* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download THE EVOLUTION OF MALE TRAITS IN SOCIAL INSECTS Jacobus J
Plant reproduction wikipedia , lookup
Female promiscuity wikipedia , lookup
Human male sexuality wikipedia , lookup
Sexual attraction wikipedia , lookup
Sexual coercion wikipedia , lookup
Body odour and sexual attraction wikipedia , lookup
Fertilisation wikipedia , lookup
Sexual selection wikipedia , lookup
Sexual reproduction wikipedia , lookup
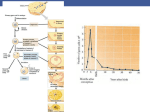
28 Oct 2004 20:17 AR AR234-EN50-17.tex AR234-EN50-17.sgm P1: GCE LaTeX2e(2002/01/18) 10.1146/annurev.ento.50.071803.130416 Annu. Rev. Entomol. 2005. 50:395–420 doi: 10.1146/annurev.ento.50.071803.130416 c 2005 by Annual Reviews. All rights reserved Copyright THE EVOLUTION OF MALE TRAITS IN SOCIAL INSECTS Jacobus J. Boomsma,1 Boris Baer,1 and Jürgen Heinze2 1 Institute of Biology, Department of Population Biology, University of Copenhagen, Universitetsparken 15, 2100 Copenhagen, Denmark; email: [email protected]; [email protected] 2 Biologie I, Universität Regensburg, D-93040 Regensburg, Germany; email: [email protected] Key Words sexual selection, multiple mating, sperm competition, female choice ■ Abstract Pair formation in social insects mostly happens early in adult life and away from the social colony context, which precludes promiscuity in the usual sense. Termite males have continuous sperm production, but males of social Hymenoptera have fixed complements of sperm, except for a few species that mate before female dispersal and show male-fighting and lifelong sperm production. We develop an evolutionary framework for testing sexual selection and sperm competition theory across the advanced eusocial insects (ants, wasps, bees, termites) and highlight two areas related to premating sexual selection (sexual dimorphism and male mate number) that have remained understudied and in which considerable progress can be achieved with relatively simple approaches. We also infer that mating plugs may be relatively common, and we review further possibilities for postmating sexual selection, which gradually become less likely in termite evolution, but for which eusocial Hymenoptera provide unusual opportunities because they have clonal ejaculates and store viable sperm for up to several decades. INTRODUCTION It is in sexual behavior that all animals can be considered “social.” A.M. Stuart (118) Most mating systems are characterized by a variance in male reproductive success that is higher than that in females (127) and by competition among males for fertilization of eggs. This strife is the driving force of sexual selection and has produced effective fighting devices, costly ornaments and elaborate courtship displays in males, and concomitant traits of mate quality assessment and choice in females (8, 112, 126). Social insects, however, seem to be exceptions to this rule. Their societies appear to represent the cumulative effort of sterile helpers 0066-4170/05/0107-0395$14.00 395 28 Oct 2004 20:17 396 AR AR234-EN50-17.tex BOOMSMA BAER AR234-EN50-17.sgm LaTeX2e(2002/01/18) P1: GCE HEINZE (workers and occasionally soldiers) and specialized egg-layers (queens), in which fatherhood is constant and males have few if any sexually selected traits. As a result, the field of insect behavioral ecology has primarily addressed questions of sexual conflict and mate choice in nonsocial insects and reproductive conflict and kin selection in social insects. Male traits in social insects have been addressed (20, 23, 32, 39, 44, 65, 111, 115), but the main emphasis was usually on the female aspect of mating systems (31, 45, 92, 93, 114, 117), except for a recent review on male bumble bees (10). This review addresses questions of male mating strategies across all groups of advanced eusocial insects—ants, social bees, social wasps, and termites. By taking this broad comparative approach, we concentrate on convergent similarities that allow us to place the available data in a common evolutionary framework. To achieve this objective in a moderately sized paper, we often had to ignore details and to cite earlier reviews rather than original sources. From this synthesis we develop the outlines of an explicit research program for testing sexual selection and sperm competition theory in social insects and we identify topics for which progress can be realized and for which social insects may offer opportunities for testing questions that are less accessible in nonsocial animals. FATHERHOOD IN INSECT SOCIETIES Partner-Commitment for Life and Other Idiosyncrasies of Male Social Insects Advanced insect societies are usually kin groups living in single or multiple nests. Although most individuals are fully or partially sterile, species have almost invariably retained sexual reproduction (23, 33, 84, 136), but with inconspicuous roles of males. In the eusocial Hymenoptera, males are short-lived and do not normally engage in social activities. They die after one or a few matings, realized during a brief mating period, and persist as long-lived sperm stored by the queen(s) with whom they mated (65, 66, 84, 136). They are haploid, produce clonal sperm, and sire only female offspring (10, 11, 23, 112). In contrast, a male termite is diploid and may live as long as the queen, with whom he mates at regular intervals (91, 125, 132). Termite workers and soldiers can be of either sex and are often immatures without sex-specific traits (103, 111). Adult sexual dimorphism is negligible, except for genital traits (91, 104), whereas queens and males of ants, bees, and wasps can be very different. The mating systems of social insects usually involve obligate partner-commitment for life (20, 23), and with such extreme and exclusive commitment, that when the female dies or loses her fertility, the male stops reproducing as well and does not obtain a new partner (in Hymenoptera because he is only represented as stored sperm, in termites because he can no longer disperse). With only few possible exceptions, queens of social Hymenoptera never remate later in life (23, 84, 106), and in as many as half of the studied species, females always mate with a 28 Oct 2004 20:17 AR AR234-EN50-17.tex AR234-EN50-17.sgm LaTeX2e(2002/01/18) SOCIAL INSECT MALES P1: GCE 397 single male and thus produce full-sibling offspring throughout their life (20, 117). Although many species maintain polygyny (here defined as multiple queens per colony and not as multiple female mates per male) by accepting newly inseminated queens (23, 66, 69, 130), these queens are already inseminated by males that have unequivocally committed their future reproductive fitness before the social status of their mate was decided. Polygyny therefore does not imply promiscuity and does not affect the forces of selection that create and maintain male-specific traits in social insects. Societies of higher termites are probably even more genetically closed than hymenopteran societies, as reproductives that have left are not allowed back into any nest (132). Inbreeding cycles with replacement reproductives occur (86, 111), but they only decrease the genetic diversity of full siblings. Cofounding of colonies by several reproductive pairs, with the possibility of promiscuity among them, rarely results in more than one surviving independent breeder of each sex (33, 91, 103, 111, 123, 124). Fusion of mature colonies in lower termites seems to be the only exception to this generalization (36, 83, 125), although it would likely induce some form of serial monogamy rather than a new round of unconstrained mate choice. Irreversible partner-commitment for life implies that sexual selection is often restricted to premating behavior, when the sexes disperse and compete for partners. Apart from the limited options of secondary mate choice in the lower termites, postmating sexual selection is possible only when females mate with multiple males and store multiple ejaculates. This occurs in various lineages of the eusocial Hymenoptera, most commonly as a facultative trait but obligatorily in some derived clades (20, 117). However, in all these mating systems, females are receptive for only a short period and males are normally unable to monopolize groups of females because they cannot control female dispersal. Selection for Long-Lived Males or Sperm Sexuals of annual social bees and wasps tend to be agile flyers and forage on their own, similarly to their solitary sister taxa, whereas sexuals of ants and higher termites are often unable to forage as effectively as workers because of their more elaborate specialization on a limited set of reproductive tasks later in perennial life (for Hymenoptera this applies only to females, as males do not survive much beyond their mating flight). This implies a higher vulnerability when workers are not present, reinforcing selection to minimize exposure to external mortality factors during partner selection and pair formation outside the colony. The interesting question is thus not why social insect pair formation happens once early in adult life, but rather why there are few reversals in clades in which pair formation secondarily became decoupled from leaving the nest to disperse, so that the vulnerability constraint became less significant. Selection for minimizing the solitary exposure of reproductives during pair formation has produced fundamentally different adaptations in the social Hymenoptera and the termites. As biparental care was ancestral in the termites (88, 28 Oct 2004 20:17 398 AR AR234-EN50-17.tex BOOMSMA BAER AR234-EN50-17.sgm LaTeX2e(2002/01/18) P1: GCE HEINZE 89, 103, 111), the