* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Colonies
Phospholipid-derived fatty acids wikipedia , lookup
Microorganism wikipedia , lookup
Disinfectant wikipedia , lookup
Magnetotactic bacteria wikipedia , lookup
Triclocarban wikipedia , lookup
Marine microorganism wikipedia , lookup
Human microbiota wikipedia , lookup
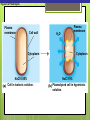
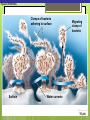
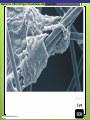
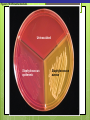
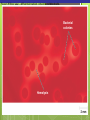
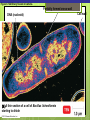
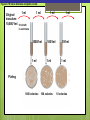
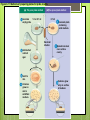
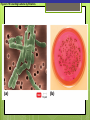
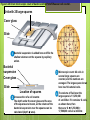
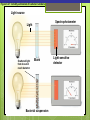
Microbial Growth Increase in number of cells, not cell size Colonies- groups of cells large enough to be seen without a microscope The Requirements for Growth Physical requirements Temperature pH Osmotic pressure Chemical requirements Carbon Nitrogen, sulfur, and phosphorous Trace elements Oxygen Organic growth factors Physical Requirements Temperature Minimum growth temperature- lowest temp. an organism will grow Optimum growth temperature- organisms grow BEST Maximum growth temperature- highest temp. an organism will grow Physical Requirements Temperature Psychrophiles- cold loving microbes; 025 C; deep oceans and ice Mesophiles- moderate temperature loving microbes; 25-40 C; PATHOGENS Thermophiles- heat loving microbes; 5060 C; hot springs and volcanoes F to °C- Deduct 32, then multiply by 5, then divide by 9 °C to °F- Multiply by 9, then divide by 5, then add 32 Figure 6.1 Typical growth rates of different types of microorganisms in response to temperature. Thermophiles Hyperthermophiles Mesophiles Psychrotrophs Psychrophiles Applications of Microbiology 6.1 A white microbial biofilm is visible on this deep-sea hydrothermal vent. Water is being emitted through the ocean floor at temperatures above 100°C. Psychrotrophs Grow between 0°C and 20–30°C (refrigerator) Cause food spoilage Mold, slime, change in colors and taste pH Most bacteria grow between pH 6.5 and 7.5 Molds and yeasts grow between pH 5 and 6 Acidophiles grow in acidic environments Osmotic Pressure Hypertonic environments, or an increase in salt or sugar, cause plasmolysis (shrinking) Extreme or obligate halophiles require high osmotic pressure Facultative halophiles tolerate high osmotic pressure Figure 6.4 Plasmolysis. Plasma membrane Cell wall Plasma membrane H2O Cytoplasm NaCl 0.85% Cell in isotonic solution. Cytoplasm NaCl 10% Plasmolyzed cell in hypertonic solution. Chemical Requirements CARBON Structural organic molecules, energy source Chemoheterotrophs- carbon from organic carbon sources Autotrophs- carbon from CO2 Chemical Requirements NITROGEN Makes amino acids and proteins Most bacteria decompose proteins to get Nitrogen Some bacteria use ammonium or nitrate A few bacteria use nitrogen gas from the atmosphere in nitrogen fixation Chemical Requirements SULFUR In amino acids, thiamine, and biotin Most bacteria decompose proteins Sulfate or Hydrogen Sulfide- source of sulfur PHOSPHORUS In DNA, RNA, ATP, and cell membranes Phosphate-source of phosphorus Chemical Requirements TRACE ELEMENTS Inorganic elements required in small amounts Usually as enzyme cofactors Table 6.1 The Effect of Oxygen on the Growth of Various Types of Bacteria Organic Growth Factors Organic compounds obtained from the environment Organism is unable to synthesize them on their own Vitamins, amino acids, purines, and pyrimidines Biofilms Microbial communities Form slime Usually attached to surfaces Quorum Sensing- bacteria are attracted to each other through chemical Coordinate their activities Benefit each other Share nutrients Sheltered from harmful factors- MORE RESISTANT Figure 6.5 Biofilms. Clumps of bacteria adhering to surface Surface Water currents Migrating clump of bacteria Applications of Microbiology 3.2 Pseudomonas aeruginosa biofilm. © 2013 Pearson Education, Inc. Biofilms in Health CDC- 70% of infections due to biofilms Nosocomial infections Indwelling catheters Heart valves Culture Media Culture medium: media with nutrients prepared for microbial growth Sterile: no living microbes; media must FIRST be this Inoculum: introduction of microbes into medium Culture: microbes growing in/on culture medium Agar Polysaccharide from algae Used as solidifying agent for culture media in Petri plates, slants, and deeps Generally not metabolized by microbes Liquefies at 100°C Solidifies at ~40°C Culture Media Must contain energy, chemicals and growth factors Chemically defined media: exact chemical composition is known Complex media: extracts and digests of yeasts, meat, or plants Nutrient broth Nutrient agar Table 6.2 A Chemically Defined Medium for Growing a Typical Chemoheterotroph, Such as Escherichia coli Anaerobic Culture Methods Reducing media Contain chemicals that combine with O2 Heated before use to get rid of any O2 Figure 6.7 An anaerobic chamber. Air lock Arm ports Capnophiles Microbes that require high CO2 conditions CO2 packet Candle jar Selective Media Suppress or inhibit unwanted microbes from growing while encouraging desired microbes Ex: MacConkey Agar Differential Media Make it easy to distinguish colonies of different microbes by creating a VISUAL change A type of SELECTIVE MEDIA Ex: Mannitol Salt Agar, Blood Agar Plate Figure 6.10 Differential medium. Uninoculated Staphylococcus epidermis Staphylococcus aureus Figure 6.9 Blood agar, a differential medium containing red blood cells. Bacterial colonies Hemolysis Enrichment Culture Encourages growth of desired microbe (SELECTIVE) Increase growth as well Used for fecal or soil samples with many microorganisms Obtaining Pure Cultures Pure Culture- contains only one species Colony (Colony Forming Unit CFU)population of cells arising from a single cell or spore or from a group of attached cells Streak plate methodused to isolate pure cultures Sterile inoculating loop is used to streak plate in 4 quadrants Figure 6.11 The streak plate method for isolating pure bacterial cultures. 