* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Exposure Response
Survey
Document related concepts
Transcript
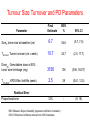
The time course of tumour size changes with gemcitabine. What can we learn about pharmacology? Nick Holford Dept Pharmacology & Clinical Pharmacology University of Auckland The Workers Lai San Tham3 Boon-Cher Goh1 Wei Peng Yong1 Ross A Soo1 Ling-Zhi Wang1 Soo-Chin Lee1 How-Sung Lee2 1Department of Hematology-Oncology, National University Hospital, Singapore 2Department of Pharmacology, National University of Singapore 3Lilly-NUS Centre for Clinical Pharmacology, Singapore Outline • What does Pharmacology mean? • Does Cancer need PKPD? • Learning and Confirming – A reminder • Tumor response study – A pharmacodynamic model for the time course of tumor shrinkage in patients with ‘big cell’ lung cancer – What can we learn about pharmacology? – How can we use tumour size to predict survival? Pharmacokinetics Pharmacodynamics Treatment Rx =Recipe or Jupiter Symbol What does this mean? Confirming or Learning? Confirming • Making sure • Outcome Expected • Analysis Assumptions Minimized E.g. Randomized Treatment Assignment • Questions for Drug Approval – E.g. • Is the drug effective? • Can it be used safely in renal failure? Learning • Exploration • Outcome Unexpected • Assumption rich analysis – E.g. PKPD model • Questions for Drug Science – E.g. • How big an effect does the drug have? • What is the clearance in renal failure? Sheiner LB. Learning versus confirming in clinical drug development. Clinical Pharmacology & Therapeutics 1997;61(3):275-91 "Why We're Losing the War on Cancer" Accelerating Anticancer Agent Development and Validation Workshop June 20-22, 2007 Keynote Address: "Learning Too Little, Too Late: Why We Need a New Paradigm for the Cancer Clinical Trial" Clifton Leaf Former Executive Editor Fortune Resisting RECIST Throwing away data? Collaborative Effort Singapore Study • Randomized, phase II trial • Carboplatin at AUC of 5mg/mL*min given over one hour on day 1 of each cycle prior to the gemcitabine infusion • Study arms – – • gemcitabine 750 mg/m2 over 75 minutes (Arm A) on days 1 and 8 every 3 weeks x 6 cycles gemcitabine 1000 mg/m2 over 30 minutes (Arm B) on days 1 and 8 every 3 weeks x 6 cycles No differences in outcome (survival or toxicity) Soo RA, Wang LZ, Tham LS, Yong WP, Boyer M, Lim HL, et al. A multicentre randomised phase II study of carboplatin in combination with gemcitabine at standard rate or fixed dose rate infusion in patients with advanced stage non-small-cell lung cancer. Ann Oncol. 2006;17(7):1128-33. Singapore Study of Tumour Size • 56 treatment naïve patients with advanced ‘big cell’ lung cancer treated with carboplatin and gemcitabine • 261 measurements of tumour size – largest dimension of the primary tumour measured from CT images using electronic calipers – Used only for RECIST category • Measurements at protocol baseline, cycles 2, 4 and 6, and bimonthly – Actual mean follow up only 3.5 months Gemcitabine Pharmacology • Gemcitabine (dFdC) – Inactive pro-drug • dFdCTP (gemcitabine triphosphate) – Intracellular, active, tri-phosphate metabolite • dFdU – Major extracellular, inactive metabolite Exposure Response • Which Exposure Measure? – Dose • Cannot distinguish PK from PD causes of variation – AUC • Can be used to identify causes of between patient variability through PK model linking Dose to AUC – C(t) • Can be used to predict schedule dependence • But long computation times preclude practical exploration of C(t) and response 8 36 6 27 Concentration Spikes With Each Cycle 4 18 2 9 Slow Tumour Response 0 0 0 10 20 30 40 Week Tumour Size Drug 50 60 