* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemistry 112
Survey
Document related concepts
Transcript
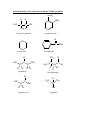
Chemistry 111/112 Laboratory Vining Instrumental Analysis Instrumental analysis is a subfield of chemistry involving a variety of techniques where samples are analyzed by observing how they absorb or emit electromagnetic radiation (light), how they act in a magnetic field, or how they respond to some other perturbation. There are many techniques- fluorescence spectroscopy, electron-paramagnetic spectroscopy, mass spectrometry, - but the two most often used to identify structural properties of organic compounds are infrared spectroscopy and nuclear magnetic resonance spectroscopy. In this experiment we will first observe spectral features that arise for different kinds of compounds. Then, we will use that information to identify an unknown compound from a list of possibilities. PART 1. Infrared Spectroscopy Different ranges of wavelengths of light have different effects on molecules. Ultraviolet and visible light, for example, cause an electron to be promoted from a filled orbital to a vacant orbital at higher energy. Infrared light serves to excite molecular vibrations. Vibrations are molecular motions involving the stretching of bonds or the compression of bond angles. The energy (and therefore the wavelength) of light absorbed depends on many things, including the strength of the bonds and the masses of the bonded atoms. For our purpose here, we will use the fact that similar bonds in different molecules usually have IR absorptions at similar energies. Therefore, we have amassed tables of typical wavelength ranges for important structural features. These are compiled in Table 1, near the end of the handout. Part 1A. Spectra are provided here for four compounds, for which the structures are given. On each, identify as many absorption bands as you can be reasonably sure of. This process is called “assigning the spectra.” It is important to note that IR spectra can be pretty complex and you will not be able to assign many of the bands, especially those on the far right of the spectrum. Part 1B. You will receive an unknown compound. Take an IR spectrum of it, as shown during the laboratory period. Using the information you have about typical spectral bands, predict what types of functional groups (molecular fragments, types of bonds, etc.) your unknown has. IR ASSIGNMENT: Part 1A IR Spectra of Known Compounds: You draw lines from the molecular structure to the peak associated with that part of the molecule. IR Spectra of Known Compounds: You draw lines from the molecular structure to the peak associated with that part of the molecule. PART 2. Nuclear Magnetic Resonance Spectroscopy We have learned that electrons have spin properties, and that compounds with unpaired electrons are paramagnetic and influenced by a magnetic field. Unfortunately, very few molecules are paramagnetic, so this property is not very useful. Fortunately, many nuclei also have spin properties, and two very important ones- 1H and 13C- do, and are very useful for determining facets of molecular structure. The NMR experiment works by placing a sample in a very strong magnetic field. This magnet aligns along with some of the nuclei, but against others. This creates an energy difference between spins that can be probed. The most useful feature of this property is that for both nuclei the energy difference depends on the amount of electron density around that atom. The signals for atoms in such different “environments” therefore come at different places on the spectrum. We call these positions “chemical shifts,” and, like in IR spectroscopy, they are very well known for common structural features. Tables 2 and 3 (the last pages in this handout) contain typical chemical shifts for important functional groups for 1H and 13C NMR experiments. The shifts given in the table are not absolute. Consider the spectrum of 1-bromopropane. shown below. The presence of the electronegative Br atom shifts the C-H peaks to the left. Notice that the closer to the Br atom, the more the shift occurs. The H atoms four bonds away from the Br are not affected at all. Those only two bonds away are shifted by over 2 ppm. In general, the presence of an electronegative element will shift peaks to the left. The more electronegative and the closer that atom is, the greater the shift. In general, the more electronegative an atom bonded near the H being measured, the greater the shift to higher ppm values. Typical signals for H atoms bonded near C, Br, and O are shown below. Notice that as the electronegativity increases, the peaks shift left. In addition, for 1H NMR spectroscopy, another feature that can be identified is the number of similar H atoms a signal represents. This process is called “integration.” In an NMR spectrum, the relative integration is normally given numerically beside each