* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download C. difficile
Antimicrobial peptides wikipedia , lookup
Plant disease resistance wikipedia , lookup
Gastroenteritis wikipedia , lookup
Urinary tract infection wikipedia , lookup
Neonatal infection wikipedia , lookup
Management of multiple sclerosis wikipedia , lookup
Infection control wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Carbapenem-resistant enterobacteriaceae wikipedia , lookup
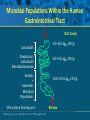
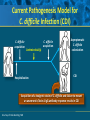
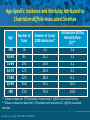
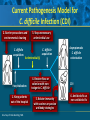
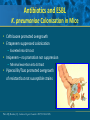
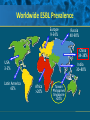
Beyond the Target Pathogen: Ecological Effects of the Hospital Formulary Ellie J.C. Goldstein, MD, FIDSA Clinical Professor of Medicine David Geffen School of Medicine at UCLA Director, R.M. Alden Research Laboratory Santa Monica, California Antimicrobial Stewardship and Infection Control Programs: Meeting New Challenges Survival of the Fittest Charles Darwin Needed: Antibiotic Stewardship Courtesy of Gary Doern, PhD Antibiotics and Gram-negative Organisms Imipenem Cephalosporins Slower diffusion due to bulk and ionic charges Rapid diffusion due to small size and zwitterionic +/- charge OmpF OmpC Beta lactamases (hydrolyzing enzymes) Penicillin-binding proteins PBP2 PBP1a PBP3 PB1b Resistant Organisms 2010: When One is Targeted, What is the Effect on the Non-Targets? Current Issues • • • • • Vancomycin MIC creep MRSA VRE ESBL P. aeruginosa Acinetobacter Increasing prevalence Pan-resistance Pan-resistance Future • E. coli • Enterobacteriaceae • K. pneumoniae carbapenemase (KPC) • ? Integrons What is the Breakpoint and What Does it Mean ? • Susceptible - level of antimicrobial activity associated with a high likelihood of therapeutic success • Intermediate - activity of uncertain therapeutic effect – Infection may be appropriately treated in body sites where the drugs are concentrated OR when a high dosage of drug can be used – It also indicates a buffer zone that should prevent small, uncontrolled, technical factors from causing major discrepancies in interpretations • Resistant - activity associated with a high likelihood of therapeutic failure • Wild type (WT) - absence of acquired and mutational resistance mechanisms to the drug in question • Non-wild type (NWT) - presence of an acquired or mutational resistance mechanism to the drug in question What is “Collateral Damage”? • A movie? • Resistant fecal flora? • C. difficile infection? • Resistant isolates? – Gram positive? – Gram negative? • Industry spin? • Whatever you want it to be? Potential “Collateral Damage” From Use of Cephalosporins and Quinolones • Class of agent, pathogen(s) selected for: – Third generation cephalosporins • Vancomycin-resistant enterococci • ESBL Klebsiella species • Beta-lactam–resistant Acinetobacter species • Clostridium difficile – Quinolones • MRSA • Quinolone-resistant gram-negative bacilli, including Pseudomonas aeruginosa Paterson DL. Clin Infect Dis. 2004;38(Suppl 4):S341-345. The Diversity of the Fecal Flora Courtesy of Sherwood Gorbach, MD Microbial Populations Within the Human Gastrointestinal Tract Oral Cavity Lactobacilli Streptococci Lactobacilli Enterobacteriaceae Aerobic + Anaerobic Microbial Populations CFU=colony-forming unit Edmiston CE, Jr, et al. Infect Dis Clin Pract. 