1 2 3 Colonies Preserving Bacterial Cultures Deep-freezing: –50° to –95°C Lyophilization (freeze-drying): frozen (–54° to –72°C) and dehydrated in a vacuum Container is sealed by heat Reproduction in Prokaryotes Binary fission Budding Conidiospores ANIMATION Bacterial Growth: Overview Figure 6.12b Binary fission in bacteria. Partially formed cross-wall DNA (nucleoid) (b) A thin section of a cell of Bacillus licheniformis starting to divide © 2013 Pearson Education, Inc. Cell wall Figure 6.13a Cell division. Generation Time The time it take for a bacteria to DOUBLE it’s population Varies from organism to organism Phases of Growth Lag Phase- cells are getting use to their new environment Not much cellular division Metabolism is occuring 1hr- several days Log Phase- period of growth and celluar division Most active Phases of Growth Stationary Phase- number of new cells being made EQUALS the number of cells dying Conditions begin to deteriorate Death Phase- number of deaths are greater than number of new cells being made All or most of the cells die as conditions continue to deteriorate Figure 6.15 Understanding the Bacterial Growth Curve. Lag Phase Log Phase Stationary Phase Death Phase Intense activity preparing for population growth, but no increase in population. Logarithmic, or exponential, increase in population. Period of equilibrium; microbial deaths balance production of new cells. Population Is decreasing at a logarithmic rate. The logarithmic growth in the log phase is due to reproduction by binary fission (bacteria) or mitosis (yeast). Staphylococcus spp. Phases of Growth ANIMATION Bacterial Growth Curve Plate Counts Most frequently used Sample is diluted several time in a process called SERIAL DILUTION before inoculated onto a plate. Inoculation Pour Plate Spread Plate After incubation, count colonies on plates that have 25–250 colonies (CFUs) Serial Dilutions FIRST dilution-10,000 bacteria/1ml SECOND dilution- add 1ml from 1st dilution to 9ml(sterile) then you would have 10,000 bacteria/10ml which would be 1000/1ml. THIRD dilution- add 1ml from 2nd dilution to 9ml(sterile) then you would have 1000ml/10ml which would be 100/1ml. This would be a COUNTABLE plate. Figure 6.16 Serial dilutions and plate counts. Original inoculum10,000/1ml 1 ml 1 ml 1 ml 1 ml 9 m broth in each tube 1000/1ml 1 ml 100/1ml 10/1ml 1 ml 1 ml Plating 1000 colonies 100 colonies 10 colonies Plate Counts 1. 2. 3. 4. Pour Plate 1ml or 0.1ml of sample Add melted agar Mix Media solidifies with colonies on and in media Only good for a count not identification. Colonies can be damaged. Spread Plate 1. 2. 3. Inoculate 1ml of sample onto solid agar. Spread evenly. Colonies only grow on the surface. Better for identification of colonies. Figure 6.17 Methods of preparing plates for plate counts. The pour plate method The spread plate method Inoculate 1.0 or 0.1 ml empty plate. 0.1 ml Inoculate plate containing solid medium. Bacterial dilution Add melted nutrient agar. Spread inoculum over surface evenly. Swirl to mix. Colonies grow on and in solidified medium. Colonies grow only on surface of medium. Filtration Used in samples with small amounts of organisms. 1. Liquid is passed through a membrane with small pores. 2. Filter is transferred to a petri dish with nutrient broth and colonies grow on the filter. Figure 6.18 Counting bacteria by filtration. Direct Microscopic Count A measured volume of bacterial suspension is placed within a defined area on a slide. Dye is added to view bacteria. Look within a large square (1ml). Count colonies. Each colony= 1,250,000 bacteria 14 colonies x 1,250,000 = 17,500,000 cells/ml Figure 6.20 Direct microscopic count of bacteria with a Petroff-Hausser cell counter. Grid with 25 large squares Cover glass Slide Bacterial suspension is added here and fills the shallow volume over the squares by capillary action. Bacterial suspension Microscopic count: All cells in several large squares are counted, and the numbers are averaged. The large square shown here has 14 bacterial cells. Cover glass Slide Location of squares Cross section of a cell counter. The depth under the cover glass and the area of the squares are known, so the volume of the bacterial suspension over the squares can be calculated (depth × area). The volume of fluid over the large square is 1/1,250,000 of a milliliter. If it contains 14 cells, as shown here, then there are 14 × 1,250,000 = 17,500,000 cells in a milliliter. Turbidity Cloudiness in a liquid which signifies growth. Spectrophotometer- light is transmitted through the liquid. Percentage is measured. 100% light transmitted- no turbidity Figure 6.21 Turbidity estimation of bacterial numbers. Light source Spectrophotometer Light Scattered light that does not reach detector Blank Bacterial suspension Light-sensitive detector Measuring Microbial Growth Direct Methods Plate counts Filtration MPN Direct microscopic count Indirect Methods Turbidity Metabolic Dry weight activity