* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Heat capacity wikipedia , lookup
Equipartition theorem wikipedia , lookup
Heat transfer wikipedia , lookup
Thermal conduction wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Conservation of energy wikipedia , lookup
Internal energy wikipedia , lookup
Adiabatic process wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Thermodynamic system wikipedia , lookup
Heat transfer physics wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Gibbs free energy wikipedia , lookup
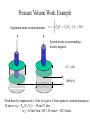
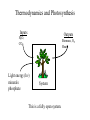
Work • Work (w): addition or extraction of non-heat energy from a system. – Work is positive if it flows into the system – Work is force times distance (in direction of force): x2 w f dx x1 – Gravitational work: w mgh (h and g in same direction) x2 – Work against a spring: w k x x 0 d x x0 x1 • k = spring constant • Spring force (pushing back) = f sp k(x x 0 ) Pressure Volume Work V2 w Pex dV V1 Pex Pex V1 V2 V Pressure-volume work is equivalent to Force-distance work Pressure Volume Work: Example V2 Expansion under constant pressure P P w Pex dV Pex V2 V1 PV V1 System works on surroundings, work is negative A V Ah hf hi h=hf-hi Work done by expansion of a 1 liter of a gas to 2 liters against a constant pressure at 10 atm is: wP= -Pex (V2-V1)= -10 atm *1 liter wp= -10 liter*atm (101.3 J/L•atm)= -1013 Joules Heat • Heat (q): the quantity of energy exchanged across the boundaries of a system that results in temperature change. – Heat is positive if it flows into the system – Heat capacity, C: the amount of thermal energy that must be added to a system per unit temperature rise under specific conditions. dq C dt dq CdT – C is different for different materials and depends on temperature T2 q CdT T1 – For a pure chemical material C nC where n is the number of moles and C is the molar heat capacity. (see table TSWP p. 26) Heat Capacity under Different Conditions The ability of a material to transfer heat depends on the conditions. For example, processes can be carried out at constant volume or constant pressure. Bench Chemistry Bomb Calorimetry Ignite CP Constant Pressure Process(isobaric) RXN Temp. CV Constant Volume Process (isochoric) Heat Capacity is an extensive property. To compare capacities we use molar heat capacities or specific heat capacities. Bomb Calorimetry Ignite RXN Temp. Consider the system: 0.1 g H2 + 0.8 g O2 We find that the heat capacity at constant volume for this amount of material is Cv=21,700 calories/°C. After ignition we find that the temperature has risen from 25 °C to 25.155°C qV=CV*T= 21,700 cal/°C *(0.155 °C)= 3360 cal But remember we choose the sign such that heat evolved by the system is negative! So really: qv= -3.36 kcal Energy • Energy is a measure of the capacity to do work. – Energy is a state variable, depends only on state, not path. • Enthalpy: H = E + PV – H has units of energy (J, kJ, cal, kcal) • Entropy: a measure of unavailable energy in a closed system, disorder. • Free energy: G = H + TS – Connects enthalpy and entropy – Reflects tendency of system to change from one state to another Equations of State An equation of state relates the variables of state (P,V,T,n). Let’s consider equations for volume. In solids and liquids dV/dP 0, dV/dT0 V(T,P) constant In gases: e.g. An ideal gas-e.g. A van der Waals gas V(T,P,n)= (nRT)/P n2a ( P 2 )(V nb) nRT V State Changes Now that we have an understanding of 1) What kinds of energy can be transferred 2) What kinds of equations define the state of a system. How do we treat changes between states? Changes include not only the heat and work, temperature and pressure with which we are now familiar but also changes in physical and chemical states. We will use two concepts: Internal Energy, E and Enthalpy, H to describe these changes. Energy and Enthalpy Types of energy that can be transferred: Heat Work We are interested in measures of the energy of our system. Remember heat and work are path dependent. Energy (E)- Energy is the capacity for doing work. Enthalpy (H)- The heat content of a system. (E+PV) Thus enthalpy is really an energy corrected for the pressure/volume work. E and H are variables of state. The Laws of Thermodynamics • Zeroth Law: two systems in equilibrium with a third system are in equilibrium with each