1996;5(suppl 1):S16. 1.0–3.0 Log10 CFU/g 3.0–5.0 Log10 CFU/g 10.0–12.0 Log10 CFU/g Rectum Quorum Sensing • Organism releases small amount of autoinducer or transcription activator [small molecules] • Concentration increases as cell density increases until a minimal threshold concentration triggers a shift in gene expression • Associated with competence, conjugation, virulence – eg, proteases, biofilm formation, antibiotic formation, motility, and sporulation Quorum Sensing in Gram-negative Bacteria LuxI – Enzymes that produce AHL (acyl homoserine lactone) autoinducer proteins (AIP) AHL Luxl LuxR Genes xyz Federle MJ, Bassler BL. J Clin Investig. 2003;112:1291-1299. LuxR – binds AIP, then activates promoter segment of target gene Clinical Aspects of C. difficile Infection Time until onset During antibiotic (abx) therapy (usually after 4-5 days) Post-Abx therapy (usually within 4 weeks) No Abx therapy Risk Factors • Advanced age • Hypoalbuminemia • Co-morbidities • Immunosuppression 80% 20% Rare Current Pathogenesis Model for C. difficile Infection (CDI) C. difficile acquisition Antimicrobial(s) Hospitalization C. difficile acquisition Asymptomatic C. difficile colonization CDI Acquisition of a toxigenic strain of C. difficile and failure to mount an anamnestic Toxin A IgG antibody response results in CDI Courtesy of Dale Gerding, MD Age-Specific Incidence and Mortality Attributed to Clostridium difficile-Associated Diarrhea Age Number of Cases Number of Cases/ 1000 Admissions* Attributable 30-Day Mortality Rate (%)** <40 41-50 51-60 61-70 71-80 81-90 >90 76 85 191 272 523 458 114 3.5 11.2 20.0 24.4 38.3 54.5 74.4 2.6 1.2 3.2 5.1 6.2 10.2 14.0 * Values are based on 1719 episodes of nosocomial C. difficile-associated diarrhea ** Values are based on data from 1703 patients with nosocomial C. difficile-associated diarrhea Loo VG, et al. N Engl J Med. 2005;353:2442-2449. Current Pathogenesis Model for C. difficile Infection (CDI) 2. Barrier precautions and environmental cleaning C. difficile acquisition Antimicrobial(s) Hospitalization 1. Keep patients out of the hospital Courtesy of Dale Gerding, MD. 3. Stop unnecessary antimicrobial use C. difficile acquisition 4. Restore flora or colonize with nontoxigenic C. difficile 5. Bolster immunity with vaccines or passive antibody strategies Asymptomatic C. difficile colonization CDI 6. Antibiotic Rx vs non-antibiotic Rx Forest Plot of 25 Randomized, Controlled Studies of Probiotics for Prevention of AAD and Pooled Risk Ratios Study Adam, 1977 Surawicz, 1989 McFarland, 1985 Kotowska, 2005 Lewis, 1998 Cremonini, 2002 Arovala, 1999 Vanderhoof, 1999 Szajewska, 2001 Thomas, 2001 Cremonini, 2002 Armuzzi, 2001 Nista, 2004 Orrhage, 1994 Seki, 2003 Wunderlich, 1989 Borgia, 1982 Witsell, 1995 Gotz, 1979 Tankanow, 1990 Orrhage, 1994 Cremonini, 2002 Correa, 2005 LaRosa, 2003 Jirapinyo, 2002 Overall Risk Ratio (95% CI) 0.26 (0.13, 0.53) 0.43 (0.21, 0.90) 0.49 (0.21, 1.17) 0.19 (0.07, 0.55) 1.53 (0.54, 4.35) 0.16 (0.02, 1.21) 0.32 (0.09, 1.11) 0.29 (0.13, 0.63) 0.20 (0.06, 0.66) 0.98 (0.68, 1.42) 0.17 (0.02, 1.27) 0.13 (0.03, 0.52) 0.88 (0.50, 1.57) 0.57 (0.24, 1.35) 0.12 (0.05, 0.28) 0.32 (0.07, 1.41) 0.28 (0.11, 0.72) 1.25 (0.81, 1.94) 0.40 (0.12, 1.36) 0.96 (0.61, 1.50) 0.29 (0.08, 1.05) 0.17 (0.02, 1.27) 0.51 (0.21, 1.23) 0.48 (0.29, 0.77) 0.47 (0.18, 1.21) 0.43 (0.31, 0.58) 0.020877 Favors probiotic McFarland LV. Anaerobe. 