signal. Carbon-13 NMR experiments can tell the number of different “types” of C atoms in a sample, but not the relative numbers of each. That is, you cannot obtain integration information in a 13C spectrum. What You Do in This Part: Part 2A. Spectra are provided here for four compounds, for which the structures are given. On each, identify as many chemical shift signal as you can be reasonably sure of. This process is again called “assigning the spectra.” In the case of NMR experiments, it is usually possible and desirable to assign all the signals. Part 2B. An 1H and 13C NMR spectra of your unknown compound (the same compound as that in the IR experiment) is given to you. Identify as many important structural features as you can from the NMR spectra. Part 2C. Combine the information from your NMR and IR experiments and determine the identity of your unknown from the list of possible compounds (on a page later in this handout). NMR ASSIGNMENT: Part 2A NMR Spectra of Known Compounds: You draw lines from the molecular structure to the peak associated with that part of the molecule. NMR Spectra of Known Compounds: You draw lines from the molecular structure to the peak associated with that part of the molecule. PART 3. Mass Spectrometry Mass spectrometry measures the mass of compounds. It does so by ionizing a sample of the molecules, forming cations. Those cations are then sent down a tube and deflected with a magnetic field. The degree that the magnetic field deflects them indicates their mass. A spectrum of a single compound seems like it would give only a single peak, at it molar mass. However, there are two complicating factors that make mass spectra more complex (which is a good thing because they therefore contain more information). Complicating Factor 1: Isotopes Consider the highest mass peaks in the mass spectrum of cyclohexane, C6H12. There are two peaks, one at a mass of 84 and a smaller one at mass 85. The peak at 84 comes from the mass of most of the molecules containing 6 atoms of 12C and 12 atoms of 1 H: 6 x 12 + 12 x 1 = 84. However, C has a second stable isotope, 13C, and H has a second stable isotope, 2H. Each of these occurs in about 1% of the atoms of those elements. Therefore, sometimes a molecule of C6H12 will have either a 13C atom or a 2H atom and will then have a mass of 85. Important isotope patterns: 1. All hydrocarbons will have small peaks with a mass 1 greater than the main molar mass. 2. Cl has two isotopes, 35Cl and 37Cl, with the 35Cl being three times more abundant. Therefore, any compound containing a single Cl atom will show a pair of peaks, two units apart, with the lower mass peak being three times as high as the greater mass peak. 3. Br has two isotopes, 79Br and 81Br, with the two being nearly equal in abundance. Therefore, any compound containing a single Br atom will show a pair of peaks, two units apart, with nearly equal height. Complicating Factor 1: Fragmentation When a molecule is ionized in the mass spec experiment, it often becomes unstable and is susceptible to a variety of fragmentation reactions. The products of these reactions vary widely and cannot be easily predicted, but they are repeatable and create a pattern of peaks that can be used to identify a particular compound. This is important because there are many different compounds that have the same molar mass. Because they fragment differently, they lead to differing mass spectra and can be distinguished. Sometimes, the fragments can be identified. Examine the mass spectrum of 1-bromopropane. The pair of peaks at 122/124 amu represent the parent molecule with the two Br isotopes. The peaks at 79 and 81 amu represent Br atoms that have fragmented from the main molecule. The peak at 43 amu is the remaining CH3CH2CH2 fragment left after removal of the Br atom. Mass Spectrum ASSIGNMENT: Part 3A Mass Spectra of Known Compounds: Indicate what molecule or fragment leads to each noted peak. In the case of pairs of peaks due to isotopes, indicate the different isotopes that lead to the different peaks. Problem 1. Consider the mass spectrum for ethyl bromoacetate, below. Indicate the formula of the molecule or fragment leading to each peak. Be sure to note the Br isotope in the species if it contains a Br atom. Problem 2. The spectrum below is for either CH3CH2CH2CH2CH2CH2OH or for CH3CH2CH2OCH2CH2CH3. Which is it, and how do you know? Or, can’t the two be told apart from this single experiment? PART 4. UV-VIS Spectrophotometry Anything you see that is colored contains compounds that absorb visible light. When visible light of all wavelengths are absorbed, an object appears black. When no visible light is absorbed, and object appears white. When only a portion of the visible wavelengths are absorbed, an object appears colored. For example, if an object absorbs blue light, it will look red. If it absorbs red light, it will appear blue. Chemists measure absorbance of light as a function of