Drug Tumour Size Why Dose by Dose Concentration Time Course Won’t Work How to Describe Delayed Drug Response? • Exposure has delayed effect – – – – Time course of drug concentration is complete within a few hours Binding of drug to DNA probably rapid Effect of DNA damage on cell proliferation probably slow Time course of tumour response takes weeks • KPD Effect Compartment Model for Exposure – What “apparent half-life” of drug would explain the effect time course? – Can be based on Dose or AUC – C(t) not required dExposure ln( 2) / T 1 / 2, effect Exposure dt Tumour Size Model Drug Effect on Tumour Formation Rate dSize 1 RateIn Effect Size Size dt Tturnover Tumour Turnover Kinetics Simple Feedback Semi-mechanistic Natural history of tumour growth has rapid growth with asymptote Feedback inhibits growth Drug effect expresses pathophysiological mechanism “KPD” Drug Effect and Tumour Turnover • Effect assumed to slow rate of proliferation of new tumour cells Dose Effect 1 Effect Compartment dCe k1 ( Dose Ce) dt KPD Model ln( 2) k1 T1/ 2,effect E max Ce Dose50 Ce Drug Effect Model Tumour Turnover Compartment dSize ( RateIn Effect k 2 Size ) Size dt Tumour Model T1/2,effect describes delay in drug effect Tturnover describes delay in tumour esponse k2 1 Tturnover “KPD” plus Turnover 8 36 6 27 4 18 Tumour Size 2 9 0 0 0 10 20 30 40 50 Week Tumour Size Ae Dose 60 Dose and Ae Tumour Size Effective Amount of Drug Variability in Gemcitabine Dose-Response Why Stop Treatment? Which Exposure Metric? • No better fit with dFdCTP (or dfdU) compared to gemcitibine AUC • No better fit with individual AUC compared with dose of gemcitabine • Dose is the simplest exposure metric Tumour Size Turnover and PD Parameters BSV % 95% CI 6.7 54.6 (5.7, 7.8) Tturnover, Tumor turnover (cm x week) 10.7 24.7 (2.0, 17.7) Dose50, Gemcitabine dose at 50% tumor size shrinkage (mg) 3156 136 (536, 16417) T1/2,effect, KPD Effect half-life (week) 2.5 29 (0.61, 12.0) 12% - (9, 16) Parameter Size0, tumor size at baseline (cm) Final Estimate Residual Error Proportional error BSV=Between Subject Variability (apparent coefficient of variation) 95%CI=Empirical confidence interval from 1000 bootstraps What Did We Learn About Pharmacology? • No evidence that differences in exposure time course [C(t)] can influence tumour response • No evidence that intracellular metabolite is better then dose as a predictor of tumour response – Anti-tumour mechanism may be different from haematological toxicities • Unable to learn about influence of dose and duration of infusion – Uninformative design Didn’t learn very much! What Could We Learn About Pharmacology? • Can quantitate individual sensitivity (ED50) and time course (effect and tumour ‘half-lives’) • Complements toxicity based models e.g. Friberg myelosuppression model (optimal dosing?) • Link to survival probability (Claret et al 2009, Wang et al 2009) FDA Model Linking Tumour Size with Survival Wang Y, Sung C, Dartois C, Ramchandani R, Booth BP, Rock E, Gobburu J. Elucidation of relationship between tumor size and survival in non-small-cell lung cancer patients can aid early decision making in clinical drug development. Clin Pharmacol Ther. 2009;86(2):167-74. Wang (FDA) Tumour Size Model “A model with mixed exponential-decay (shrinkage) and linear-growth (progression) components described the time course of tumor change where TSi(t) is the tumor size at time t for the ith individual, BASEi is the baseline tumor size, SRi is the exponential tumor shrinkage rate constant, and PRi is the linear tumor progression rate.” Empirical model No dose or exposure information used Claret Tumour Size Model Log growth for tumor (no asymptote unlike