2009;15:274–280. 1 Risk ratio Favors placebo 47.8984 Antibiotics and ESBL K. pneumoniae Colonization in Mice • Ceftriaxone promoted overgrowth • Ertapenem suppressed colonization – Excreted into GI tract • Imipenem – no promotion nor suppression – Minimal excretion into GI tract • Piperacillin/Tazo promoted overgrowth of resistant but not susceptible strains Pultz MJ, Donskey CJ. Antimicrob Agents Chemother. 2007;51:3044-3045. OASIS I • Design – Prospective, multicenter, randomized, open-label trial (OASIS I) • Patients – 370 hospitalized adults with intra-abdominal infections requiring surgery • Therapy – Ertapenem 1 g once daily versus piperacillin/tazobactam 3.375 g every 6 hours or 4.5 g every 8 hours • Primary endpoint – Proportion of microbiologically evaluable patients with favorable clinical and microbiologic assessments at test of cure 2 weeks after completion of therapy OASIS = Optimizing Intra-Abdominal Surgery with INVANZ Studies Adapted from Dela Pena AS, et al. J Gastrointest Surg 2006;10:567–574. OASIS I Therapy Resistant Enterobacteriaceae Subanalysis Ertapenem 14 Piperacillin/Tazobactam 12.2 12 Percent 10 8 6 4.5 4 2.6 2 0 0 n= 162 0.6 0 155 133 Resistant Baseline 0.6 162 0 155 0.8 0.6 133 160 ESBL Producers End of Therapy DiNubile MJ, et al. Eur J Clin Microbiol Infect Dis. 2005;24:443-449. 0.8 0.6 156 133 Resistant 160 156 133 ESBL Producers 2 Weeks Post Therapy OASIS I VRE Subanalysis: Minimal Risk of Colonization with VRE Patients With VRE, % 10 8 6 4 2.4 2.7 2 0 0 0 Ertapenem (n=37) Piperacillin/Tazobactam (n=42) Baseline VRE = vancomycin-resistant Enterococci Adapted from DiNubile MJ, et al. Diagn Microbiol Infect Dis. 2007;58:491-494. 2 Weeks Post Therapy OASIS II Therapy Resistant Enterobacteriaceae Subanalysis Ceftriaxone/Metronidazole Ertapenem 25 22.4 20 17.2 Percent 17.1 15 9.3 10 4.0 5 0 n= 0.5 0.5 0 201 196 182 Resistant Baseline 2.2 2.6 182 195 2.1 0 201 196 ESBL Producers End of Therapy 193 174 Resistant 195 174 ESBL Producers 2 Weeks Post Therapy Adapted from DiNubile MJ, et al. Eur J Clin Microbiol Infect Dis 2005;24:443–449. 193 Tigecycline Fecal Concentrations, mg/kg in 12 Healthy Subjects Day 2 Day 5 Day 8 Day 10 Day 12 Day 15 Mean 4.7 5.0 6.0 4.1 2.4 0.1 SD 3.4 3.7 2.9 1.9 1.1 0.2 Median 4.5 3.5 5.6 3.7 2.8 0 Range 0-9.9 0-11.3 3-14.1 1.1-7.2 0.5-4.2 0-0.4 50 mg IV q 12 h x 10 days Nord CE, et al. Antimicrob Agents Chemother. 2006;50:3375-3380. Tigecycline Effect on Fecal Flora By Day 8, 12 subjects • • • • • E. coli and enterococci reduced Other enterobacteria and yeast increased Lactobacilli and bifidobacteria decreased Bacteroides no change Resistance development [MIC ≥8 ug/ml]: 2 K. pneumoniae; 5 Enterobacter cloacae Nord CE, et al. Antimicrob Agents Chemother. 2006;50:3375-3380. Worldwide ESBL Prevalence Europe 9-54% Russia 40-90% China 34-38% USA 3-5% Latin America 45% India 30–80% Africa >20% Taiwan Philippines Singapore >20% Effect of Ertapenem on the Hospital Ecology • Clinical Studies – – – – – Crank, 44th IDSA Annual meeting, Toronto, 2006. # 285. Goff, J Infection. 