increased longevity of queens induced selection for a similar life span for males (111, 125). However, only the females build nests and provision offspring in the Hymenoptera (115, 120), so that the longevity of stored sperm had to increase with female life span. This happened multiple times in ants, social bees, and social wasps and made somatic survival of males increasingly superfluous in advanced clades in which males often survive for only a few hours after leaving the nest to mate. Males of both social and nonsocial Hymenoptera have generally completed their lifetime spermatogenesis when they reach sexual maturity (37, 61, 65) and cannot increase their supplement of sperm afterward. Male sperm limitation therefore did not evolve as a result of social fatherhood, although a further reduced time frame for mating probably reinforced selection to maintain this trait. The long-lived queens of perennial hymenopteran societies in particular need an efficient sperm storage process to maintain high sperm viability. Queen longevity may be several decades in ants (66, 70, 95), so that the survival of stored sperm matches the equally impressive survival of “kings” (resident males) in termites. Survival of stored sperm for such long periods is a unique trait for ants and probably incurs significant metabolic costs for maintenance (128). However, neither sperm viability nor maintenance costs have yet been quantified or approximated. To conclude, the result of once-in-a-lifetime partner selection after or during dispersal is that established insect societies are normally inaccessible for immigrating single (i.e., unmated) reproductives. Although many efforts of mature colonies are concerned with reproduction, the mate choice activities are normally a solitary activity (23) or rather the minimum extent of social interaction that most organisms experience (118; see quotation, above). This is in marked contrast with social vertebrates, in which most if not all individuals are potential reproducers and in which many of the society’s social activities interact with partner selection and mating. However, pair formation within the nest and without preceding dispersal has evolved multiple times, both as inbreeding cycles in the higher termites and in various derived mating systems of ants, which is dealt with in later sections. MALE MATING STRATEGIES Mate Location Mating systems of social Hymenoptera can normally be categorized as either resource defense polygamy or scramble competition polygamy (126). Many bumble bees and social wasps have resource defense polygamy, in which males patrol the females’ foraging ranges or flight corridors (5, 17, 106). These mating systems are typical when females emerge asynchronously from scattered sites and live solitarily for a number of days or weeks before colony foundation or hibernation. Scramble competition polygamy is usually associated with mating swarms at landmarks, i.e., synchronized mass-emergence of females over a short period across 28 Oct 2004 20:17 AR AR234-EN50-17.tex AR234-EN50-17.sgm LaTeX2e(2002/01/18) SOCIAL INSECT MALES P1: GCE 399 Figure 1 A classification of social insect mating systems based on the density of virgin females present in aggregations for pair formation and/or mating (determined in large part by colony density and size and by whether mating is individually or mass initiated), and the extent to which virgin females disperse on the wing before mating, a variable that closely reflects where pair formation (mating in social Hymenoptera) takes place. entire habitats, and is particularly common in ants and termites, but occurs also in social bees and wasps (3, 16). Hölldobler & Bartz (65) divided ant mating systems into a female-calling syndrome, in which individual females emit pheromones to attract males, and a male aggregation syndrome, in which males first attract additional males and the mass buildup of pheromones later attracts females (9, 64). Sivinski & Petersson (113) argued that insects generally have two types of mating swarms, aerial swarms and substrate-based swarms, a distinction that makes it relatively straightforward to extend the Hölldobler & Bartz (65) scheme to bees and wasps and to syndromes of mating in the nest, which have only recently become relatively well studied (Figure 1). The latter include all situations in which females mate before dispersal or without dispersing at all. In some ants (see Male Territoriality and Lethal Fighting as Derived Conditions in Ants, below) this implies that territorial ergatoid (fighting) males can monopolize groups of females, but more generally this category refers to males hovering around the entrances of nests, where clutches of virgin females mature. This occurs in some social bees and wasps (2, 5, 9, 108) but also in polygynous (often unicolonial) ants (71). Two key variables, local density and premating dispersal of females, seem to explain most of the variation in social insect mate location systems. The lines 28 Oct 2004 20:17 400 AR AR234-EN50-17.tex BOOMSMA BAER AR234-EN50-17.sgm LaTeX2e(2002/01/18) P1: GCE HEINZE separating the categories (Figure 1) are tentative and will likely be neither straight nor sharp in real life (compare with Reference 24). For the social Hymenoptera, the premating dispersal distance of females usually determines the location of mating, but this is not so for termites, for which dispersal, mate choice, and mating are strictly separated in time (118). For example, termites normally start with aerial swarms to disperse before pair formation is initiated, but separation of these two phases is less clear when males sit on branches and pounce on females passing by and remain attached to them during the rest of the dispersal flight (91, 118). In other termites, females call after a dispersal flight (91). Social Hymenoptera therefore usually have a single position in the parameter space of Figure 1, whereas termites generally start their dispersal activity somewhere in the upper center and complete their mate choice activities toward the lower left of the diagram. However, dispersal followed by calling is also known in some ants (53, 101). The strength of sexual selection is dependent on female aggregation in space and time (42): It increases with nonaggregation in time (asynchrony) and with aggregation in space. Both factors allow a higher variance in male mating success, with the best males focusing on a single location at a time and inseminating one female after another. These criteria suggest that the strength of sexual selection decreases from left to right in Figure 1, although the issue is complicated by the fact that females are attracted by male aggregations in the top 80% of the figure, whereas males are attracted by females at the bottom. Sexual Dimorphism and Premating Sexual Selection The different mate location systems allow predictions about the relative magnitude of sexual dimorphism. In spite of the relative ease of collecting data and the importance of estimating female-to-male cost ratios for studies of sex allocation (18), sexual dimorphism is notoriously understudied in social insects (115, 120). To encourage such work, we offer a crude comparative analysis to demonstrate that simple measures of body size may give important information on the mate location system and on the forces of premating sexual selection that would typically apply. With females (queens) under consistent selection for high fertility (and thus large body size) and males unable to monopolize groups of females, it seems unlikely to find species in which males are larger than females. Indeed, as a rule of thumb, social insect males have a body size that is identical to or smaller than that of females (120), although they are rarely smaller than workers. However, the variation in sexual dimorphism across species differs significantly among the major groups of social insects (Figure 2). The rectangles in the figure show that ants have high variation in sexual size dimorphism, ranging from both sexes being about equal in size to queens having ∼3 times the body length and ∼25 times the body mass of males. Variation in sexual dimorphism is much lower in the social bees, in which queens are at best about 1.5 times as long and 5 times as heavy as males, and minimal in our small sample of social wasps (difference in mass is less than or equal to a factor of two). Sexual size dimorphism is universally low in termites, most likely as a direct result of bi-parental care (90). The ant 28 Oct 2004 20:17 AR AR234-EN50-17.tex AR234-EN50-17.sgm LaTeX2e(2002/01/18) SOCIAL INSECT MALES P1: GCE 401 Figure 2 Patterns of sexual dimorphism at the time of mating in the major groups of social Hymenoptera. The ant sample (n = 20) is from Reference 18, the samples for bumble bees (n = 10) and social wasps (n = 4) are from Reference 63, and the sample for honey bees (n = 3) is from Reference 74. Only a single species per genus (the first one alphabetically in the lists) was included for the ants and social wasps to exclude most taxonomic confounding. Sexual dimorphism was measured as the cube root of the weight of a typical male divided by the weight of a typical queen, to be roughly equivalent to the ratio of body lengths as reported in some handbooks. Species-specific sexual dimorphism was plotted in a ranked order with different symbols (ants, black circles; social bees, open circles; social wasps, open squares). The rectangles illustrate the total variation per group. genera that have degrees of sexual dimorphism beyond the range of values for social bees tend to be characterized by mass aerial swarming without a need for landmarks, a mate location system that does not seem to occur in the social bees (except in honey bees) and wasps. These groups predominantly occupy the more competitive mating niches, including male territoriality, which is extremely rare in ants. Pre-mating sexual selection has been documented in Pogonomyrmex harvester ants, in which larger males mate more often and transfer more sperm during substrate-based swarming (1a, 35, 133, 134). A negative correlation between thorax weight and sperm content was found in Atta males, which suggests that trade-offs with flight ability may occur (44). However, in honey bees and a tropical bumble bee, male sperm load was positively correlated with body size (55, 110). Ants with a female-calling system, honey bees, and the army ants also have low degrees of sexual dimorphism. The last two show colony fission and extremely male-biased sex ratios, selecting for male performance in flight or interference competition (see The Number of Mating per Male, below), so that males of about the same size as queens are expected (52, 98). Overall, these comparative data indicate that sexual dimorphism is often a good predictor variable for mating systems (120) and that a more systematic study of sex-specific variation in body size parameters of social insects would give valuable insights into the factors that determine male and female fitness during pair formation and mating. 