wavelength and report the results as a spectrum: a graph of how much light is absorbed (on the y axis) vs. wavelength (on the x axis). On the molecular scale, absorption of visible (or UV) light leads to promotion of an electron from one orbital in a molecule to another. The wavelength of light absorbed therefore tells us something about the relative energies of orbitals in the molecule. If the gap between the low and high energy orbitals is large, short wavelength light is absorbed. If the energy gap is small, long wavelength light is absorbed. E = h Recall: = c = 3.00 108 m/s Part 4A. Transition metal compounds often have nice colors due to absorption of visible light. The photons of light promote an electron from one set of d orbitals in the metal to another. The size of the energy gap between the two sets of orbitals can be measured by the energy of the light that is absorbed. This is interesting because the energy gap observed is indicative of the strength of the bonding between the metal and its complexing agents (the small molecules or ions that bind to the metal are called ligands, and the strength of their bonding to a metal varies). The larger the energy gap between the two sets of orbitals, the stronger the metal ligand bonding. Below are UV-VIS spectra for Cu(H2O)42+ and Cu(NH3)42+. Use the spectra to find: a. the energy gap between the orbitals, in kJ/mol b. which of the two ligands, H2O or NH3, bonds more strongly to the Cu2+ ion. Part 5. Electrochemistry Electrochemical techniques involve adding or removing one or more electrons from a molecule: that is, reducing or oxidizing a sample. What is measured is the voltage required to either reduce or oxidize the sample. The larger to voltage, the more difficult to cause the gain or loss of the electrons. The voltages measured tell us about how well a molecule holds on to its electrons, or how much it wants to add additional ones. That is, the voltage measurements tell us about orbital energies in the molecule. The most popular technique for this is cyclic voltammetry. An annotated voltammogram of the compound Fe(bpy)32+ is shown below. The compound has an Fe2+ metal center. As the voltage is increased above 0.40 V, the compound loses an electron, forming Fe(bpy)33+ ion. This is observed as a deflection downward. Above 0.40 V, the compound is in this oxidized form. When the voltage sweep turns around and approaches 0.40 V again, the compound is re-reduced (the electron is given back). This is seen as a deflection upwards. Part 5A. Cyclic voltammograms for three similar compounds are given below. Each compound consists of a metal center, surrounded by three bipyridine ligands. The metal is attached to six N atoms (two from each bipyridine ligand). The ions analyzed are: Fe(bpy)32+ Ru(bpy)32+ Co(bpy)32+ Use the voltammograms below to rank the three compounds in order of the difficulty of oxidizing them. Also, rank the three compounds in ascending order of the energy of their highest filled orbital (this is the orbital from which an electron is removed). Cyclic Voltammograms of Fe(bpy)32+, Ru(bpy)32+, and Co(bpy)32+. INSTRUMENTAL ANALYSIS LABORATORY CHECKSHEET Be sure you have done the following. Check each as you complete it to feel good. _____ Part 1A. Assign peaks in the provided IR spectra. _____ Part 1B. Take an IR spectrum of your unknown and assign its peaks. _____ Part 2A. Assign peaks in the provided NMR spectra. _____ Part 2B. Obtain a copy of the NMR spectrum of your unknown and assign the peaks. _____ Part 2C. Use the results of your IR and NMR spectra to identify your unknown from the list given. _____ Part 3. Problem 1. Identify the noted peaks in the given mass spectrum. _____ Part 3. Problem 2. Use the mass spectrum to decide which of the two compounds is the one studied. _____ Part 4A. Calculate the energy gap for the two spectra, in kJ/mol. _____ Part 4B. Use the results from part 4A to decide which compound has the stronger bonding ligands. _____ Part 5. Indicate the oxidation voltage of the three compounds. _____ Part 5. Rank the three compounds in terms of difficulty of oxidation and in terms of the energy of the highest filled orbital. Possible Identities of the Unknowns for IR and NMR Experiments OCH3 H3C H H C C NH2 OH CH3 H 2-methyl-1-propanol o-methoxyaniline O C cyclohexene acetophenone O H3C C C H C H CH3 H3C C C H H H 3-methylbutanal H H3C C H O H O C CH3 C H H 3-pentanone H3C CH3 OH C CH3 H OH propanoic acid 2-propanol Typical 1H Chemical Shift Values (ppm) H H C C sp3 C 0.9 - 1.5 O O C C next to a carbonyl group 2.0 - 2.3 next to an ether 3.3 - 4.5 O H H C H C C aromatic 6.5 - 8.0 sp2 carbon 4.6 - 6.4 H aldehyde 9.5 - 10.0 Typical 13C Chemical Shift Values (ppm) H H R C R H R C R H H 0 - 30 5 - 35 C O 45 - 95 R C R H H R2C CR2 R R C 160 - 180 C NH2 RC CR 60 - 90 R C Cl H RC 20 - 60 N 105 - 135 O NR2 H 25 - 70 O C H H 15 - 50 100 - 145 aromatic 100 - 170 H C 160 - 190 O OR R C 190 - 220 R (or H)