Gompertz) D(t)*exp(-λ*t) is identical to the effect compartment model R(t) ‘resistance’ function cannot be distinguished from drug elimination Tumour Size and Survival Wang/Claret Tumour Size At 8 weeks FDA Advisory Committee for Pharmaceutical Sciences and Clinical Pharmacology Meeting, March 18–19, 2008. http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4351b1-01-FDA.pdf Tumour Size Predictions FDA Advisory Committee for Pharmaceutical Sciences and Clinical Pharmacology Meeting, March 18–19, 2008. http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4351b1-01-FDA.pdf Tumour Size Improves Prediction of Survival FDA Advisory Committee for Pharmaceutical Sciences and Clinical Pharmacology Meeting, March 18–19, 2008. http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4351b1-01-FDA.pdf Which Patient Will Survive Longer? Wang et al. and Claret et al. use tumour size at a fixed time to predict survival. These patients have the same 8 week tumour size but different response time course Survival models should include time course of tumour size! Way to go! Backup Commentary Clinical Pharmacology and Therapeutics 1. “Drug-independent models that link biomarker response to clinical end points are critical to support early (end of phase II) clinical decisions. 2. In oncology, change in tumor size (a biomarker of drug effect evaluated in phase II) is linked to survival (a phase II end point) in some solid tumors. 3. Change in tumor size can be used as a primary end point in the design and evaluation of phase II studies and in supporting go/no-go decisions and phase II study design.” Bruno R, Claret L. On the use of change in tumor size to predict survival in clinical oncology studies: toward a new paradigm to design and evaluate phase II studies. Clin Pharmacol Ther. 2009;86(2):136-8. FDA Conclusions FDA Advisory Committee for Pharmaceutical Sciences and Clinical Pharmacology Meeting, March 18–19, 2008. http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4351b1-01-FDA.pdf Clinical Response Table 2. Summary of clinical response Response Arm A, 750mg/m2 over 75mins (n= 38) Arm B, 1000mg/m2 over 30mins (n=38) Partial Response Rate (%) 34 42 Median Survival (days) 212 287 1-year Survival Rate (%) 31.6 35.6 Conclusion – no significant difference between treatments Toxicity Table 3. Hematologic toxicities expressed as percentage of patients Arm A, 750mg/m2 over 75min Arm B, 1000mg/m2 over 30min Toxicity Grade 3-4 Grade 3-4 p values Anemia 31% 33% 1.0 Neutropenia 68% 75% 0.61 Thrombocytopenia 69% 52% 0.10 Conclusion – no significant difference between treatments Which Exposure Metric? Structural Model Description Objective Function Values 2 Compartments - KPD, Turnover Emax AUCgemcitabine 521.2 AUCdFdU 524.0 AUCdFdCTP 522.0 Dosegemcitabine 520.8 Sigmoid Emax AUCgemcitabine 522.0 AUCdFdU 523.4 AUCdFdCTP 522.9 Dosegemcitabine 532.2 AUCgemcitabine AUCdFdU AUCdFdCTP Dosegemcitabine gemcitabine dFdU dFdCTP gemcitabine AUC-driven model AUC-driven model AUC-driven model Dose-driven model Tumour Size – Effect of Gemcitabine Visual Predictive Check Scatterplot Comparison Median and 95% Interval Comparison 100 cm cm 100 10 1 10 1 0 4 8 12 16 20 24 28 32 36 40 44 48 52 0 4 8 Week Hi Median 12 16 Week Lo Size Hi Median Lo ObsHi ObsMedian ObsLo Tumour Size Gemcitabine Dose Tumour Size Gemcitabine Dose-Response Tumour Size Gemcitabine Dose-Response Tumour Size Gemcitabine Dose-Response Tumour Size Gemcitabine Dose-Response Tumour Size Gemcitabine Dose-Response Visual Predictive Check FDA Advisory Committee for Pharmaceutical Sciences and Clinical Pharmacology Meeting, March 18–19, 2008. http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4351b1-01-FDA.pdf