28 Oct 2004 20:17 402 AR AR234-EN50-17.tex BOOMSMA BAER AR234-EN50-17.sgm LaTeX2e(2002/01/18) P1: GCE HEINZE Male Territoriality and Lethal Fighting as Derived Conditions in Ants Territorial males of social bees and wasps may attack each other (126) and males of harvester ants may physically compete for access to receptive queens (1a, 35, 126, 133, 134), but the outcome of such interferences always relies on body size and stamina, whereas adaptations to harm or kill rival males seem almost completely absent in social insects (115). This makes sense, as the evolution of male weaponry tends to be associated with the possibility to control groups of females and to eliminate rival males intermittently and one by one (8). For social insects this requires pair formation and/or mating in the confined area of the nest before dispersal of either sex, a situation that is found only in some ants. Below, we review the selective forces that have shaped the males of these rare ants and the general concepts on which this understanding builds. Mating in confined areas has led to the evolution of multiple male morphs in numerous nonsocial arthropods, in which winged disperser males coexist with local fighter males with reduced wings (34, 41, 58, 126). Such male polymorphisms are evolutionarily stable when the number of reproductives eclosing in a specific compartment is small and variable, so that broods might occasionally have only females by chance (58). Winged males are then maintained by selection because they can obtain more fitness by inseminating females in distant compartments than by competing with brothers for a limited number of intranidal matings. A comparative analysis in fig wasps has recently corroborated this hypothesis (28). In ants, multiple male morphs and the complete replacement of winged males by wingless ones are also associated with mating in the natal nest without prior dispersal. As long as wingless males compete with brothers, fighting is unlikely to evolve, because selection through local mate competition reduces the number of males per clutch to the bare minimum needed to inseminate all females (57). For example, colonies of some parasitic ants produce only a few peaceful males that inseminate all their sisters (60, 135), and in another species wingless males and virgin queens eclose after the death of the mother queen and mate in the nest without any male-male aggression (139). Wingless males that do engage in lethal fighting have evolved independently in Hypoponera (58, 140) and Cardiocondyla ants (62, 72, 119). In some of these species, aggressive wingless fighter males coexist with docile winged disperser males, whereas related species have completely lost the winged male morph. Figure 3 summarizes the mating system variation in Cardiocondyla ants as a function of relatedness and colony size (7, 58). The tropical species Cardiocondyla obscurior, C. wroughtonii, C. emeryi, and C. minutior have typically small colonies of less than 50 workers. The first two have up to 15 queens per colony and the last two somewhat fewer. Worker relatedness is therefore variable (particularly in C. obscurior and C. wroughtonii owing to the unstable nest sites of these species) and relatively low on average. All four species produce sexuals in small numbers throughout the year. Under normal conditions a single territorial ergatoid male monopolizes mating with emerging nestmates by killing rival males. In the first 28 Oct 2004 20:17 AR AR234-EN50-17.tex AR234-EN50-17.sgm LaTeX2e(2002/01/18) SOCIAL INSECT MALES P1: GCE 403 Figure 3 Colony size (log scale) and relatedness among nestmates as key variables that affect the occurrence and polymorphism of dispersing winged males and territorial fighting (ergatoid) males in Cardiocondyla ants. The triangle indicates that high-relatedness colonies are rarer than low-relatedness colonies. The relatedness limits of the rectangles and triangle are approximate, as they are inferences based on queen number per colony. LMC, local mate competition. two species, ergatoid males have elongated mandibles, with which they injure and kill callow males and also hold adult rivals while marking them with a pheromone so that they are subsequently killed by nestmate workers (72, 119). The last two species have shorter sturdy mandibles, which are used to kill callow rivals but are less efficient against rivals that have managed to survive long enough to harden their cuticle. This implies that several ergatoid males may coexist in larger colonies. In all four species, successful ergatoid males live for several weeks, until their tenure is taken over by a younger male. Normal winged males are also produced, but probably only under stressful conditions (29). They disperse, presumably to mate with virgin queens in other small colonies that have accidentally remained without an ergatoid male (72). Winged males often mate with nestmate females before dispersing, apparently escaping attack from the ergatoid male by mimicking the odor of virgin queens (30). Colonies of C. mauritanica have up to 200 workers and produce sexuals seasonally (59). With such larger clutches it is highly unlikely that broods remain without any males. Winged males are thus never produced, but as in C. emeryi and C. minutior, more than one ergatoid male is occasionally found in larger colonies. 28 Oct 2004 20:17 404 AR AR234-EN50-17.tex BOOMSMA BAER AR234-EN50-17.sgm LaTeX2e(2002/01/18) P1: GCE HEINZE C. mauritanica is also similar to these two species in that its ergatoid males kill only callow rivals. Colonies of Cardiocondyla elegans, C. ulianini, and C. batesii are even larger (100 to 400 workers), but they have a single queen (and thus high relatedness) and contain several mutually tolerant ergatoid males when the female sexuals emerge (82). Multiple queens per colony and low relatedness have likely been ancestral in the genus Cardiocondyla (J. Heinze, A. Trindl, B. Seifert & K. Yamauchi, unpublished data), so we infer that males remained polymorphic until colony size passed a certain threshold at which winged males were lost completely. High relatedness and mutually tolerant ergatoid males (top-right corner in Figure 3) would thus be a derived condition, associated with inbreeding, so that relatedness could potentially increase to values above 0.75. No Cardiocondyla species seems to follow the most simple local mate competition model with small colonies, female-biased sex ratios, and one or a few wingless, nonfighting males. Rare and transient monogynous colonies of C. obscurior and C. wroughtonii tend to have these colony characteristics but always produce a few ergatoid males, all of which but one are eliminated by fighting. Although their typical colony sizes are unknown, similar scenarios may apply in the two Hypoponera species that have male fighting morphs coexisting with normal males (58, 140), such that they would fit somewhere at the bottom-left corner of Figure 3. Several other Hypoponera species (47, 79, 141) have extensive mate guarding as an alternative to male fighting: Wingless nonfighting males cling to the cocoons of queen pupae for hours or even days while inserting their genitalia through an opening in the cocoon in order to mate before these queens eclose. Another case of male polymorphism in which both morphs have retained their wings and differ only slightly in dispersal has been reported in two Formica ant species (48). Cardiocondyla ants have evolved yet another remarkable adaptation together with male fighting. Their ergatoid males are the only Hymenoptera known to date that have life-long spermatogenesis (61), such that the lifetime number of matings of these males is no longer constrained. Although these social insects are tiny, this is a spectacular development comparable to that of the hypothetical descendants of whales, which become terrestrial by re-evolving proper legs. This illustrates that the forces of sexual selection in social insects can be strong when the right combination of conditions (low relatedness and the possibility to monopolize groups of females) is present. The Number of Matings Per Male Earlier reviews have hypothesized that most ant males mate only