* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Variation in auditory neuropathy spectrum disorder
Survey
Document related concepts
Noise-induced hearing loss wikipedia , lookup
Sound localization wikipedia , lookup
Sensorineural hearing loss wikipedia , lookup
Speech perception wikipedia , lookup
Audiology and hearing health professionals in developed and developing countries wikipedia , lookup
Lip reading wikipedia , lookup
Transcript
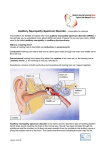
Variation in Auditory Neuropathy Spectrum Disorder: Implications for Evaluation and Management Linda J. Hood, Ph.D.1 ABSTRACT Downloaded by: SASLHA. Copyrighted material. Auditory neuropathy spectrum disorder (ANSD) has an effect, either directly or indirectly, on the neural processing of auditory stimuli. Physiological measures are needed to accurately identify and assess ANSD. Sound processing in patients with ANSD is highly variable, and relationships between hearing sensitivity and ability to process speech do not follow the typical hearing loss rules. Auditory function may show changes over time, with progression or fluctuation, or may remain stable. Considerable variation in presentation is observed across patients of all ages and includes milder forms of ANSD. Based on these observations, management should proceed with thorough assessment of individual capabilities. It is important to distinguish between detection (sensitivity) and discrimination of sound, especially in noise, when evaluating function and benefit from various forms of management. Visual information is important for most patients and cochlear implants provide significant benefit when that option is selected. As the understanding of ANSD and the inherent variation among patients advances, the knowledge and methods that we bring to our clinical practices should allow us to more accurately characterize function and implement appropriate management. KEYWORDS: Auditory neuropathy, auditory dys-synchrony, auditory neuropathy spectrum disorder, cochlear hair cell response, auditory neural response Learning Outcomes: As a result of this activity, the participant will be able to (1) identify the characteristics of auditory neuropathy spectrum disorder and appreciate the variation among patients, and (2) make appropriate recommendations for the management of patients with auditory neuropathy spectrum disorder. 1 Professor, Department of Hearing and Sciences; Associate Director for Research, National Center for Childhood Deafness; Vanderbilt University, Tennessee. Address for correspondence and reprint requests: Linda J. Hood, Ph.D., Associate Director for Research, National Center for Childhood Deafness; Vanderbilt University, Nashville, TN 37212 (e-mail: linda.j.hood@Vanderbilt. edu). Proceedings of the Widex Pediatric Audiology Congress; Guest Editor, André M. Marcoux, Ph.D. Semin Hear 2011;32:117–122. Copyright # 2011 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New York, NY 10001, USA. Tel: +1(212) 584-4662. DOI: http://dx.doi.org/10.1055/s-0031-1277232. ISSN 0734-0451. 117 SEMINARS IN HEARING/VOLUME 32, NUMBER 2 2011 P atients with present cochlear responses and absent/abnormal auditory brain stem responses (ABRs) are classified as having auditory neuropathy (AN),1 AN/dys-synchrony (AD),2 or AN spectrum disorder (ANSD).3 Though these terms have somewhat different intended meanings and origins, at present they often are used interchangeably. More specific and accurate descriptors will likely emerge with better understanding of underlying mechanisms. Clinical presentation in AN (AN/AD, ANSD) typically involves inordinate difficulty listening in noise, possible fluctuation in hearing over time, and/or delayed speech and language development. Some patients with fluctuations in auditory responses accompanying elevated temperature are reported.4 Physiological responses are key factors in accurately identifying and characterizing AN. Presence of cochlear responses is documented by otoacoustic emissions (OAE) and cochlear microphonics. Neural responses are either directly or indirectly affected, and abnormalities are demonstrated by absent or highly abnormal ABRs, middle ear muscle reflexes (MEMRs), and medial olivocochlear reflexes (noted via OAE suppression). Behavioral responses are variable as