* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Definition of Corrosion
Survey
Document related concepts
Transcript
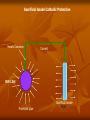
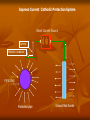
Definition of Corrosion Corrion is a destructive attack of a metal by chemical or electrochemical reaction with its environment. Types of Corrosions Uniform Attack Galvanic Corrosion Crevice Corrosion Intergranular Corrosion Stress Corrosion High Temperature Corrosion Uniform Attack Uniform attack is a form of electrochemical corrosion that occurs with equal intensity of the entire surface of the metal. Iron rust when exposed to air and water, and silver tarnishes due to exposure to air. Galvanic corrosion Galvanic corrosion occurs when two metals having different composition are electrically coupled in the presence of an electrolyte. The more reactive metal will experience sever corrosion while the more noble metal will be quite well protected. Perhaps the most infamous examples of this type of combination such as steel and brass or copper and steel. Crevice Corrosion Crevice corrosion is consequence of concentration difference of ions or dissolved gases in an electrolytic solution. A solution became trapped between a pipe and the flange on the left. The stagnant liquid in the crevice eventually had a lowered dissolved oxygen concentration and crevice corrosion took over and destroyed the flange. Intergranular Corrosion Occurring along grain boundaries for some alloy, the heating of the some material causes chromium carbide to form from the chromium and carbon in the metal. This leaves a chromium deficient boundary just shy of the where the metal was heated for welding. PREVENTION OF CORROSION By Material Selection By Environmental modification By Corrosion inhibitors By Paints By Coating By Cthodic protection By Interaction between the above methods Principle of Cathodic Protection Cathodic Protection is to make the potential of the whole surface of the steel structure sufficiently negative with respect to the surround medium to ensure that no current flows from the metal into the medium. Method of Cathodic Protection Sacrificial Anode System Impressed direct current System Sacrificial Anode System With the Sacrificial anode method, use is made of galvanic action to provide the cathodic protection current. The surface of the structure is made by connecting it electrically to mass of less noble metal buried or immersed in to common electrolyte. Sacrificial Anode Cathodic Protection Metallic Conductor Current PIPE LINE Protected pipe Sacrificial Anode ZINIC Impress Direct Current System Impress direct current method, the structure is connected to the negative of a direct current power supply and the positive of the supply is connected to a number of anodes buried in ground or impressed in water. Impress Current Cathodic Protection System Direct Current Source Current Metallic Conductor PIPE LINE Protected pipe Ground Bed Anode THE ELECTROMOTIVE SERIES Electrodes Electrode Potential, volt Sodium -2.712 Magnesium -2.340 Aluminum -1.670 Zinc -0.760 Chromium (Trivalent) - 0.710 Iron (divalent) -0.440 Nickel -0.250 Tin -0.136 Lead -0.126 Hydrogen 0.000 Copper (divalent) +0.345 Copper (monovalent) +0.552 Silver + 0.800 Platinum +1.200 Gold (monovalent) +1.680 Base End or Anodic end Noble End Cathodic End THE GALVANIC SERIES Magnesium Anode - Corroding End Aluminum Zinc Least Noble, Cadmium Electro - negative Steel Cast iron Chromium (Active) Stain less Steel (Active) Soft Solder Tin Lead Nickel Brass Bronze Copper Silver solder Chromium (Passive) Cathode - Protected End Silver Graphite Most Noble, Gold Electro - Positive Platinum Types of Anodes Graphite Anode High Silicon cast iron Anode Mixed- metal oxide Anode Platinum coated Anode Lead alloy Anode Types of ground Bed Deep Ground Bed Ground bed more then 50feet deep. Shallow Ground Bed Ground bed up to 50 feet depth Types of backfill Standard metallurgical coke breeze Petroleum coke backfill Natural or manufactured graphite Corrosion cell due to dissimilarity of metals Protective current to old pipe-Large Cathode Old pipe Old pipe New pipe Corrosion current to new pipe- Small Anode RESISTIVITY A resistor is an element that resist the movement of electrical charge. The unit of resistance is the ohm. A resistance of one ohm will allow one ampere to pass when one volt of potential difference is applied. Electrical resistivity (ρ) has the ohm-cm and is physical property of the material. R= ρ L/A R = resistanenc (ohm) L = length (cm) of the current flow path A = area (cm2) perpendicular to current flow path ρ = Resistivity (ohm-cm) SOIL RESISTIVITY The resistivity of the soil( electrolyte) largely determine the magnitude of corrosion current in any corrosion cell. Thus lower the resistivity the greater would be the intensity of the corrosive attack. And if cathodic protection is required, the soil resistivity will determine the magnitude of current generated by sacrificial anode or the resistance to ground of impressed current anode. Cathodic Protection A technique to prevent corrosion of a metal surface by making it the cathode of an electrochemical cell