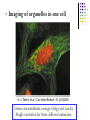
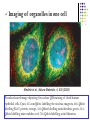
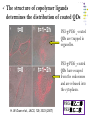
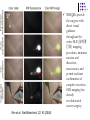
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 7.2 Radiative Recombination and Non
Condensed matter physics wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Density of states wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
Heat transfer physics wikipedia , lookup
Thermophotovoltaic wikipedia , lookup
Nanochemistry wikipedia , lookup
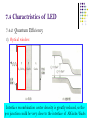
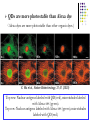
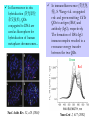
7.2 Radiative Recombination and Nonradiative Recombination 7.2.1 Radiative recombination of non-equilibrium carriers 3)Recombination of donor-acceptor pairs (D-A pairs) • The ionization energy of D-A pairs depends on the impurity atoms and the ways of substitution. E.g., for GaP, if donor O and acceptor C substitute P, at T=1.6K, ionization energy = 941meV. If donor O substitutes for P and acceptor Zn substitues for Ga, at T=1.6K, ionization energy = 956.6 meV. • At room temperature, due to the strong interaction with phonons, the line spectrum of D-A pairs is hard to detect. But at low temperatures, it is easy to observe the line spectrum. 7.2 Radiative Recombination and Nonradiative Recombination 7.2.1 Radiative recombination of non-equilibrium carriers 4)Recombination via deep levels • The emitted photon energy is much smaller than the bandgap, for recombination via deep levels, and the wavelength of emission is far from the absorption edge. • The recombination via deep levels is useful for wide bandgap materials, e.g., it gives red emission in GaP. • The deep levels usually result in non-radiative recombination, obvious in direct bandgap semiconductors. In practice, one should try to eliminate deep levels to enhance the fluorescence efficiency. 7.2 Radiative Recombination and Nonradiative Recombination 7.2.1 Radiative recombination of non-equilibrium carriers 5)Recombination of excitons • If photon energy is lower than the bandgap, electrons can be excited to the exciton levels, where electron and hole are bound together due to Coulomb interactions. The bound electron hole pairs, excitons, can move freely in the crystal. • Excitons are neutral, whose movements will not give rise to currents. But the energies of excitons can be released in the form of radiation or non-radiation. 7.2 Radiative Recombination and Nonradiative Recombination 7.2.1 Radiative recombination of non-equilibrium carriers 5)Recombination of excitons • Two types of excitons: 1. Frenkel exiton or tightly-bound exciton: the distance between electron and hole is on the order of crystal constants. 2. Wannier exciton: weekly bound excitons. The distance is much larger than the crystal constants. Wannier excitons are commonly present in semiconductors. • Bound exciton: the movements of excitons are not free in crystals. Excitons can be bound by the donors, acceptors, D-A pairs and isoelectronic traps. 7.2 Radiative Recombination and Non-radiative Recombination n Eexc 7.2.1 Radiative recombination of nonequilibrium carriers Eg 5)Recombination of excitons • Exciton energy: Hydrogen-like atom model n Eexc Ionization energy of ground state of 1 mr* EH hydrogen atom. 2 2 r m n 1 * 1 * 1 * mq 4 EH 2 2 13.6(eV) mr mn mp 8 0 h Dielectric constant of crystal Effective mass of Effective mass of electrons holes Exciton energy is discrete. n=1: ground state. n=, exciton energy=0 bottom of conduction band, electron and hole is not bound together. 7.2 Radiative Recombination and Nonradiative Recombination 7.2.1 Radiative recombination of non-equilibrium carriers 5)Recombination of excitons • For free excitons, the recombination of electron and hole will emit photons. • For direct bandgap semiconductor, the photon energy is: hv Eg E n exc n Eexc Eg • For indirect bandgap semiconductor, the emitted photon energy is: hv Eg E n exc NE p Energy of N phonons absorbed or released. 7.2 Radiative Recombination and Nonradiative Recombination 7.2.1 Radiative recombination of non-equilibrium carriers 5)Recombination of excitons • For bound excitons, if the binding energy with impurity atoms is Ebx, the peak position of the emission spectrum is: hv Eg E n exc Ebx • It was found that bound excitons are important to emissions, leading to high emission efficiency. • E.g., in GaP-LED, the main emissions are red emission from Zn-O pair bounded excitons, and green emission from N isoelectronic trap bounded excitons, which greatly enhanced the emission efficiency of GaP. 7.2 Radiative Recombination and Nonradiative Recombination 7.2.1 Radiative recombination of non-equilibrium carriers 6)Isoelectronic trap recombination • Isoelectronic impurity: atom that in the same group of the matrix atom in the periodic table, which has the same number of valence electrons as the matrix atom. E.g., N is the isoelectronic impurity for P in GaP. • Isoelectronic trap: bound states induced by the isoelectronic imurities. • The substitution of matrix atoms with the isoelectronic impurity will not offer more free electrons and holes, but form neutral centers. 