once (23, 32, 65) and are thus equally monogamous as termite males. However, exceptions seem common and show male mating frequencies of up to 10 times (64, 135, 137). Similar inferences in leafcutting ants (19, 45, 99) were recently confirmed by showing that multiple ejaculations could be provoked from a specialized part of the accessory testis, which is refilled after each ejaculation (11). Multiple mating 28 Oct 2004 20:17 AR AR234-EN50-17.tex AR234-EN50-17.sgm LaTeX2e(2002/01/18) SOCIAL INSECT MALES P1: GCE 405 by males is common in bumble bees and social wasps (2, 105, 126), whereas male honey bees and stingless bees mate singly (84, 115). The probability that an average male obtains more than one mating is a function of the operational sex ratio (23, 126). In most mating systems this ratio depends on sexual dimorphism (Figure 2), because the same equilibrium sex ratio in terms of investment may translate into different numerical sex ratios depending on sexual dimorphism (18, 63). Local mate competition and lethal male fighting may shift the operational male-to-female sex ratio to values far below 1, whereas colony fission induces extremely male-biased sex ratios (23). Selection always tends to maximize the probability of obtaining a first mating, but this is unlikely to be the case for additional matings (23, 126). Assuming that every male mating is essentially a random event, the probability of mating 0, 1, 2, or 3 times should be Poisson distributed. When the operational sex ratio becomes more male-biased, the mean number of matings per male decreases, but the probability of obtaining multiple matings decreases much faster than the probability of mating once (Figure 4). Selection thus increasingly promotes traits that maximize the chance of obtaining any mating instead of traits that allow additional matings. The occurrence of short-lived males with fixed amounts of sperm must therefore imply that the ability to mate multiply is lost when the operational male-to-female sex ratio is more male-biased than some threshold value. The vertical arrows in Figure 4 illustrate the additional effect of interference competition (relaxing the assumption of randomness). This increases the variance in male mating success, i.e., increases the proportion of multiple matings for the males that manage to mate at all. Suicidal mating of male honey bees and stingless bees (115) is consistent with this model, and the same must apply to army ants, which shed their wings when entering a foreign colony and copulate for hours (23). In ants with sex allocation ratios between the respective worker and queen optima of 3:1 and 1:1, we expect that species with aerial swarms and high degrees of sexual size dimorphism also have males that can mate only once. Expectations such as this are given in Figure 4, but they need to be explicitly tested by further comparative studies of the size and compartmentalization of the accessory testes (11). The species that mate in the nest without either sex dispersing before mating (see Male Territoriality and Lethal Fighting as Derived Conditions in Ants, above) are at the far left of Figure 4. This illustrates once more that the Hamiltonian (57) decoupling of mating and dispersal shapes these mating systems. That females stay in their maternal colony allows males to monopolize groups of them, either uncontested (when relatedness is high; 60, 135, 139) or after killing rivals (when relatedness is low; Figure 3). POSTMATING SEXUAL SELECTION Accessory Glands: Have Mating Plug Functions Been Overlooked? The accessory glands of male insects are now generally considered to be an extra set of manipulative chemical genitalia that may affect virtually all aspects of female 28 Oct 2004 20:17 406 AR AR234-EN50-17.tex BOOMSMA BAER AR234-EN50-17.sgm LaTeX2e(2002/01/18) P1: GCE HEINZE Figure 4 The Poisson probability of mating more than once (i.e., 2, 3, 4. . . times: (eµ -µ-1)/µ) divided by the Poisson probability of mating once (µ/eµ ) expressed as proportion, 1-(µ/eµ -1), for operational (male-to-female, m/f) sex ratios between 1:10 and 100:1. µ is the mean number of matings that a male can statistically expect assuming that queens mate once. Multiple queen-mating has the same effect as shifting the operational sex ratio toward females. Vertical arrows indicate the effect of interference competition among males, which is expected when territoriality increases toward the left-hand side of the figure. The expected position of various mating systems of social Hymenoptera is indicated. LMC, local mate competition. reproduction (40, 56). In many insects these glands produce spermatophores or mating plugs, which are meant to monopolize paternity of a clutch (56, 126). Similar mechanisms have been hypothesized to help males of social Hymenoptera to monopolize paternity of a queen’s lifetime reproduction (23). Males of Carebara and Diacamma ants leave spermatophores in the female genital tract (6, 100, 101). In Diacamma, male mating is suicidal (6), which suggests that excessive accessory gland use for paternity guarding may make males lose the ability to mate more than once even if the operational sex ratio is not male-biased. Male bumble bees deposit mating plugs, which effectively prevent intromission by subsequent males and demotivate queens to continue sexual activity (13, 14, 25, 109). Similar mating plugs occur in fire ants (85) and most likely in 28 Oct 2004 20:17 AR AR234-EN50-17.tex AR234-EN50-17.sgm LaTeX2e(2002/01/18) SOCIAL INSECT MALES P1: GCE 407 fungus-growing ants (11). Females are obligatorily singly mated in most of these cases except Bombus hypnorum (25) and Acromyrmex and Atta leafcutter ants (131). In the last two, accessory gland size is reduced, so that the size ratio of the accessory testes (the enlarged parts of the seminal vesicles that store mature sperm) and accessory glands (mating plug investment) can be used as a predictor of single and multiple mating (11). The inquiline social parasite Acromyrmex insinuator has reverted almost completely to single queen-mating (121), but its male genitalia only partly reflect this reversal (11). Investments in sperm and accessory gland mass are both intermediate, but together they exceed an investment that would fit on a linear trade-off line. This suggests that partial multiple mating of queens imposes disproportionate costs that render an intermediate mating system unstable in fungus-growing ants. Partial multiple mating of queens has indeed never been found in any free-living species (131), in spite of this being a relatively common mating system in other social Hymenoptera (20, 117). Similar measurements in other social insect tribes that combine single and multiple queen-mating and have both free-living and socially parasitic species would be valuable to test the generality of these trends. Mating plugs can be highly functional even when invisible from the outside (14, 38, 109). This implies that they may be much more widespread than previously assumed and that explicit studies of their presence and function should have high priority to establish whether they do occur in most if not all social Hymenoptera with obligatorily singly mated queens (20, 117). A general association of mating plugs and single queen-mating would imply that there is much more male-control over mating in social Hymenoptera than has previously been acknowledged. This conclusion would stand even when secondary developments occasionally reverse male-control, as seems to have happened in Apis honey bees with their derived “mating signs” (73, 138), but not in the predominantly singly mating stingless bees (96, 97). In the lower termites, accessory gland compounds with unknown functions are expelled with the sperm (132), but the accessory glands have apparently been lost in the higher termites. It would be interesting to establish whether this loss precedes, coincides with, or follows the emergence of aflagellate sperm (see below) and whether either of these transitions is linked to the emergence of central site nesting, i.e., the separation of nest site and foraging range, which effectively precluded colony mixing and enforced lifelong functional monogamy. The direction of change suggests that accessory glands are ultimately redundant in an exclusively monogamous mating system. Further studies of accessory glands in social insects may also help to shed light on male precedence in paternity. This has been extensively studied in nonsocial insects (112), but it is difficult to address in social insects, because copulations can be observed in only a few species. The studies that are available only concern patterns of multiple paternity and show significant inequalities in paternity among males (21, 25, 43, 46, 49, 51, 94, 121). In Lasius and Formica ants the extent of paternity skew tends to be negatively correlated with the frequency of multiple 28 Oct 2004 20:17 408 AR AR234-EN50-17.tex BOOMSMA BAER AR234-EN50-17.sgm LaTeX2e(2002/01/18) P1: GCE HEINZE mating (21, 22). This pattern may be consistent with mating plugs becoming less effective when multiple mating increases in frequency (so that later males can store more equal amounts of sperm; 25), but a number of other explanations are also potentially applicable (21, 22). Paternity Manipulation, But No Sperm Displacement Displacement of sperm, a typically promiscuous trait in nonsocial insects, seems to be absent in social insects (23) and the active killing of rival males appears rare (Figure 3). In species with multiply mated queens, sperm of different males is normally deposited in the bursa copulatrix some distance away from