetus due to vancomycin. In a controlled clinical study, the potential ototoxic and nephrotoxic effects of vancomycin hydrochloride on infants were evaluated when the drug was administered to pregnant women for serious staphylococcal infections complicating intravenous drug abuse. Vancomycin hydrochloride was found in cord blood. No sensorineural hearing loss or nephrotoxicity attributable to vancomycin was noted. One infant, whose mother received vancomycin in the third trimester, experienced conductive hearing loss that was not attributable to vancomycin. Because vancomycin was administered only in the second and third trimesters, it is not known whether it causes foetal harm. Vancomycin should be given in pregnancy only if clearly needed and blood levels should be monitored carefully to minimise the risk of foetal toxicity. It has been reported, however, that pregnant patients may require significantly increased doses of vancomycin to achieve therapeutic serum concentrations. Breastfeeding: Vancomycin hydrochloride is excreted in human milk. Caution should be exercised when vancomycin is administered to a nursing woman. It is unlikely that a nursing infant can absorb a significant amount of vancomycin from its gastro-intestinal tract. 4.7 Effects on ability to drive and use machines Not applicable. 4.8 Undesirable effects The most common adverse reactions are phlebitis and pseudo-allergic reactions in connection with too rapid intravenous use (by infusion) of vancomycin. Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness. The adverse reactions listed below is defined using the following MedDRA convention and system organ class database: very common ( 1/10); common ( 1/100 to < 1/10); uncommon ( 1/1,000 to < 1/100); rare ( 1/10,000 to < 1/1,000); very rare (< 1/10,000), not known (cannot be estimated from the available data). Blood and the lymphatic system disorder: Rare (≥ 10,000 to ≤1/1,000): Thrombocytopenia, neutropenia, agranulocytosis, eosinophilia. Immune system disorders: Rare (≥ 10,000 to ≤1/1,000): Anaphylactic reactions, hypersensitivity reactions. Ear and labyrinth disorders: Uncommon (≥ 1,000 to ≤1/1 00): Transient or permanent loss of hearing. Rare (≥ 10,000 to ≤1/1,000): Tinnitus, dizziness. Cardiac disturbances: Very rare (≤1/10,000): Cardiac arrest Vascular disorders: Common (>1/100 to ≤1/10): Decrease in blood pressure, thrombophlebitis. Rare (≥ 10,000 to ≤1/1,000): Leukocytoclastic vasculitis. Respiratory, thoracic and mediastinal disorders: Common (>1/100 to ≤1/10): Dyspnoea, stridor. Gastrointestinal disorders: Rare (≥ 10,000 to ≤1/1,000): Nausea. Very rare (≤1/10,000): Pseudomembranous enterocolitis. Skin and subcutaneous tissue disorders: Common (>1/100 to ≤1/10): Exanthema and mucosal inflammation, pruritus, urticaria. Very rare (≤1/10, 000): Exfoliative dermatitis, Stevens-Johnson syndrome, Lyell’s syndrome, vasculitis, IgA induced bullous dermatitis, Rash maculopopular. Renal and urinary disorders: Common (>1/100 to ≤1/10): Renal insufficiency manifested primarily by increased serum creatinine or serum urea concentrations. Rare (≥ 10,000 to ≤1/1,000): Interstitial nephritis, acute renal failure. General disorders and administration site conditions: Common (>1/100 to ≤1/10): Red man syndrome (Redness of the upper body and the face). Pain and spasm of the chest and back muscles. Rare (≥ 10,000 to ≤1/1,000): Drug fever, shivering. During or shortly after rapid infusion anaphylactic reactions may occur. The reactions abate when administration is stopped, generally between 20 minutes and 2 hours after having stopped administration. Drug rash with eosinophilia and systemic symptoms (DRESS syndrome) has been reported during postmarketing experience. Ototoxicity has primarily been reported in patients given high doses, or concomitant treatment with other ototoxic medicinal products, or had pre-existing reduction in kidney function or hearing. 4.9 Overdose Supportive care is advised, with maintenance of glomerular filtration. Vancomycin is poorly removed from the blood by haemodialysis or peritoneal dialysis. Haemoperfusion with Amberlite resin XAD-4 has been reported to be of limited benefit. 