7.2 Radiative Recombination and Nonradiative Recombination 7.2.1 Radiative recombination of non-equilibrium carriers 6)Isoelectronic trap recombination • Reasons for “traps” or “bound states” ? The differences in the electronnegativity and atomic radius between the isoelectronic impurity atom and the matrix atoms will result in distortion of lattice potential, which could bound carriers (electrons and holes) and form charged centers. 7.2 Radiative Recombination and Nonradiative Recombination 7.2.1 Radiative recombination of non-equilibrium carriers 6)Isoelectronic trap recombination • How to distinguish electron bound state and hole bound states ? Electronnegativity of isoelectronic impurity> electronnegativity of matrix atom electron bound states, isoelectronic electron trap, isoelectronic acceptor. Electronnegativity of isoelectronic impurity< electronnegativity of matrix atom hole bound states, isoelectronic hole trap, isoelectronic donor. E.g., N substitutes P in GaP: electron bound states, isoelectronic acceptor. Bi substitutes P in GaP: hole bound states, isoelectronic donor. 7.2 Radiative Recombination and Nonradiative Recombination 7.2.1 Radiative recombination of non-equilibrium carriers 6)Isoelectronic trap recombination • Light emission Isoelectronic trap becomes charged center after capturing certain type of carriers, and captures oppositely charged carriers due to Colomb interaction. bounded exciton to the isoelectronic impurity. The recombination of exciton could emit light. E.g., in GaP:N, atom N is the isoelectronic electron trap, capturing electron first, and then capturing hole bound exciton. The recombination of exciton gives green emission. The photon energy nearly equals the bandgap of GaP (2.22eV)。 h=2.22-0.01-0.037=2.17 eV 7.2 Radiative Recombination and Nonradiative Recombination 7.2.1 Radiative recombination of non-equilibrium carriers 6)Isoelectronic trap recombination • Isoelectronic trap can effectively enhance the emission efficiency of indirect bandgap semiconductors. E.g., GaP is a indirect bandgap semiconductor, whose emission efficiency is very low. The doping with N atoms can greatly enhance its emission efficiency. Possible reasons: The formation of isoelectronic electron trap due to the substitution of P in GaP by N; Due to the short range force, the bound electrons are confined in the vicinity of the N atoms; Based on Heisenberg uncertainty relation, if the electron wavefunction is localized in the real space, it will spread in a wide range of the momentum space. x p / 2 7.2 Radiative Recombination and Nonradiative Recombination 7.2.1 Radiative recombination of non-equilibrium carriers 6)Isoelectronic trap recombination Higher probability density at wave vector of the top of valence band, which greatly enhance the direct transition probability and the emission efficiency of GaP:N. 7.2 Radiative Recombination and Nonradiative Recombination 7.2.1 Radiative recombination of non-equilibrium carriers 6)Isoelectronic trap recombination • The transition probability is still small in GaP:N, compared to the direct transition. • Besides, two or more N atoms can also form isoelectronic traps, e.g., GaAs1-xPx:NN and GaAs1-xPx:NN3 7.2 Radiative Recombination and Nonradiative Recombination 7.2.1 Radiative recombination of nonequilibrium carriers 6)Isoelectronic trap recombination V(x) -a 0 V0 a • Conditions for electron or hole bound states? The potential at the isoelectronic impurity can be regarded as a square potential well with depth of V0 and radius of a. Calculations show, the condition for bound states is the following, 2 2 V0 a 2 * 8m Isoelectronic traps are more probable for electrons with larger effective mass. Generally, electrons in wide bandgap materials have larger effiective mass, so isoelectronic traps are usually present in wide bandgap materials. x 7.2 Radiative Recombination and Nonradiative Recombination 7.2.1 Radiative recombination of non-equilibrium carriers 6)Isoelectronic trap recombination • How to form isoelectronic traps that can strongly bind carriers? If there is a large difference in the radius of isoelectronic impurity atom and the matrix atom, large lattice distortion will lead to strongly bound states. 