shown by audiometric configurations from normal to profound ranges and variable but generally poor speech recognition, particularly in noise. VARIATION AMONG PATIENTS WITH ANSD Variation is broad among ANSD patients.5,6 Factors related to variation include time of onset, underlying mechanisms, genetics, possible risk factors, ability to understand speech, and changes over time. Some patients display no overt delays or auditory complaints until adulthood or in some cases until, by chance, MEMR or ABR testing is completed. Patients at the other end of the continuum display an apparent total lack of sound awareness, reflected in their severely affected communication and speech production abilities. However, most ANSD patients fall between these two extremes, showing inconsistent auditory responses with best responses in quiet and poor- est in noise. Their audiograms can be misleading or fluctuate. The ABR is desynchronized and middle-ear muscle reflexes are absent or in some cases elevated. These characteristics, and particularly their variation, present the clinician with several challenges. Accurate characterization of ANSD includes understanding variation among and within particular forms of ANSD along with the ability to identify factors contributing to the variation. As our understanding of the underlying mechanisms, physiological responses, and genetic factors improves, so should our ability to better characterize and distinguish among forms of ANSD. More accurate characterization should also have a positive impact on management planning. To understand the characteristics and variation of ANSD, we (the group at the Kresge Hearing Research Laboratory) developed a database of patients and recently summarized data related to 260 of those patients.6 Most of these patients are under 12 years of age, though some adults also are included. The majority of the patients are pediatric because these are the groups who are actively being tested through newborn hearing and school screening programs. We believe that there are adults with ANSD who simply have not been identified. Patients were included in the database based on presence of OAEs (currently or at some point in their history) and cochlear microphonics with absent or abnormal MEMRs and ABRs. Review of OAE data showed that 76% of the patients had present OAEs when the diagnostically definitive testing for AN/AD was completed. About 16% had absent OAEs, which in most cases was accompanied by abnormal middle-ear test results, and the remaining patients had partially present OAEs. MEMRs were absent in 90% of patients.7 When MEMRs were elicited, none were normal; they were elevated or in combination with elevated or absent responses at other frequencies. The medial olivocochlear reflex, measured via suppression of OAEs, is also substantially reduced or absent in patients with ANSD.8 Most patients have absent ABRs, though some show responses only to high-intensity stimuli. In our database, 75% of the ANSD Downloaded by: SASLHA. Copyrighted material. 118 patients had absent ABRs, and 25% showed some evidence of neural responses. In these cases, ABRs typically showed only low-amplitude wave V and, as noted earlier, only at higher (i.e., 75 dB hearing level in normal listeners for brief signals and above) intensities. The variation among individuals in behavioral responses highlights the importance of physiological responses in assessment and makes management of ANSD particularly challenging. Thresholds for tonal stimuli (e.g., pure-tone audiometric thresholds) range from normal to profound. Speech recognition is typically poorer than seen in other forms of hearing loss, though some patients display quite good word recognition in quiet. All ANSD patients tested thus far in our experience have very poor (i.e., poorer than expected based on threshold sensitivity) speech understanding ability in noise. As a part of our database analysis, we summarized speech recognition ability for 68 subjects over the age of 4 years.6 Our rationale for limiting the analysis to this age range related to the ability to use standardized assessment measures. In this group, 38 of the 68 individuals had no word recognition ability for monosyllables in quiet. Thus, the remainder of the analysis focused on the remaining 30 