the spermatheca so that sperm competition for storage will occur. Accessory gland compounds that play an active role in this competition process are increasingly discovered (56), but the evolution of such traits in social Hymenoptera is constrained. In nonsocial insects, mechanisms of sperm competition that negatively affect female survival might be selected when oviposition takes place shortly after mating (26, 56), but not in social insects in which male reproductive fitness comes with a considerable time lag because colonies will not produce reproductives unless a number of cohorts of sterile workers have been produced first (10, 11). As a result, arms races between males and females (26, 67) are less likely to evolve in social insects, so that postmating sexual selection is more likely to be based on cryptic female choice (40). The only mechanism for directly manipulating the sperm storage process during or immediately after mating that has been demonstrated is ejaculation directly into the spermatheca. This has been documented in two species of dwarf honey bees (75, 76), in which drones are significantly more powerful in flight performance than drones of other honey bees (98). A similar mechanism has evolved in Atta leafcutting ants (B. Baer, unpublished data). Sperm Length Versus Sperm Number: Production and Storage Constraints When competing for storage, longer sperm likely moves faster but is more expensive to produce and store. Although the generality of this idea is currently being disputed (112), it is almost unimaginable that it would not apply to social insects because of the idiosyncratic production and storage constraints in the Hymenoptera and the general monogamy of the termites. When males eclose with a fixed amount of sperm, there is a trade-off between making many short sperm and fewer longer sperm, and when the size of mature colonies increases, shorter sperm is likely to be favored because more of it can be stored to fulfill the lifetime reproductive potential of a hymenopteran queen and her mate. In social Hymenoptera, sperm length should thus decrease with colony size as long as there is one singly mated queen per colony. This trend is expected to reverse when polygyny evolves in species with medium-to-large colonies, as this would relax the fertility demands 28 Oct 2004 20:17 AR AR234-EN50-17.tex AR234-EN50-17.sgm LaTeX2e(2002/01/18) SOCIAL INSECT MALES P1: GCE 409 of queens, and when multiple queen-mating evolves, as this would add a novel directional selection component for faster sperm. In termites, however, there are neither production nor storage constraints, such that the need for longer and faster sperm should reflect the likelihood of deviations from lifetime monogamy. Concepts such as this assume that there is additive genetic variation for sperm length so that it can respond to selection, a contention that was recently confirmed for the bumble bee Bombus terrestris (B. Baer, G. de Jong, P. Schmid-Hempel, R. Schmid-Hempel, J.T. Høeg & J.J. Boomsma, unpublished manuscript). Bombus hypnorum has facultatively multiply mated queens (25) and significantly longer sperm than B. terrestris and B. lucorum, which have singly mated queens (15). No obligatorily multiply mating bumble bees are known, but sperm length is significantly longer in Apis honey bees than in any of these bumble bees (80), which suggests that higher queen-mating frequencies in social bees are associated with longer sperm. Sperm length in termites has apparently evolved in the opposite direction. The most basal termite family still has flagellate sperm, but sperm tails were lost subsequently when amoeboid sperm evolved. The most derived termites (Rhinotermitidae and Termitidae) have completely nonmotile round-shaped sperm (68). The termite data thus indicate that a permanent absence of sperm competition allows sperm to get shorter, even when males do not hatch with a fixed amount of sperm. In the fungus-growing ants, sperm is long in the most basal genera with small, short-lived colonies. It decreases in length with increasing colony size in the more derived clades, only to increase again in some Atta leafcutting ants (B. Baer & J.J. Boomsma, manuscript in preparation). This fits the trade-off and constraint assumptions outlined above (Figure 5). The ancestral fungus-growing ants had single queen-mating and small, relatively short-lived colonies (11, 131). Small ejaculates thus sufficed and rapid sperm storage probably had some fitness advantage, maintaining long sperm in the absence of significant storage constraints. When colony size and queen longevity increased, shorter sperm evolved because the advantage of storing more sperm probably became significant. Attine colonies never became large before multiple queen-mating evolved, but the sperm of Solenopsis fire ants with obligatorily singly mating queens (107) and large colonies is shorter than that of any attine ant, which is consistent with expectation (81). Multiple queen-mating evolved in the ancestor of the Acromyrmex and Atta leafcutter ants (131) and is expected to select for longer sperm, unless spermatheca storage constraints preclude such development. These constraints are illustrated by the two vertically arrowed rectangles in Figure 5, indicating that their position may be anywhere between the typical sperm length before multiple mating evolved and some biologically feasible maximum. The data seem to confirm this, with Acromyrmex sperm having not increased in length, whereas sperm length is significantly longer in some Atta species than in sister genera of higher attine ants that have maintained single queen-mating (B. Baer & J.J. Boomsma, manuscript in preparation). Spermatheca size (both absolute and relative to queen body size and fecundity) may explain part of this variation, as the size of the sperm storage 28 Oct 2004 20:17 410 AR AR234-EN50-17.tex BOOMSMA BAER AR234-EN50-17.sgm LaTeX2e(2002/01/18) P1: GCE HEINZE Figure 5 The inferred trade-off between sperm length and sperm number in eusocial Hymenoptera, assuming that longer sperm moves faster and reaches the storage organ more effectively but has higher production costs. The vertical line marks the transition from single to multiple mating, as happened, for example, in the ancestor of the leafcutting ants and the honey bees. The concave, decreasing curve represents the normal sperm storage constraint, applying to all species. The two curves toward the right are hypothetical increases in sperm length after multiple mating evolved, until stopped by some dynamic ultimate storage constraint (vertically arrowed rectangles). organ may vary considerably even among species with queens of similar size (e.g., C. obscurior and C. minutior; E. Darouzzet, unpublished data). Sexual selection for longer sperm in social Hymenoptera with obligate multiple mating is expected to reach some dynamic equilibrium with the female storage constraints. However, these constraints may become relaxed when queen body size increases, which is likely as obligate multiple mating of queens is associated with large colony size (20). At such dynamic equilibrium, males may well produce sperm longer than necessary for the lifetime reproductive success of queens. This may be one of the few female-male arms races that can be maintained in social Hymenoptera, because its consequences (a lower number of lifetime fertilized eggs) affect the survival of colonies that have already reached the reproductive phase and not the probability of colonies reaching that stage. The evolution of dwarf honey bee males depositing their sperm directly into the spermatheca would 28 Oct 2004 20:17 AR AR234-EN50-17.tex AR234-EN50-17.sgm LaTeX2e(2002/01/18) SOCIAL INSECT MALES P1: GCE 411 be an ultimate male move in this arms race to which females would probably have no response. It would be interesting to know whether these bees have shorter sperm, as the strength of selection for longer sperm is likely to be lower when competition for storage no longer applies. Sperm Competition after Storage and Cryptic Female Choice In termites, sperm mortality has been hypothesized to be potentially significant (40) because females mate repeatedly with the same male so that sperm is abundantly available and not selected for longevity. In contrast, the Hymenoptera have limited amounts of long-lived sperm and use it efficiently after storage, an economy that is undoubtedly influenced by the lack of intraejaculate sperm competition due to the clonal nature of hymenopteran sperm (10, 11, 112). Some social Hymenoptera are incredibly economic, such as monandrous Solenopsis fire ant queens, which use on average only 2.6 to 3.5 sperm per fertilized egg (128, 129). Honey bee queens mate with 10 to 20 males, store one male’s worth of sperm, and use 5 to 14 sperm per egg (10). This implies that even with multiple matings, prestorage and poststorage sperm competition are of the same order of magnitude. In Atta colombica both figures are also similar and probably below 5 sperm per egg (44, 45), whereas Dolichovespula wasps with facultative multiple mating of queens (49) seem intermediate with approximately 7 sperm per egg (116). Overall, these sperm-to-egg ratios are similar to those found in nonsocial, parasitoid Hymenoptera (27), but that they can be maintained after years or even decades of sperm storage illustrates the amazing viability of sperm in social Hymenoptera and suggests that stored sperm may be physiologically quite different from sperm found in noneusocial Hymenoptera (56). However, even though sperm viability is universally high, both genetic and environmental variations are likely to exist. These variations and their possible causes and results deserve to be explicitly studied, either by comparing sperm storage and reproductive fitness of queens inseminated with semen of specific males (15, 77) or by comparing the representation of patrilines throughout the years in long-lived colonies (122). A re-analysis of comparative data (32, 54, 92) for ants (n = 5), social bees (n = 28), and social wasps (n = 11) showed that the number of sperm stored by a queen tends to increase isometrically (log-log slope ∼ 1) or allometrically (loglog slope >1) with the sperm complement of a typical conspecific male. However, these steep slopes are an artifact of the taxonomic heterogeneity of these overall data sets, as slopes within bee genera were lower than 1 (Bombus: slope = 0.632, standard error (SE) = 0.152, n = 7, probability (P) = 0.052 for difference with a slope of 1; Apis: slope = 0.398, SE = 0.095, n = 7, P < 0.001 for difference with a slope of 1). The latter slopes indicate that stored sperm does not increase proportionally to male sperm stores, either because males of larger Bombus species mate with more females so that ejaculate size is a smaller fraction of their sperm store, or because queens of larger Apis species mate with more males but without storing more sperm. 