7.2 Radiative Recombination and Nonradiative Recombination 7.2.2 Non-radiative recombination • Non-radiative recombination centers are also called killer centers (消光中心), harmful to emissions. • The impurities, lattice defects, and the combination of defect and impurity can become non-radiative recombination centers. • The following types of non-radiative recombination can be clearly explained: multi-phonon transitions, Auger process, and surface recombination. 7.2 Radiative Recombination and Nonradiative Recombination 7.2.2 Non-radiative recombination 1)Multi-phonon transition • The released energy of electron-hole recombination can excite more phonons. • The bandgap of fluorescent semiconductors are usually larger than 1 eV, while one phonon energy is about 0.06eV. If the released energy of electron-hole recombination completely form phonons, the probability of generating numerous phonons is very low. • The crystal defects and impurities introduce discrete energy levels in the forbidden band, in favor of electron transitions to these levels, and subsequent generation of phonons. multi-phonon transition. • Multi-phonon transition is a multilevel process with a low probability. 7.2 Radiative Recombination and Nonradiative Recombination 7.2.2 Non-radiative recombination 2)Auger process N-type • Auger recombination: The released energy from the recombination of an electron with a hole can excite a third carrier to higher energy levels of the conduction or valence band. The relaxation of the third carrier is the multi-phonon transition in the continuous states of the band. • Auger process includes the interactions between two electrons (or holes) and one hole (electron). So it is prominent when electron (or hole) density is very high. Therefore, the doping density of p-n junction LED should not too high. Band-band Auger Process P-type 7.2 Radiative Recombination and Nonradiative Recombination 7.2.2 Non-radiative recombination 2)Auger process • Band-impurity level Auger process: the recombination of electron and hole in the conduction/valence band and the impurity levels. • In heavily doped wide bandgap materials, e.g., GaAs, band-band or band-impurity level Auger process is the primary non-radiative recombination process. N-type P-type 7.2 Radiative Recombination and Nonradiative Recombination 7.2.2 Non-radiative recombination 2)Auger process • The impurity level Auger process is similar to the D-A pair recombination, however, the released energy is transferred to a free carrier, but not to a photon. • E.g, for the red emission in GaP: Zn-O, if the acceptor density is too high, the emission efficiency will drop, due to the Auger process. 7.2 Radiative Recombination and Nonradiative Recombination 7.2.2 Non-radiative recombination 3)Surface recombination • Dangling bonds at the crystal surface can generate high density of deep and shallow levels to serve as recombination centers. • Continuous distribution of surface levels: within the diffusion length at the surface, the recombination of electron and hole is the continuous transitions across the continuous surface levels, in favor of non-radiative recombination. • Surface treatments and protections are very important in improving the emission efficiency of LEDs. 7.3 Basic Structure and Principle of LED LED is a p-n junction under forward bias. Fabricated by using planar technology. Basic structure: 7.3 Basic Structure and Principle of LED Operational principle: e.g., interband radiative recombination. Forward bias minority injection recombination of non-equilibrium carriers photon emission. Injection electroluminescence (注入式电致发光): conversion of electrical energy to photon energy. P N P N No Bias Forward Bias P-side: recombination of injected electrons and the holes in the valence band. N-side: recombination of injected holes and the electrons in the conduction band. The emitted phonon energy is Eg 7.4 Charactristics of LED 7.4.1 I-V Characteristics Similar to that of an ordinary diode. Low cur-in voltage (开启电压)~1-2 V working current (工作电流)~10 mA. LED can be very small in size. Point light source, very useful for optical display 7.4 Charactristics of LED 7.4.2 Quantum Efficiency Quantum efficiency reflects the efficiency of photon generation through the recombination the injected carriers. External quantum efficiency: the ratio of the number of output photons to the number of injected carriers per unit time. Internal quantum efficiency: the ratio of the number of generated photons due to recombination to the number of injected carriers per unit time. Quantum efficiency is related to the injection efficiency, radiation efficiency, and the escape efficiency. 