subjects. Of those, 25 had measureable word recognition only in quiet (i.e., 0% in noise). Word recognition in quiet averaged 45% in this group, with some patients having very poor word recognition ability (i.e., < 20%) and others quite good (i.e., > 80%). The five subjects of the original 30 with word recognition ability in quiet and in noise are our best performers and, despite quite good ability in quiet, they still fall below those with other forms of hearing loss in noise. These findings are quite consistent with those reported by Rance et al.9 Difficulty in understanding speech has been linked to marked abnormalities observed on temporal processing tasks.10,11 The incidence of ANSD is 1 in 10 patients with desynchronized ABRs. This is based on several studies that have included data from newborn screening as well as evaluation of children enrolled in schools for the deaf.12–15 Several factors have been explored in relation to ANSD. In our database, 6.2% display unilat- eral ANSD, with the majority having normal hearing in the other ear. Some risk factors are often seen in the history of individuals with ANSD, and we and others have summarized those.6,16,17 Several genetic mutations have been identified that are associated with ANSD.18,19 Families display recessive and dominant inheritance patterns, as well as some that are likely mitochondrial in nature. ANSD occurs alone, as in nonsyndromic recessive ANSD, as well as part of a syndrome, such as hereditary motor sensory neuropathies. Among the various genes linked to ANSD, OTOF mutations, responsible for production of the protein otoferlin, with localized expression at the inner hair cell synaptic juncture, have been of particular interest. IMPLICATIONS OF VARIABLE FUNCTION FOR MANAGEMENT OF PATIENTS WITH ANSD Management of ANSD differs from typical sensorineural hearing loss. In many cases, discrimination of sound is affected to a much greater degree than detection of sound. For example, some patients have quite good sound detection with threshold sensitivity in the mild hearing loss range but have very poor or no word and sentence understanding. Variation in clinical presentation is further highlighted by differences among patients in the effectiveness of various management approaches. Although some patients are reported to demonstrate benefit from amplification, the majority of patients with ANSD in our practices (Louisiana State University Health Sciences Center–New Orleans and Vanderbilt University) have not been successful in using amplification alone to facilitate the development of speech and language or general communication. We evaluate the effectiveness of amplification in all of our ANSD patients who demonstrate reduced threshold sensitivity to sound. In our practice, we fit amplification using targets based on behavioral thresholds, which typically can be obtained at an age of around 6 months. A key factor in assessing benefit is separating improvement from detection of sound, where we typically observe 119 Downloaded by: SASLHA. Copyrighted material. VARIATION IN AUDITORY NEUROPATHY SPECTRUM DISORDER/HOOD SEMINARS IN HEARING/VOLUME 32, NUMBER 2 2011 changes postfitting, from ability related to discrimination of sound. Because discrimination of sound is necessary for communication and speech and language development, we closely follow each individual’s progress in assessing the effectiveness of amplification. Assistive listening (FM) devices are very helpful, which is consistent with the particular difficulty that ANSD patients experience in noisy situations. The improved signal-to-noise ratios provide a clearer signal to an auditory system that cannot cope with interference.20 In addition, use of visual information is strongly encouraged in the management of ANSD patients. Communication systems, such as cued speech or sign languages, facilitate language development in infants and children.21 Both children and adults with ANSD demonstrate improved speech perception and significant benefit from cochlear implants.22–25 Despite variation among patients and early reports of limited benefit, ANSD patients generally derive considerable benefit from cochlear implants in improved hearing and speech perception abilities. As might be expected, more favorable postimplantation speech perception