5. PHARMACOLOGICAL PROPERTIES 5.1 Pharmacodynamic properties Pharmacotherapeutic group: Glycopeptide antibacterials ATC code: J01XA01 for intravenous use and A07 AA09 for oral use Vancomycin is a glycopeptide antibiotic derived from Amycolatopsis orientalis. The primary mode of action of vancomycin is inhibition of cell-wall synthesis. In addition, vancomycin may alter bacterial cell membrane permeability and RNA synthesis. There is no cross-resistance between vancomycin and other classes of antibiotics. EUCAST Clinical MIC Breakpoints The non-species related breakpoints for susceptible (S) and resistant (R) species are: S <4 mg/L and R >8 mg/L. For Staphylococcus spp. S ≤4 mg/L and R >8 mg/L For Enterococcus spp. S ≤4 mg/L and R >8 mg/L For Streptococcus ABCG S ≤4 mg/L and R >4 mg/L For S. pneumoniae S ≤4 mg/L and R >4 mg/L The prevalence of acquired resistance may vary geographically and with time for selected species and local information on resistance is desirable, particularly when treating severe infections. As necessary, expert advice should be sought when the local prevalence of resistance is such that the utility of the agent in at least some types of infections is questionable. Commonly susceptible species: Gram-positive aerobes Enterococcus faecalis Staphylococcus aureus Coagulase-negative staphylococci Streptococcus group B Streptococcus group C Streptococcus group G Streptococcus pneumoniae Streptococcus pyogenes Viridans streptococci Species for which acquired resistance may be a problem Gram-positive aerobes Enterococcus faecium Clostridium difficile (e.g. toxigenic strains implicated in pseudomembranous colitis) is a target species for oral use where high intraluminal concentrations of vancomycin are achieved. 5.2 Pharmacokinetic properties Vancomycin is given intravenously for therapy of systemic infections. In subjects with normal renal function, multiple intravenous dosing of 1 g of vancomycin (15 mg/kg) infused over 60 minutes produces mean plasma concentrations of approximately 63 mg/L immediately after the completion of infusion, mean plasma concentrations of approximately 23 mg/L 2 hours after infusion. Multiple dosing of 500 mg infused over 30 minutes produces mean plasma concentrations of about 49 mg/L at the completion of infusion, mean plasma concentrations of about 19 mg/L 2 hours after infusion, and mean plasma concentrations of about 10 mg/L 6 hours after infusion. The plasma concentrations during multiple dosing are similar to those after a single dose. The mean elimination half-life of vancomycin from the plasma is 4 to 6 hours in patients with normal renal function. About 75% of an administered dose of vancomycin is excreted in urine by glomerular filtration in the first 24 hours. Mean plasma clearance is about 0.058 L/kg/h, and mean renal clearance is about 0.048 L/kg/h. Renal vancomycin clearance is fairly constant and accounts for 70% to 80% of vancomycin elimination. The volume of distribution ranges from 0.39 to 0.97 L/kg. There is no apparent metabolism of the drug. Vancomycin is 55% protein bound as measured by ultrafiltration at vancomycin serum levels of 10 to 100 mg/L. After IV administration of vancomycin hydrochloride, inhibitory concentrations are present in pleural, pericardial, ascitic, atrial appendage tissue and synovial fluid, as well as urine and peritoneal fluid. Vancomycin does not readily penetrate the cerebrospinal fluid unless the meninges are inflamed. Renal dysfunction slows excretion of vancomycin. In anephric patients, the average half-life of elimination is 7.5 days. The total systemic and renal clearance of vancomycin may be reduced in the elderly due to the natural decrement of glomerular filtration. Vancomycin is not significantly absorbed from the normal gastro-intestinal tract and is therefore not effective by the oral route for infections other than staphylococcal enterocolitis and pseudomembranous colitis due to Clostridium difficile. Orally administered vancomycin does not usually enter the systemic circulation even when inflammatory lesions are present. Measurable serum concentrations may occur infrequently in patients with active C. difficile- induced pseudomembranous colitis and, in the presence of renal impairment, the possibility of accumulation exists. Administration of vancomycin oral solution, 2 g daily for 16 days to anephric patients with no inflammatory bowel disease, gave serum levels of <0.66 μg/ml. With doses of 2 g daily, concentration of 3,100 mg/kg can be found in the faeces and levels of <1 μg/ml can be found in the serum of patients with normal renal function who have pseudomembranous colitis. 5.3 Preclinical safety data Although no long-term studies in animals have been performed to evaluate carcinogenic potential, no mutagenic potential of vancomycin was found in standard laboratory tests. No definitive fertility studies have been performed. 6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients Hydrochloric acid (for pH adjustment). 