7.4 Charactristics of LED 7.4.2 Quantum Efficiency Injection efficiency —— the ratio of the current that can generate radiative recombination to the total current. Band tail in heavily doped cases, ~1018 cm-3 For n-type, Eph is slightly larger than Eg, easy to be absorbed again. While for p-type, Eph is slightly smaller than Eg, hard to be absorbed again. So LEDs should be designed to realize p-side emission. 7.4 Charactristics of LED 7.4.2 Quantum Efficiency In: the injected electron current on the p-side. In Injection Ip: the injected hole current on the n-side. efficiency: I n I p I R I : recombination current in the depletion region. R Ways to improve the injection efficiency: 1) Donor density in the n-side > acceptor density in the p-side N+P junction. 2) Reduce IR: the materials and fabrication procedures should retain crystal perfection, avoiding doping with unwanted impurities. 3) Materials selection: electron mobility> hole mobility, e.g., GaAs, electron mobility/hole mobility ~30. 7.4 Charactristics of LED 7.4.2 Quantum Efficiency Radiation efficiency r—— On the p-side, the ratio of the number of recombined electrons to the total number of injected electrons. Ur Ur: radiative recombination rate. r U r U nr Unr: non-radiative recombination rate. R1, R2 : radiative recombination, Eph ~Eg. R3 : radiative or non-radiative recombination, Eph <Eg. 7.4 Charactristics of LED 7.4.2 Quantum Efficiency For the case of competition between R1 and R3, one way to improve the radiation efficiency is to increase the doping level on the p-side. However, higher doping level more crystal defects more non-radiative recombination centers. Higher doping level on the p-side lower injection efficiency. For GaP LED, highest efficiency doping level Na=2.51017 cm-3 Internal quantum efficiency r=r 7.4 Charactristics of LED 7.4.2 Quantum Efficiency Escape efficiency o —— The ratio of the number of escaped photons to the total number of photons due to radiative recombination. External quantum efficiency e=io =ro Interface reflection/ Absorption total reflection 1) Small depth of junction. But surface recombination can reduce i if the depth is less than one diffusion length. Ways to improve o: 2) Low absorption coefficient: emitted photon energy less than Eg. 7.4 Charactristics of LED 7.4.2 Quantum Efficiency 3) Optical window. Interface recombination center density is greatly reduced, so the p-n junction could be very close to the interface of AlGaAs/GaAs 7.4 Charactristics of LED 7.4.2 Quantum Efficiency 3) Optical window. Dome-like shape can eliminate the total reflectance. Epoxy (n=1.5) is used to make the dome – reduce interface reflectance, low cost. 7.5 Semiconductor Laser 7.5.1 Semiconductor Laser and its structures Based on the operation materials, laser can be classified into solid laser, liquid laser, gas laser, and semiconductor laser. Compared to other lasers, semiconductor laser has small volume, high efficiency, simple and substantial structure, and can be directly modulated. 7.5 Semiconductor Laser 7.5.2 Conditions for Stimulated Emission Stimulated emission of laser: external stimulation induced carrier recombination. Coherent light emission: same frequency, phase, polarization, and direction. Superior monochromaticity, directionality, and brightness, compared to LED. The operation of semiconductor laser depends on carrier injection. Three basic conditions for laser generation: 1) Distribution for population inversion: number of particles at high energy level>>number of particles at low energy level. 2) Resonance cavity: feedback effect, multiplication of simulated emission laser ocillation. 3) Threshold condition: to realize photon multiplication >= photon loss. 7.5 Semiconductor Laser 7.5.2 Conditions for Stimulated Emission 1) Distribution for population inversion Spontaneous emission V.S. stimulated emission In the process of simulated emission, the incident light is magnified and the output light is coherent. Semiconductor laser works based on stimulated emission. Initial State Final State Spontaneous Emission Stimulated Emission 7.5 Semiconductor Laser 7.5.2 Conditions for Stimulated Emission 1) Distribution for population inversion In semiconductors, light absorption, spontaneous emission, and stimulated emission coexist. Conditions for light gain (光增益): stimulated emission rate > absorption rate the probability of occupying a level in the conduction band by electrons > the probability of occupying a level in the valence band by electrons (related to radiative transition) —— distribution for population inversion. 