scores are reported for ANSD subjects with normal cochlear nerves as compared with those with cochlear nerve anomalies.26,27 Other techniques such as clear speech25 and envelope enhancement28 show some promise in improving word recognition. FUTURE NEEDS: ISSUES AND CHALLENGES Although our understanding of ANSD has advanced, several issues and challenges remain. The observed variation among patients highlights the importance of developing information and methods that will allow clinicians to accurately distinguish among the various forms of ANSD. Progress in this area will likely include the development of methods that can accurately distinguish normal from abnormal function at inner hair cell, synaptic juncture, and neural levels and the identification of specific risk factors related to ANSD. Even within various forms of ANSD, we expect that variation will exist, and we need to understand the range of variation, the role of genetics and variation within and among genetic mutations, and other contributing physiological and environmental factors. Another challenge in evaluating and managing ANSD in infants is distinguishing between neuromaturation of the auditory system, where neural responses improve over the first year or so of life, and true ANSD. Are there characteristics of physiological responses, patient history, or other factors that can distinguish neuromaturation from ANSD? In infants and children with ANSD, how can we predict who will develop speech/language with minimal intervention despite poor neural synchrony reflected in absent or highly abnormal ABRs? In developing appropriate management strategies and an evidence base, guidelines are needed for determination of appropriate recommendations and fitting practices for hearing aids, frequency modulation systems, cochlear implants, and appropriate use of various intervention strategies. Studies of patients with ANSD have demonstrated sensitivity of electrocochleography and compound action potential physiological responses from the cochlea and VIIIth nerve in characterizing variation in function among patients.29,30 Psychophysical tasks, particularly those related to temporal resolution, and cortical responses provide insight into underlying mechanisms and functional abilities. Cortical evoked potentials also show promise in characterizing auditory function objectively and in establishing links with speech recognition.31,32 As future clinical assessment facilitates greater use of novel stimuli and paradigms, more sensitive approaches, and development of clinically feasible methods that include these advances, we should be able to more accurately characterize and understand the variation we observe in ANSD. This understanding also should support more informed approaches to patient management. REFERENCES 1. Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain 1996;119(Pt 3): 741–753 Downloaded by: SASLHA. Copyrighted material. 120 2. Berlin C, Hood L, Rose K. On renaming auditory neuropathy as auditory dys-synchrony: implications for a clearer understanding of the underlying mechanisms and management options. Audiology Today 2001;13:15–17 3. Hayes D, Sininger YS. Identification and management of infants and young children with auditory neuropathy spectrum disorder. Presented at: The Newborn Hearing Systems Conference; June 19–21, 2008; Lake Como, Italy 4. Starr A, Sininger Y, Winter M, Derebery MJ, Oba S, Michalewski HJ. Transient deafness due to temperature-sensitive auditory neuropathy. Ear Hear 1998;19(3):169–179 5. Starr A, Sininger YS, Pratt H. The varieties of auditory neuropathy. J Basic Clin Physiol Pharmacol 2000;11(3):215–230 6. Berlin CI, Hood LJ, Morlet T, et al. Multi-site diagnosis and management of 260 patients with auditory neuropathy/dys-synchrony (auditory neuropathy spectrum disorder). Int J Audiol 2010; 49(1):30–43 7. Berlin CI, Hood LJ, Morlet T, et al. Absent or elevated middle ear muscle reflexes in the presence of normal otoacoustic emissions: a universal finding in 136 cases of auditory neuropathy/dys-synchrony. J Am Acad Audiol 2005;16(8):546–553 8. Hood LJ, Berlin CI, Bordelon J, Rose K. Patients with auditory neuropathy/dys-synchrony lack efferent suppression of transient evoked otoacoustic emissions. J Am Acad Audiol 2003;14(6):302– 313 9. Rance