6.2 Incompatibilities Vancomycin solution has a low pH that may cause chemical or physical instability when it is mixed with other compounds. Mixing with alkaline solutions should be avoided. Each parenteral solution should be checked visually for precipitation and discolouration prior to use. Mixtures of solutions of vancomycin and beta-lactam antibiotics have been shown to be physically incompatible. The likelihood of precipitation increases with higher concentrations of vancomycin. It is recommended to adequately flush the intravenous lines between the administrations of these antibiotics. It is also recommended to dilute solutions of vancomycin to 5 mg/mL or less. Although intravitreal injection is not an approved route of administration for vancomycin, precipitation has been reported after intravitreal injection of vancomycin and ceftazidime for endophthalmitis using different syringes and needles. The precipitates dissolved gradually, with complete clearing of the vitreous cavity over two months and with improvement of visual acuity. This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6. 6.3 Shelf life Powder as packaged for sale 500mg vial: 3 years 1000 mg vial: 30 months After reconstitution /dilution From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage items and conditions prior to use are the responsibility of the user. Chemical and physical in-use stability has been demonstrated for 24 hours at 2-8 oC. 6.4 Special precautions for storage Powder: Store below 25°C. Keep the vial in the outer carton to protect from light. After reconstitution /dilution: For storage conditions of the reconstituted/diluted medicinal product, see section 6.3. Prior to administration, parenteral medicinal products should be inspected visually for particulate matter and discolouration whenever solution or container permits. Solutions of the parenteral powder intended for oral administration may be stored in a refrigerator (2°-8°C) for 96 hours. 6.5 Nature and contents of container 500 mg presentation: Colourless, glass (Type I) vials of 19 ml, closed with rubber (Type I) closures and sealed with aluminium caps and a blue plastic flip-off top. 1 g presentation: Colourless, glass (Type I) vials of 30.5 ml, closed with rubber (Type I) closures and sealed with aluminium caps and a red plastic flipoff top. Pack Sizes: 1, 5, 10 and 20. Not all pack sizes may be marketed. 6.6 Special precautions for disposal and other handling Preparation of the reconstituted concentrate At the time of use, the content of each 500 mg vial is dissolved in 10 ml of sterile water for injections. One ml of reconstituted solution contains 50 mg of vancomycin. At the time of use, the content of each 1000 mg vial is dissolved in 20ml Water for Injections Ph. Eur. One ml of reconstituted solution contains 50 mg of vancomycin. For storage conditions of the reconstituted medicinal product, see section 6.3. FURTHER DILUTION IS REQUIRED. Read instructions which follow: 1. Intermittent infusion is the preferred method of administration. Reconstituted solutions containing 500 mg vancomycin must be diluted with at least 100 ml diluent. Sodium Chloride Intravenous Infusion BP or 5% Dextrose Intravenous Infusion BP are suitable diluents. Reconstituted solutions containing 1000 mg vancomycin must be diluted with at least 200 ml diluent. Sodium Chloride Intravenous Infusion BP or 5% Dextrose Intravenous Infusion BP are suitable diluents. The desired dose should be given by intravenous infusion, over a period of at least 60 minutes. If administered over a shorter period of time or in higher concentrations, there is the possibility of inducing marked hypotension in addition to thrombophlebitis. Rapid administration may also produce flushing and a transient rash over the neck and shoulders. 2. Continuous infusion (should be used only when intermittent infusion is not feasible). 1000 mg -2000 mg can be added to a sufficiently large volume of Sodium Chloride Intravenous Infusion BP or 5% Dextrose Intravenous Infusion BP to permit the desired daily dose to be administered slowly by intravenous drip over a 24 hour period. For storage conditions of the diluted medicinal product, see section 6.3. Before administration, the reconstituted and diluted solutions should be inspected visually for particulate matter and discoloration. Only clear and colourless solution free from particles should be used. 3. Oral administration The contents of vials for parenteral administration may be used. Common flavouring syrups may be added to the solution at the time of administration to improve the taste. Disposal Vials are for single use only. Unused product must be discarded. Any unused product or waste material should be disposed of in accordance with local requirements. 7. MARKETING AUTHORISATION HOLDER Noridem Enterprises Ltd, Evagorou & Makariou, Mitsi Building 3, Office 115, 1065 Nicosia, Cyprus. 8. MARKETING AUTHORISATION NUMBER(S) 500mg: PL 24598/0015, PA1122/008/001 1g: PL 24598/0016, PA1122/008/002 9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION 03/06/2011 10. 01/2016 DATE OF REVISION OF THE TEXT