7.5 Semiconductor Laser 7.5.2 Conditions for Stimulated Emission 1) Distribution for population inversion How to realize distribution for population inversion: EFn EFp Eg Quasi-Fermi levels enter the conduction and valence bands. For injection semiconductor laser, to realize the above condition, 1) semiconductor should be heavily doped, 2) the applied voltage should satisfy, qV EFn EFp Eg 7.5 Semiconductor Laser 7.5.2 Conditions for Stimulated Emission 1) Distribution for population inversion Active area Band diagram of heavily doped GaAs p-n junction laser 7.5 Semiconductor Laser 7.5.2 Conditions for Stimulated Emission 2)Optical resonance cavity Spontaneous emission in the active area a small amount of photons can serve as the excitation source for simulated emission (coherent light). Optical resonance cavity: photons can reflect back and forth between the two parallel interfaces, and amplified. Emission are confined inside the p-n junction plane. 7.5 Semiconductor Laser 7.5.2 Conditions for Stimulated Emission 3)Threshold condition for resonance Laser has end loss and internal loss. When light gain larger than loss after light propagating back and forth once, laser is formed. The gain factor (增益系数) should reach a certain value for laser formation. 1 1 g a ln 2 L R1 R2 Gain factor Absorption coefficient g dI g /( Idx) Reflection of the two end surfaces L 7.5 Semiconductor Laser 7.5.2 Conditions for Stimulated Emission 4)Threshold current For GaAs junction laser, forward current is applied to provide light gain. Threshold current: only when gain factor g increases to a certain values, laser could occure. Outline Chap. 1 Introduction Chap. 2 Basics of Semiconductor Physics Chap. 3 P-N Junctions Chap. 4 Metal-Semiconductor Junctions Chap. 5 Semiconductor Heterojunctions Chap. 6 Semiconductor Solar Cells & Photodiodes Chap. 7 Light Emitting Diodes & Semiconductor Lasers Chap. 8 Quantum Dots for Biological Fluorescent Probes Chapter 5 Quantum Dots for Biological Fluorescent Probes Optical Properties of QDs Quantum confinement effect (QCE)— Discrete energy, widen bandgap Bulk QDs with different sizes Single molecule The variation of bandgap of PbS QDs with their sizes ACS Nano 3, 3023(2009) Optical Properties of QDs Blue shift of the absorption edges and PL peaks. CdS CdSe/ZnS Quantum Dots for Biological Fluorescent Probes Introduction to biological fluorescent probes QDs V.S. traditional organic dye fluorescent probes Labeling and detection of biomacromolecules by QDs Imaging of biological tissues and cells by QDs NIR fluorescent QDs for biomedical imaging in living tissue Quantum Dots for Biological Fluorescent Probes Introduction to biological fluorescent probes QDs V.S. traditional organic dye fluorescent probes Labeling and detection of biomacromolecules by QDs Imaging of biological tissues and cells by QDs NIR fluorescent QDs for biomedical imaging in living tissue Introduction to biological fluorescent probes In bioscience, fluorescence spectroscopy (荧光光谱学) provides the structures, functions and interactions of biomacromolecules by studying the chromophores of biomacromolecules or the fluorescent labels and combining relevant fluorescent techniques. Fluorescent probes have been widely used in AIDS virus detection, human genome sequencing, protein solution conformations, intracellular activity and so on. Generally, organic dye fluorescent molecules are used as probes, i.e., rhodamine. Introduction to biological fluorescent probes QDs as biological fluorescent probes Medintz et al., Nature Materials, 4, 435 (2005) Quantum Dots for Biological Fluorescent Probes Introduction to biological fluorescent probes QDs V.S. traditional organic dye fluorescent probes Labeling and detection of biomacromolecules by QDs Imaging of biological tissues and cells by QDs NIR fluorescent QDs for biomedical imaging in living tissue Organic fluorescent dyes Narrow PL peaks, tunable peak position. Fluorescent QDs CdSe/ZnS QDs Medintz et al., Nature Materials, 4, 435 (2005) QDs have much higher fluorescence intensity than Alexa dye (Alexa dyes are reported to be brighter than any other known organic dyes.) X. Wu et al., Nature Biotechnology, 21,41 (2003) Fluorescence intensity comparison between Alexa and QDs QDs are more photostable than Alexa dye (Alexa dyes are more photostable than other organic dyes.) X. Wu et al., Nature Biotechnology, 21,41 (2003) Top row: Nuclear antigens labeled with QD (red), microtubules labeled with Alexa 488 (green). Top row: Nuclear antigens labeled with Alexa 488 (green), microtubules labeled with QD (red). Advantages of QDs as fluorescent probes Fluorescent QDs Organic fluorescent dyes Excitation spectrum Wide and continuous Narrow Emission spectrum Symmetrically distributed, narrow and tunable emission peaks Wide emission peaks Photostability High, hard to decompose