G, Barker E, Mok M, Dowell R, Rincon A, Garratt R. Speech perception in noise for children with auditory neuropathy/dys-synchrony type hearing loss. Ear Hear 2007;28(3):351–360 10. Zeng FG, Oba S, Garde S, Sininger Y, Starr A. Temporal and speech processing deficits in auditory neuropathy. Neuroreport 1999;10(16): 3429–3435 11. Rance G, McKay C, Grayden D. Perceptual characterization of children with auditory neuropathy. Ear Hear 2004;25(1):34–46 12. Berlin CI, Hood LJ, Morlet T, et al. The search for auditory neuropathy patients and connexin 26 patients in schools for the deaf. ARO Abstracts 2000;23:23 13. Lee JSM, McPherson B, Yuen KCP, Wong LLN. Screening for auditory neuropathy in a school for hearing impaired children. Int J Pediatr Otorhinolaryngol 2001;61(1):39–46 14. Rance G, Beer DE, Cone-Wesson B, et al. Clinical findings for a group of infants and young children with auditory neuropathy. Ear Hear 1999;20(3): 238–252 15. Sininger YS. Auditory neuropathy in infants and children: implications for early hearing detection 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. and intervention programs. Audiology Today 2002;14:16–21 Deltenre P, Mansbach AL, Bozet C, Clercx A, Hecox KE. Auditory neuropathy: a report on three cases with early onsets and major neonatal illnesses. Electroencephalogr Clin Neurophysiol 1997;104(1):17–22 Madden C, Rutter M, Hilbert L, Greinwald JHJr, Choo DI. Clinical and audiological features in auditory neuropathy. Arch Otolaryngol Head Neck Surg 2002;128(9):1026–1030 Varga R, Kelley PM, Keats BJ, et al. Nonsyndromic recessive auditory neuropathy is the result of mutations in the otoferlin (OTOF) gene. J Med Genet 2003;40(1):45–50 Starr A, Michalewski HJ, Zeng FG, et al. Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene (Tyr145> Ser). Brain 2003;126(Pt 7):1604–1619 Hood LJ, Wilensky D, Li L, Berlin CI. The role of FM technology in the management of patients with auditory neuropathy/dys-synchrony. In: Fabry DA, De Conde Johnson C, eds. ACCESS: Achieving, Clear Communication Employing Sound Solutions–2003. Proceedings of the 1st International FM Conference; 2004; Chicago, IL Berlin CI, Li L, Hood LJ, Morlet T, Rose K, Brashears S. Auditory neuropathy/dys-synchrony: after the diagnosis, then what? Semin Hear 2002; 23:209–214 Trautwein PG, Sininger YS, Nelson R. Cochlear implantation of auditory neuropathy. J Am Acad Audiol 2000;11(6):309–315 Shallop JK, Peterson A, Facer GW, Fabry LB, Driscoll CLW. Cochlear implants in five cases of auditory neuropathy: postoperative findings and progress. Laryngoscope 2001;111(4 Pt 1):555– 562 Peterson A, Shallop J, Driscoll C, et al. Outcomes of cochlear implantation in children with auditory neuropathy. J Am Acad Audiol 2003;14(4): 188–201 Zeng FG, Liu S. Speech perception in individuals with auditory neuropathy. J Speech Lang Hear Res 2006;49(2):367–380 Buchman CA, Roush PA, Teagle HF, Brown CJ, Zdanski CJ, Grose JH. Auditory neuropathy characteristics in children with cochlear nerve deficiency. Ear Hear 2006;27(4):399–408 Walton J, Gibson WP, Sanli H, Prelog K. Predicting cochlear implant outcomes in children with auditory neuropathy. Otol Neurotol 2008; 29(3):302–309 Narne VK, Vanaja CS. Perception of envelopeenhanced speech in the presence of noise by individuals with auditory neuropathy. Ear Hear 2009;30(1):136–142 121 Downloaded by: SASLHA. Copyrighted material. VARIATION IN AUDITORY NEUROPATHY SPECTRUM DISORDER/HOOD SEMINARS IN HEARING/VOLUME 32, NUMBER 2 2011 29. McMahon CM, Patuzzi RB, Gibson WP, Sanli H. Frequency-specific electrocochleography indicates that presynaptic and postsynaptic mechanisms of auditory neuropathy exist. Ear Hear 2008;29(3):314–325 30. Santarelli R, Starr A, Michalewski HJ, Arslan E. Neural and receptor cochlear potentials obtained by transtympanic electrocochleography in auditory neuropathy. Clin Neurophysiol 2008;119(5): 1028–1041 31. Michalewski HJ, Starr A, Nguyen TT, Kong YY, Zeng FG. Auditory temporal processes in normalhearing individuals and in patients with auditory neuropathy. Clin Neurophysiol 2005;116(3):669– 680 32. Rance G, Cone-Wesson B, Wunderlich J, Dowell R. Speech perception and cortical event related potentials in children with auditory neuropathy. Ear Hear 2002;23(3):239–253 Downloaded by: SASLHA. Copyrighted material. 122