28 Oct 2004 20:17 412 AR AR234-EN50-17.tex BOOMSMA BAER AR234-EN50-17.sgm LaTeX2e(2002/01/18) P1: GCE HEINZE For ants it has further been established that the number of stored sperm increases allometrically (slope = 1.599, SE = 0.231, n = 26, P = 0.0163 for difference with a slope of 1) with queen ovariole number (128). Also this steep slope may be an artifact of the heterogeneity of the data set, but an alternative explanation may be that the rate of oocyte production per ovary increases in species with more fertile queens (87). Atta and Acromyrmex leafcutting ants store more sperm than predicted on the basis of their typical number of ovarioles (128), but this does not necessarily imply a steeper allometric slope, as the two basal attine ants in the same data set have positive residuals as well. A re-analysis (ANCOVA) of the data (128) separating attine (n = 4) and nonattine (n = 22) ants indicated that the slopes were homogeneous (P = 0.984) but that the difference of intercepts was approaching significance (P = 0.095). A larger sample is needed to settle this issue, but there is yet no convincing evidence that multiply mated attine queens store and use more sperm per egg than singly mated attine queens, for example, to allow for competition among the sperm of different fathers for the fertilization of eggs. In the honey bee, evidence for such sperm competition is also negative or ambiguous (51 and references therein). Sperm mixing is already substantial in the bursa copulatrix and tends to be almost complete a few months after storage (50, 78). In contrast, considerable sperm clumping and variable patterns of paternity across years were observed in the facultatively multiply mating ant Formica truncorum (122). Here, however, relatedness-induced split sex ratios make sperm clumping a direct fitness interest for the fathers. This adaptive response suggests that sperm clumping is a heritable trait that can be selected if there is a clear fitness interest. Most known mechanisms of female choice (40) are not applicable to the social insects, but some seem to offer realistic possibilities for cryptic postmating female choice. First, queens may resist the transport of sperm from unwanted males to the sperm storage organ. This seems likely, as the sperm storage process is affected by active and passive female factors in honey bees (75). Second, multiply mating queens may have evolved sperm storage barriers, for example, in the form of a long, contorted, and complicated spermathecal duct, which may serve for both cryptic female choice in sperm storage and differential sperm use for fertilization (40). Third, male ants (23) and social bees (102) may have elaborate genital architecture. These traits are in part naturally selected to help males stay attached to queens during mating, but they may also represent Fisherian runaway sexual selection following cryptic female choice (67), particularly when the extent of elaboration is higher in species with multiply mated females, as data for bees (102) indicate. This contrasts with genital morphology in termites, which is simple compared with their cockroach-like ancestors (40, 132). CONCLUSIONS A key question in sexual selection is whether it is the males or the females that determine the identity of successful males (24). Also, in social insects there is no simple answer to this question, but we have offered some contours of a 28 Oct 2004 20:17 AR AR234-EN50-17.tex AR234-EN50-17.sgm LaTeX2e(2002/01/18) SOCIAL INSECT MALES P1: GCE 413 conceptual framework that may ultimately allow an integrated understanding of the male and female components of sexual selection across the different groups of eusocial insects. In all four major groups considered here, sexual selection seems relatively weak in both males and females, as entire groups such as the higher termites and the obligatorily singly mating ants, bees, and wasps may not have any sexual selection beyond premating partner choice. However, the ample availability of these monogamous groups allows a relatively precise comparative evaluation of sexually selected traits in sister groups in which one or both sexes mate multiply. Other interesting contrasts are provided by bizarre adaptations in some derived taxa, such as the fighting Cardiocondyla males with continuous sperm production, and by the major differences in longevity and sperm storage between the annual and perennial social Hymenoptera and between the perennial ants and termites. The short available time frame for lifetime mate choice in social insects may well imply that queens can discriminate only according to innate minimum criteria of male quality and mate with the first male(s) that fulfills these. Starr (115) argued that this leaves little room for female choice, and Alexander et al. (4) added that this was particularly true for arbitrary female choice of male traits shaped by runaway selection. However, the social Hymenoptera do not leave much room for the alternative of female choice for direct benefits or good genes either, although these mechanisms may play a role in termite mate choice. On the basis of the data reviewed here, we tentatively conclude that (cryptic) female choice seems to be a more attractive general framework than sexual conflict models, although antagonistic sexual coevolution may happen occasionally. We further stress that premating male manipulation strategies via mating plugs have probably been systematically overlooked, such that the mating systems of social Hymenoptera may be more male controlled than previously acknowledged. Both detailed comparative analyses and in-depth experimental studies are badly needed. Of these, the comparative study of sexual dimorphism and male genitalia seem relatively easy and straightforward, whereas others, such as the explicit study of male precedence, sperm traits, female genitalia and spermathecae, and the selectiveness and cost of long-term sperm storage, will be technically challenging. ACKNOWLEDGMENTS Our work was supported by a research award from the Alexander von Humboldt Foundation (JJB), grants from the Danish Natural Science Research Council (JJB and BCB) and the Deutsche Forschungsgemeinschaft (JH: He 1623/12–2), and the EU-Research-Training Network INSECTS (contract HPRN-CT-2000–00,052). Walter Tschinkel and Rob Page provided unpublished data from previous reviews, and David Nash helped with the formatting of the figures. The final version benefited from comments by Duur Aanen, Sophie Armitage, Barbara Baer Imhoof, Lisbeth Børgesen, Sylvia Cremer, Patrizia D’Ettorre, Mischa Dijkstra, Susanne 28 Oct 2004 20:17 414 AR AR234-EN50-17.tex BOOMSMA BAER AR234-EN50-17.sgm LaTeX2e(2002/01/18) P1: GCE HEINZE Foitzik, Judith Korb, Daniel Kronauer, David Nash, Jes Pedersen, and Michael Poulsen. The Annual Review of Entomology is online at http://ento.annualreviews.org LITERATURE CITED 1. Abe T, Bignell DE, Higashi M, eds. 2000. Termites: Evolution, Sociality, Symbiosis, Ecology. Dordrecht: Kluwer Academic 1a. Abell AJ, Cole BJ, Reyes R, Wiernasz DC. 1999. Sexual selection on body size and shape in the western harvester ant, Pogonomyrmex occidentalis Cresson. Evolution 53:535–45 2. Akre RD. 1982. Social wasps. See Ref. 63a, 4:1–105 3. Alcock J, Smith AP. 1987. Hilltopping, leks and female choice in the carpenter bee Xylocopa (Neoxylocopa) varipuncta. J. Zool. 211:1–10 4. Alexander RD, Marshall DC, Cooley JR. 1997. Evolutionary perspectives on insect mating. See Ref. 27a, pp. 4–31 5. Alford DV. 1975. Bumblebees. London: Davie-Poynter. 352 pp. 6. Allard D, Gobin B, Ito F, Tsuji K, Billen J. 2002. Sperm transfer in the Japanese queenless ant Diacammasp. (Hymenoptera: Formicidae). Neth. J. Zool. 52:77– 86 7. Anderson C, Cremer S, Heinze J. 2003. Live and let die: why fighter males of the ant Cardiocondyla kill each other but tolerate their winged rivals. Behav. Ecol. 14:54–62 8. Andersson M. 1994. Sexual Selection. Princeton, NJ: Princeton Univ. Press. 599 pp. 9. Ayasse M, Paxton RJ, Tengö J. 2001. Mating behavior and chemical communication in the order Hymenoptera. Annu. Rev. Entomol. 46:31–78 10. Baer B. 2003. Bumblebees as model organisms to study male sexual selection in social insects. Behav. Ecol. Sociobiol. 