Easy photobleacing and photodecomposition (products are usually harmful to biomolecules) Linkage to biomolecules Facile and easy Specific linkage methods for each organic florescent dyes. Quantum Dots for Biological Fluorescent Probes Introduction to biological fluorescent probes QDs V.S. traditional organic dye fluorescent probes Labeling and detection of biomacromolecules by QDs Imaging of biological tissues and cells by QDs NIR fluorescent QDs for biomedical imaging in living tissue Labeling and detection of biological macromolecules by QDs The application of QDs in biology started from labeling and detection of simple biological macromolecules. The superior fluorescent properties and appropriate dimensions of QDs make them ideal in studying the structures, functions and interactions of biomacromolecules, by combining other techniques, such as fluorescence spectrum, fluorescence polarization, energy transfer, etc. Conjugation of DNA to silanized colloidal QDs CdSe core W. J. Parak et al., Chem Mater, 14, 2113 (2002) The conjugation of single- or double-stranded DNA to QDs had little effect on the optical properties of the nanocrystals. In fluorescence in situ hybridization (荧光原位 杂交技术), QDs conjugated to DNA are used as fluorophore for hybridizaiton of human metaphase chromosomes.。 In immunofluorescence (荧光免 疫), S. Wang et al. conjugated red- and green-emitting CdTe QDs to antigen (BSA) and antibody (IgG), respectively. The formation of BSA-IgG immunocomplex resulted in a resonance energy transfer between the two QDs. Green Red Nucl. Acids Res. 32, e28 (2004) Nano Lett. 2, 817 (2002) Quantum Dots for Biological Fluorescent Probes Introduction to biological fluorescent probes QDs V.S. traditional organic dye fluorescent probes Labeling and detection of biomacromolecules by QDs Imaging of biological tissues and cells by QDs NIR fluorescent QDs for biomedical imaging in living tissue Imaging of biological tissues and cells by QDs Cellular labelling and imaging for the observation of cellular activity . Multicolor labelling with QDs for the observation of live cell surface and the intracellular activities and mobility, and the study of the realization of biological functions and their interactions. Imaging of yeast cells As one of the natural polysaccharids, chitosan (壳聚糖) possesses many important biological properties, such as biocompatibility, biodegradability, and bioactivity, and thus has found a wide application in the field of biological medicine. CdSe/ZnS core/shell QDs carboxymethyl chitosan 羧甲基壳聚糖 M. Xie et al., Chem Commun., 44, 5518 (2005) Imaging of yeast cells M. Xie et al., Chem Commun., 44, 5518 (2005) CdSe/ZnS-labeled carboxymethyl chitosan as bioprobes for live yeast cell imgaing Imaging of organelles in one cell H. J. Tanke et al., Curr Opin Biotech, 16, 49 (2005) Green, microtublules; orange, Golgi; red, nuclei. Single excitation for three different emissions. Imaging of organelles in one cell Medintz et al., Nature Materials, 4, 435 (2005) Pseudocoloured image depicting five-colour QD staining of fixed human epithelial cells. Cyan, 655-nm Qdots labelling the nucleus; magenta, 605-Qdots labelling Ki-67 protein; orange, 525-Qdots labelling mitochondria; green, 565Qdots labelling microtubules; red, 705-Qdots labelling actin filaments. The structure of copolymer ligands determines the distribution of coated QDs t=0 t=0 t=1~2h t=1~2h H. W. Duan et al., JACS, 129, 3333 (2007) PEI-g-PEG4 -coated QDs are trapped in organelles. PEI-g-PEG2-coated QDs have escaped from the endosomes and are released into the cytoplasm. PEG:聚乙二醇 PEI:聚乙二胺 Quantum Dots for Biological Fluorescent Probes Introduction to biological fluorescent probes QDs V.S. traditional organic dye fluorescent probes Labeling and detection of biomacromolecules by QDs Imaging of biological tissues and cells by QDs NIR fluorescent QDs for biomedical imaging in living tissue NIR fluorescent QDs for biomedical imaging in living tissue • Biomedical imaging in living tissue requires low absorption and scattering of the incident excitation light and the emission from the QDs. • Visible lights can penetrate tissue with a largest thickness of mm, while for NIR light, the penetration depth reaches cm. • NIR QDs with emissions at 700-900 nm permit imaging of living tissue and thus allow investigations on the internal situation of tissue, in favor of disease diagnosis. NIR QDs provide the surgeon with direct visual guidance throughout the entire SLN (前哨淋 巴结) mapping procedure, minimize incision and dissection inaccuracies, and permit real-time confirmation of complete resection. SLN mapping has already revolutionized cancer surgery. Kim et al., Nat Biotechnol, 22, 93 (2004)