other. • First Law: total energy of a system plus surrounds is conserved. • Second Law: total entropy of the system plus surroundings never decreases. • Third Law: the total entropy of all pure, perfect crystals at absolute zero temperature (0 Kelvin) is zero. The Zeroth Law • Two systems in equilibrium with a third system are in equilibrium with each other. – This provides an operational definition of temperature and is the basis of thermometry. P1 P2 V1 V2 V1 Isolated P1 V1 P2 V2 P2’ P1’ V2 In thermodynamic equilibrium P3 V3 P1 V1 P3 V3 Introduction to First Law The state of system changes when heat is transferred to or from the system or work is done. If these are the only forms of energy in the system (e.g. no mass is transferred) then it seems reasonable that energy must be conserved! E2-E1= q + w (with the proper sign conventions) But it was not obvious early on that this “Law” held. Joule in around 1843 did the definitive experiment: T Essentially, the amount of heat generated in the vessel by the movement of the paddle is exactly the potential energy lost by the weight. The First Law • The total energy of the system plus surroundings is conserved. • Energy is neither created nor destroyed. – Energy can be transferred • Heat (q) • Work (w) – Change in energy is equal to the sum of heat and work: E E1 E2 q w In a public lecture, Joule rejoiced in this understanding: "...the phenomena of nature, whether mechanical, chemical or vital, consist almost entirely in a continual conversion of attraction through space [PE], living force [KE], and heat into one another. Thus it is that order is maintained in the universe-nothing is deranged, nothing ever lost, but the entire machinery, complicated as it is, works smoothly and harmoniously. ...every thing may appear complicated and involved in the apparent confusion and intricacy of an almost endless variety of causes, effects, conversions, and arrangements, yet is the most perfect regularity preserved...." Thermodynamics and Photosynthesis Inputs Outputs H2O, CO2 Biomass, O2, Heat Light energy (h) minerals phosphate System This is a fully open system. Thermodynamics and Photosynthesis Inputs Outputs H2O, CO2 h minerals phosphate Biomass, O2, Heat We assume the major conversion process is: System H2O + CO2 O2 + (CH2O) The enthalpy change for the process is H°= 485 Joules/mol In order to get this process to go we need: light, and a catalytic system. This takes place in the chloroplast of the plant. Thermodynamics and Photosynthesis We assume the major conversion process is: H2O + CO2 O2 + (CH2O) H°= 485 Joules/mol It has been shown that it takes about 8-9 photons to make one O2. The first law tells us that the total energy input must equal total energy output. Photon Energy = Chemical Energy + Heat Efficiency= Chemical Energy/Photon Energy Thermodynamics and Photosynthesis So to calculate the efficiency of production of 1 mole of O2: 1 mole of photons= 1 einstein Photon Energy= (8-9 einsteins)(6*1023 photons/mol) h h= planck’s constant= 6*10-34 Js = c/=(3*108 m/s)/(680*10-9 m) Photon Energy= 1400-1570 kJ Chemical Energy= 485 kJ/mol * 1 mol O2 %Efficiency= 485/1570 * 100 = 31% Path Dependence and Independence q and w are path dependent variables. State 1 A state change that occurs over the blue path has qblue, wblue. Over the red path we get qred, wred. State 2 All we know from the 1st Law is qblue+wblue= qred+wred Reversible or Irreversible State Change Reversible: State is changed by differential amounts along a path. At any moment a small change in the opposing force will alter the direction of the state change. Irreversible: “All at once”-- the method of the change is such that it is not possible to reverse the direction. Heat State A Ethermal =+ State B Emech. =0 Ethermal =0 Emech.= + Reversibility of Paths: PV work Here in the irreversible case you would have to do significant mechanical work to restore the initial state. In the reversible case, a small differential change in the weight can cause a reversal. State A Heat Ethermal 0 Ethermal =- State B Emech. =0 Emech. =+ Work from Reversible vs. Irreversible Processes State 1 State 1 Ideal Isotherm P P P State 2 V1 V V2 Constant pressure expansion State 2 V1 Isothermal irreversible expansion w PdV V1 If the opposing pressure is 0, w = 0 thus E q V2 V V2 w Pop dV nRT V V2 V1 nRT dV V V2 w nRT lnV2 ln V1 nRT ln V1