54:521–33 11. Baer B, Boomsma JJ. 2004. Male reproductive investment and queen mating frequency in fungus growing ants. Behav. Ecol. 15:426–32 12. Deleted in proof 13. Baer B, Maile R, Schmid-Hempel P, Morgan ED, Jones GR. 2000. Chemistry of a mating plug in bumblebees. J. Chem. Ecol. 26:1869–75 14. Baer B, Morgan ED, Schmid-Hempel P. 2001. A non-specific fatty acid within the bumblebee mating plug prevents females from remating. Proc. Natl. Acad. Sci. USA 98:3926–28 15. Baer B, Schmid-Hempel P, Hoeg JT, Boomsma JJ. 2003. Sperm length, sperm storage and mating system characteristics in bumblebees. Insectes Soc. 50:101–8 16. Beani L, Cervo R, Lorenzi CM, Turillazzi S. 1992. Landmark-based mating systems in four Polistes species (Hymenoptera: Vespidae). J. Kans. Entomol. Soc. 65:211–17 17. Beani L, Turillazzi S. 1990. Overlap at landmarks by lek-territorial and swarming males of two sympatric polistine wasps (Hymenoptera: Vespidae). Ethol. Ecol. Evol. 2:419–31 18. Boomsma JJ. 1989. Sex investment ratios in ants: Has female bias been systematically overestimated? Am. Nat. 133:517– 32 19. Boomsma JJ, Fjerdingstad EJ, Frydenberg J. 1999. Multiple paternity, relatedness and genetic diversity in Acromyrmex leafcutter ants. Proc. R. Soc. London Sci. Ser. B 266:249–54 20. Boomsma JJ, Ratnieks FLW. 1996. Paternity in eusocial Hymenoptera. Philos. Trans. R. Soc. London Ser. B 351:947–75 28 Oct 2004 20:17 AR AR234-EN50-17.tex AR234-EN50-17.sgm LaTeX2e(2002/01/18) SOCIAL INSECT MALES 21. Boomsma JJ, Sundström L. 1998. Patterns of paternity skew in Formica ants. Behav. Ecol. Sociobiol. 42:85–92 22. Boomsma JJ, van der Have TM. 1998. Queen mating and paternity variation in the ant Lasius niger. Mol. Ecol. 7:1709– 18 23. Bourke AFG, Franks NR. 1995. Social Evolution in Ants. Princeton, NJ: Princeton Univ. Press. 529 pp. 24. Bradbury JW. 1985. Contrasts between insects and vertebrates in the evolution of male display, female choice, and lek mating. See Ref. 65a, pp. 273–89 25. Brown MJF, Baer B, Schmid-Hempel R, Schmid-Hempel P. 2002. Dynamics of multiple mating in the bumblebee Bombus hypnorum. Insectes Soc. 49:315–19 26. Chapman T, Arnqvist G, Bangham J, Rowe L. 2003. Sexual conflict. Trends Ecol. Evol. 18:41–47 27. Chevrier C, Bressac C. 2002. Sperm storage and use after multiple mating in Dinarmus basalis (Hymenoptera: Pteromalidae). J. Insect Behav. 15:385–98 27a. Choe JC, Crespi BJ, eds. 1997. Mating Systems in Insects and Arachnids. Cambridge, UK: Cambridge Univ. Press 27b. Choe JC, Crespi BJ, eds. 1997. Social Behavior in Insects and Arachnids. Cambridge, UK: Cambridge Univ. Press 28. Cook JM, Compton SG, Herre EA, West SA. 1997. Alternative mating tactics and extreme male dimorphism in fig wasps. Proc. R. Soc. London Sci. Ser. B 264:747– 54 29. Cremer S, Heinze J. 2003. Stress grows wings: environmental induction of winged and dispersal males in Cardiocondyla. Curr. Biol. 13:219–23 30. Cremer S, Sledge MF, Heinze J. 2002. Male ants disguised by the queen’s bouquet. Nature 419:897 31. Crozier RH, Fjerdingstad EJ. 2001. Polyandry in social Hymenoptera: disunity in diversity? Ann. Zool. Fenn. 38:267–85 32. Crozier RH, Page RE. 1985. On being the 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. P1: GCE 415 right size: male contributions and multiple mating in social Hymenoptera. Behav. Ecol. Sociobiol. 18:105–15 Crozier RH, Pamilo P. 1996. Evolution of Social Insect Colonies. Oxford, UK: Oxford Univ. Press. 306 pp. Danforth BF, Desjardins CA. 1999. Male dimorphism in Perdita portalis (Hymenoptera, Andrenidae) has arisen from preexisting allometric patterns. Insectes Soc. 46:18–28 Davidson DW. 1982. Sexual selection in harvester ants (Formicidae: Pogonomyrmex). Behav. Ecol. Sociobiol. 10:245– 50 DeHeer CJ, Vargo EL. 2004. Colony genetic organization and colony fusion in the termite Reticulitermes flavipes as revealed by foraging patterns over time and space. Mol. Ecol. 13:431–41 Dumser JB. 1980. The regulation of spermatogenesis in insects. Annu. Rev. Entomol. 25:341–69 Duvoisin N, Baer B, Schmid-Hempel P. 1999. Sperm transfer and male competition in a bumblebee. Anim. Behav. 58:743–49 Eberhard WG. 1985. Sexual Selection and Animal Genitalia. Cambridge, MA: Harvard Univ. Press. 244 pp. Eberhard WG. 1996. Female Control: Sexual Selection by Cryptic Female Choice. Princeton, NJ: Princeton Univ. Press. 501 pp. Emlen DJ. 1997. Alternative reproductive tactics and male-dimorphism in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Behav. Ecol. Sociobiol. 41:335–41 Emlen ST, Oring LW. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197:215–23 Fernández-Escudero I, Pamilo P, Seppä P. 2002. Biased sperm use by polyandrous queens of the ant Proformica longiseta. Behav. Ecol. Sociobiol. 51:207–13 Fjerdingstad EJ, Boomsma JJ. 1997. Variation in size and sperm content of sexuals 28 Oct 2004 20:17 416 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. AR AR234-EN50-17.tex BOOMSMA BAER AR234-EN50-17.sgm LaTeX2e(2002/01/18) P1: GCE HEINZE in the leafcutter ant Atta colombica. Insectes Soc. 44:209–18 Fjerdingstad EJ, Boomsma JJ. 1998. Multiple mating increases the sperm stores of Atta colombica leafcutter ant queens. Behav. Ecol. Sociobiol. 42:257–61 Fjerdingstad EJ, Gertsch PJ, Keller L. 2003. The relationship between multiple mating by queens, within-colony genetic variability and fitness in the ant Lasius niger. J. Evol. Biol. 16:844–53 Foitzik S, Heinze J, Oberstadt B, Herbers JM. 2002. Mate guarding and alternative reproductive tactics in the ant Hypoponera opacior. Anim. Behav. 63:597–604 Fortelius W, Pamilo P, Rosengren R, Sundström L. 1987. Male size dimorphism and alternative reproductive tactics in Formica exsecta ants (Hymenoptera, Formicidae). Ann. Zool. Fennici 24:45–54 Foster KR, Ratnieks FLW. 2001. Paternity, reproduction and conflict in vespine wasps: a model system for testing kin selection predictions. Behav. Ecol. Sociobiol. 50:1–8 Franck P, Coussy H, Le Conte Y, Solignac M, Garnery L, Cornuet J-M. 1999. Microsatellite analysis of sperm in honeybee. Insect Mol. Biol. 8:419–21 Franck P, Solignac M, Vautrin D, Cornuet J-M, Koeniger G, Koeniger N. 2002. Sperm competition and last-male precedence in the honeybee. Anim. Behav. 64:503–9 Franks NR, Hölldobler B. 1987. Sexual competition during colony reproduction in army ants. Biol. J. Linn. Soc. 30:229–43 Franks NR, Sendova-Franks AB, Sendova-Vassileva M, Vassilev L. 1991. Nuptial flights and calling behaviour in the ant Leptothorax acervorum (Fabr.). Insectes Soc. 38:327–30 Garofalo CA. 1980. Reproductive aspects and evolution of social behavior in bees (Hymenoptera, Apoidea). Rev. Bras. Genet. 3:139–52 Garofalo CA, Zucchi R, Muccillo G. 1986. Reproductive studies of a neotrop- 56. 57. 58. 59. 60. 61. 62. 63. 63a. 64. 65. 65a. 66. ical bumblebee, Bombus atratus (Hymenoptera, Apidae). Rev. Bras. Genet. 9:231–43 Gillott C. 2003. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 48:163–84 Hamilton WD. 1967. Extraordinary sex ratios. Science 156:477–88 Hamilton WD. 1979. Wingless and fighting males in fig wasps and other insects. In Sexual Selection and Reproductive Competition in Insects, ed. MS Blum, NA Blum, pp. 167–220. New York: Academic Heinze J. 1999. Male polymorphism in the ant Cardiocondyla minutior (Hymenoptera: Formicidae). Entomol. Gener. 23:251–58 Heinze J. 2000. Testes degeneration and limited sperm supply in ant males with intranidal mating. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 12:207–10 Heinze J, Hölldobler B. 1993. Fighting for a harem of queens: physiology of reproduction in Cardiocondyla male ants. Proc. Natl. Acad. Sci. USA 90:8412–14 Heinze J, Hölldobler B, Yamauchi K. 1998. Male competition in Cardiocondyla ants. Behav. Ecol. Sociobiol. 42:239–46 Helms KR. 1994. Sexual size dimorphism and sex ratios in bees and wasps. Am. Nat. 143:418–34 Hermann HR. 1982. Social Insects, Vols. 3, 4. New York: Academic Hölldobler B. 1976. The behavioral ecology of mating in harvester ants (Hymenoptera: Formicidae: Pogonomyrmex). Behav. Ecol. Sociobiol. 1:405–29 Hölldobler B, Bartz SH. 1985. Sociobiology of reproduction in ants. See Ref. 65a, pp. 237–57 Hölldobler B, Lindauer M, eds. 1985. Experimental Behavioral Ecology and Sociobiology. Stuttgart/New York: Gustav Fischer Hölldobler B, Wilson EO. 1990. The Ants. Cambridge, MA: Harvard Univ. Press. 732 pp. 28 Oct 2004 20:17 AR AR234-EN50-17.tex AR234-EN50-17.sgm LaTeX2e(2002/01/18) SOCIAL INSECT MALES 67. Hosken DJ, Stockley P. 2004. Sexual selection and genital evolution. Trends Ecol. Evol. 19:87–93 68. Jamieson BGM. 1987. The Ultrastructure and Phylogeny of Insect Spermatozoa. Cambridge/New York: Cambridge Univ. Press. 320 pp. 69. Keller L, ed. 1993. Queen Number and Sociality in Insects. Oxford: Oxford Univ. Press. 439 pp. 70. Keller L. 1998. Queen lifespan and colony characteristics in ants and termites. Insectes Soc. 45:235–46 71. Keller L, Passera L. 1992. Mating system, optimal number of matings, and sperm transfer in the Argentine ant Iridomyrmex humilis. Behav. Ecol. Sociobiol. 31:359– 66 72. Kinomura K, Yamauchi K. 1987. Fighting and mating behaviors of dimorphic males in the ant Cardiocondyla wroughtoni. J. Ethol. 5:75–81 73. Koeniger G. 1990. The role of the mating sign in honey bees, Apis mellifera L.: Does it hinder or promote multiple mating? Anim. Behav. 39:444–49 74. Koeniger G, Koeniger N, Tingek S, Kelitu A. 2000. Mating flights and sperm transfer in the dwarf honeybee Apis andreniformis (Smith, 1858). Apidologie 31:301–11 75. Koeniger N, Koeniger G. 2000. Reproductive isolation among species of the genus Apis. Apidologie 31:313–39 76. Koeniger N, Koeniger G, Wongsiri S. 1989. Mating and sperm transfer in Apis florea. Apidologie 20:413–18 77. Korner P, Schmid-Hempel P. 2003. Effects of sperm on female longevity in the bumblebee Bombus terrestris L. Proc. R. Soc. London Sci. Ser. B (Suppl.) 270: S227–29 77a. Krishna K, Weesner FM, eds. 1969. Biology of Termites, Vol. 1. New York: Academic 78. Laidlaw HH, Page RE. 1985. Polyandry in honey bees (Apis mellifera L.): sperm utilization and intracolony genetic relationships. Genetics 108:985–97 P1: GCE 417 79. LeMasne G. 1956. La signification des reproducteurs aptères chez la fourmi Ponera eduardi Forel. Insectes Soc. 3:239–59 80. Lensky Y, Ben David E, Schidler H. 1979. Ultrastructure of the spermatozoan of the mature drone honey bee Apis mellifera. J. Apic. Res. 18:264–71 81. Lino Neto J, Dolder H. 2002. Sperm structure and ultrastructure of the fire ant Solenopsis invicta (Buren) (Hymenoptera, Formicidae). Tissue Cell 34:124– 28 82. Marikovskii PI, Yakushkin VT. 1974. Muraveij Cardiocondya uljanini Em., 1889 i sistematicheskoe polozhenie “paraziticheskogo murav’ya Xenometra”. Izv. Akad. Nauk Kazakhskoy SSR Ser. Biol. 3:57–62 83. Matsuura K, Nichida T. 2001. Colony fusion in a termite: What makes the society “open”? Insectes Soc. 48:378–83 84. Michener CD. 1974. The Social Behavior of the Bees. Cambridge, MA: Harvard Univ. Press. 558 pp. 85. Mikheyev AS. 2003. Evidence for mating plugs in the fire ant Solenopsis invicta. Insectes Soc. 50:401–2 86. Miles TG, Nutting WL. 1988. Termite eusocial evolution: a re-examination of Bartz’s hypothesis and assumptions. Q. Rev. Biol. 63:1–23 87. Murakami T, Higashi S, Windsor D. 2000. Mating frequency, colony size, polyethism and sex ratio in fungusgrowing ants (Attini). Behav. Ecol. Sociobiol. 48:276–84 88. Nalepa CA, Bandi C. 2000. Characterizing the ancestors: paedomorphosis and termite evolution. See Ref. 1, pp. 53– 76 89. Nalepa CA, Bell WJ. 1997. Postovulation parental investment and parental care in cockroaches. See Ref. 27b, pp. 26–51 90. Nalepa CA, Miller LR, Lenz M. 2001. Flight characteristics of Mastotermes darwiniensis (Isoptera, Mastotermitidae). Insectes Soc. 48:144–48 91. Nutting WL. 1969. Flight and colony foundation. See Ref. 77a, 1:233–82 28 Oct 2004 20:17 418 AR AR234-EN50-17.tex BOOMSMA BAER AR234-EN50-17.sgm LaTeX2e(2002/01/18) P1: GCE HEINZE 92. Page RE Jr. 1986. Sperm utilization in social insects. Annu. Rev. Entomol. 31:297– 320 93. Page RE Jr, Metcalf RA. 1982. Multiple mating, sperm utilization, and social evolution. Am. Nat. 119:263–81 94. Palmer KA, Oldroyd BP. 2001. Mating frequency in Apis florea revisited (Hymenoptera, Apidae). Insectes Soc. 48:40– 43 95. Pamilo P. 1991. Life span of queens in the ant Formica exsecta. Insectes Soc. 38(2):111–20 96. Paxton RJ. 2000. Genetic structure of colonies and a male aggregation in the stingless bee Scaptotrigona postica, as revealed by microsatellite analysis. Insectes Soc. 47:63–69 97. Peters JM, Queller DC, ImperatrizFonseca VL, Roubik DW, Strassmann JE. 1999. Mate number, kin selection and social conflicts in stingless bees and honeybees. Proc. R. Soc. London Sci. Ser. B. 266:379–84 98. Radloff SE, Hepburn HR, Koeniger G. 2003. Comparison of flight design of Asian honeybee drones. Apidologie 34:1– 6 99. Reichardt AK, Wheeler DE. 1996. Multiple mating in the ant Acromyrmex versicolor: a case of female control. Behav. Ecol. Sociobiol. 38:219–25 100. Robertson HG. 1995. Sperm transfer in the ant Carebara vidua F. Smith (Hymenoptera: Formicidae). Insectes Soc. 42:411–18 101. Robertson HG, Villet M. 1989. Mating behavior in three species of myrmicine ants (Hymenoptera: Formicidae). J. Nat. Hist. 23:767–73 102. Roig-Alsina A. 1993. The evolution of the apoid endophallus, its phylogenetic implications, and functional significance of the genital capsule (Hymenoptera, Apoidea). Boll. Zool. 60:169–83 103. Roisin Y. 2000. Diversity and evolution of caste patterns. See Ref. 1, pp. 95–119 104. Roonwal ML. 1975. Sex ratios and sexual 105. 106. 107. 108. 109. 110. 111. 112. 113. 114. 115. dimorphism in termites. J. Sci. Ind. Res. 34:402–16 Röseler PF. 1973. Die Anzahl der Spermien im Receptaculum Seminis von Hummelköniginnen (Hymenoptera, Apidea, Bombinae). Apidologie 4:267–74 Ross KG, Carpenter JM. 1991. Population genetic structure, relatedness, and breeding systems. In The Social Biology of Wasps, ed. KG Ross, RW Matthews, pp. 451–79. Ithaca, NY: Cornell Univ. Press Ross KG, Vargo EL, Fletcher DC. 1988. Colony genetic structure and queen mating frequency in fire ants of the subgenus Solenopsis (Hymenoptera: Formicidae). Biol. J. Linn. Soc. 34:105–17 Sakagami SF. 1982. Stingless bees. See Ref. 63a, 3:361–423 Sauter A, Brown MJF, Baer B, SchmidHempel P. 2001. Males of social insects can prevent queens from multiple mating. Proc. R. Soc. London Sci. Ser. B 268:1449–54 Schlüns H, Schlüns EA, van Praagh J, Moritz RFA. 2003. Sperm numbers in drone honeybees (Apis mellifera) depend on body size. Apidologie 34(6):577– 84 Shellman-Reeve JS. 1997. The spectrum of eusociality in termites. See Ref. 27b, pp. 52–93 Simmons LW. 2001. Sperm Competition and Its Evolutionary Consequences in the Insects. Princeton, NJ: Princeton Univ. Press. 434 pp. Sivinski JM, Petersson E. 1997. Mate choice and species isolation in swarming insects. See Ref. 27a, pp. 294–309 Starr CK. 1979. Origin and evolution of insect sociality: a review of modern theory. In Social Insects, Vol. 1, ed. HR Hermann, pp. 35–79. New York: Academic Starr CK. 1984. Sperm competition, kinship and sociality in the aculeate Hymenoptera. In Sperm Competition and the Evolution of Animal Mating Systems, ed. RL Smith, pp. 427–64. London: Academic 28 Oct 2004 20:17 AR AR234-EN50-17.tex AR234-EN50-17.sgm LaTeX2e(2002/01/18) SOCIAL INSECT MALES 116. Stein KJ, Fell RD, Holtzman GI. 1996. Sperm use dynamics of the baldfaced hornet (Hymenoptera: Vespidae). Environ. Entomol. 25:1365–70 117. Strassmann J. 2001. The rarity of multiple mating by females in the social Hymenoptera. Insectes Soc. 48:1–13 118. Stuart AM. 1969. Social behavior and communication. See Ref. 77a, 1:193– 232 119. Stuart RJ, Francoeur A, Loiselle R. 1987. Lethal fighting among dimorphic males of the ant, Cardiocondyla wroughtonii. Naturwissenschaften 74:548–49 120. Stubblefield JW, Seger J. 1994. Sexual dimorphism in the Hymenoptera. In The Differences Between the Sexes, ed. RV Short, E Balaban, pp. 77–103. Cambridge, UK: Cambridge Univ. Press 121. Sumner S, Hughes WOH, Pedersen JS, Boomsma JJ. 2004. Ant parasite queens revert to mating singly. Nature 428:35– 36 122. Sundström L, Boomsma JJ. 2000. Reproductive alliances and posthumous fitness enhancement in male ants. Proc. R. Soc. London Sci. Ser. B 267:1439–44 123. Thorne BL. 1985. Termite polygyny: the ecological dynamics of queen mutualism. See Ref. 65a, pp. 325–41 124. Thorne BL. 1997. Evolution of eusociality in termites. Annu. Rev. Ecol. Syst. 28:27– 54 125. Thorne BL, Breisch NL, Haverty MI. 2002. Longevity of kings and queens and first time of production of fertile progeny in dampwood termite (Isoptera; Termopsidae; Zootermopsis) colonies with different reproductive strategies. J. Anim. Ecol. 71:1030–41 126. Thornhill R, Alcock J. 1983. The Evolution of Insect Mating Systems. Cambridge, MA: Harvard Univ. Press. 547 pp. 127. Trivers RL. 1972. Parental investment and sexual selection. In Sexual Selection and the Descent of Men 1871–1971, ed. B Campbell, pp. 136–79. Chicago: Aldine 128. Tschinkel WR. 1987. Relationship be- 129. 130. 131. 132. 133. 134. 135. 136. 137. 138. 139. P1: GCE 419 tween ovariole number and spermathecal sperm count in ant queens: a new allometry. Ann. Entomol. Soc. Am. 80:208– 11 Tschinkel WR, Porter SD. 1988. Efficiency of sperm use of queens of the fire ant, Solenopsis invicta (Hymenoptera Formicidae). Ann. Entomol. Soc. Am. 81:777–81 Turillazzi S, West Eberhard MJ. 1996. Natural History and Evolution of Paper Wasps. Oxford: Oxford Univ. Press. 400 pp. Villesen P, Murakami T, Schultz TR, Boomsma JJ. 2002. Unraveling the transition between single and multiple mating in attine ants. Proc. R. Soc. London Sci Ser. B 269:1541–48 Weesner FM. 1969. The reproductive system. See Ref. 77a, 1:125–60 Wiernasz DC, Sater AK, Abell AJ, Cole BJ. 2001. Male size, sperm transfer, and colony fitness in the western harvester ant, Pogonomyrmex occidentalis. Evolution 55:324–29 Wiernasz DC, Yencharis J, Cole BJ. 1995. Size and mating success in males of the western harvester ant Pogonomyrmex occidentalis (Hymenoptera: Formicidae). J. Insect Behav. 8:523–31 Winter U, Buschinger A. 1983. The reproductive biology of a slavemaker ant, Epimyrma ravouxi, and a degenerate slavemaker, E. kraussei (Hymenoptera: Formicidae). Entomol. Gener. 9:1–15 Wilson EO. 1971. The Insect Societies. Cambridge, MA. Harvard Univ. Press. 558 pp. Woyciechowski M. 1990. Mating behaviour in the ant Myrmica rubra (Hymenoptera: Formicidae). Acta Zool. Cracov. 33:565–74 Woyciechowski M, Kabat L, Król E. 1994. The function of the mating sign in honey bees, Apis mellifera L.: new evidence. Anim. Behav. 47:733–35 Yamauchi K, Furukawa T, Kinomura K, Takamine H, Tsuji K. 1991. Secondary 28 Oct 2004 20:17 420 AR AR234-EN50-17.tex BOOMSMA BAER AR234-EN50-17.sgm LaTeX2e(2002/01/18) P1: GCE HEINZE polygyny by inbred wingless sexuals in the dolichoderine ant Technomyrmex albipes. Behav. Ecol. Sociobiol. 29:313– 19 140. Yamauchi K, Kimura Y, Corbara B, Kinomura K, Tsuji K. 1996. Dimorphic ergatoid males and their reproductive behavior in the ponerine ant Hypoponera bondroiti. Insectes Soc. 43:119–30 141. Yamauchi K, Oguchi S, Nakamura Y, Suetake H, Kawada N, Kinomura K. 2001. Mating behavior of dimorphic reproductives of the ponerine ant, Hypoponera nubatama. Insectes Soc. 48:83–87