2008;57:123-126. Goldstein, AAC. 2009;53:5122-5126. Carmeli, 47th ICAAC, Chicago, 2007. # K-396. Eagye KJ, Nicolau DP, 49th ICAAC, San Francisco, 2009. • Conclusions – Use of ertapenem did not decrease susceptibilities of Pseudomonas aeruginosa to carbapenems. St Johns Health Center Santa Monica, CA • Community teaching hospital • 334 Licensed beds • 200 Average daily census • Active oncology ward – 51 in-patient beds – Research programs • 32 ICU beds • 32 Step-down beds Goldstein EJ, et al. Antimicrob Agents Chemother. 2009;53:5122-5126. Study of Susceptibility of Aerobic Gramnegative Rods After 3 Years on Formulary Design Retrospective analysis of hospital susceptibility data from June 2002 to December Setting 344-bed community teaching hospital in Santa Monica, California, US Methods In vitro susceptibilities of gram-negative rods to formulary antibiotics determined Primary endpoint Susceptibility of gram-negative rods to imipenem, ertapenem, levofloxacin, cefepime, gentamicin, and piperacillin/tazobactam Goldstein EJ, et al. Antimicrob Agents Chemother. 2009;53:5122-5126. Usage of Antibiotics: 3 Years of Formulary Inclusion Gentamicin Cefepime Levofloxacin Clindamicin Cefoxitin Amp/Sulbactam Pip/Tazo Metronidazole Ertapenem 2002 2005 Imipenem DDD/1000 Patient Days 0 50 100 150 200 250 Ertapenem was added to formulary in 2002, and in 2003 an auto-substitution policy was established Goldstein EJ, et al. Antimicrob Agents Chemother. 2009;53:5122-5126. Prevalence of ESBLs: 3 Years of Formulary Inclusion 7 Isolates, % 6 Klebsiella spp ESBLs 5 4 3 2 E. coli ESBLs 1 0 2002 Ertapenem added 2003 2004 Ertapenem auto substitution Goldstein EJ, et al. Antimicrob Agents Chemother. 2009;53:5122-5126. 2005 Susceptibility of P. aeruginosa: 3 Years of Formulary Inclusion Bar = Doses Line = %s 100.0 225 200 80.0 175 150 125 60.0 100 40.0 75 50 Susceptible, % DDD/1000 Patient Days 250 20.0 25 0 0.0 2002 1 2002 2 Ertapenem added 2002 3 2002 4 2003 1 2003 2 2003 3 2003 4 2004 1 2004 2 2004 3 2004 4 2005 1 2005 2 2005 3 2005 4 Ertapenem auto substitution Quarter Levofloxacin Piperacillin/Tazobactam Imipenem Cefepime Tobramycin Levofloxacin Piperacillin/Tazobactam Imipenem Cefepime Tobramycin Goldstein EJ, et al. Antimicrob Agents Chemother. 2009;53:5122-5126. Ertapenem Univariate ARIMA Model Fitted to the Usage Series Susceptibility P. aeruginosa Imipenem (%) Month Min Max Mean Median Standard Error 0-9 60.00 81.00 70.00 69.0 2.69 After Ertapenem was added, Before the substitution 10-20 63.00 91.00 77.00 77.00 2.90 After the substitution 21-48 67.00 100.00 87.86 89.0 1.62 Before Ertapenem added Goldstein EJ, et al. Antimicrob Agents Chemother. 2009;53:5122-5126. Conclusions: Susceptibility • No change in the susceptibility patterns of E. coli, P. mirabilis, K. pneumoniae, K. oxytoca, Enterobacter species isolates to imipenem since the inclusion of ertapenem on formulary [100% susceptible to imipenem, ertapenem] • P. aeruginosa improved activity of imipenem (0.38%) for every unit decrease in DDD of imipenem usage (P=.008) – Levofloxacin, cefepime and pip-tazo susceptibilities improved Goldstein EJ, et al. Antimicrob Agents Chemother. 2009;53:5122-5126. Ertapenem Utilization and Resistance Emergence among Collateral Antimicrobials (EURECA) • Study Period: – 3 year prior & 3 years after ertapenem adoption • Data Sources: – USE: Commercial database (Premier, Inc., Charlotte, NC) – SUSCEPTIBILITY: Individual (25) hospital antibiograms • Antimicrobial use collected for: – Ertapenem and other carbapenems – Aminoglycosides – Fluoroquinolones – Other Beta-lactams • P. aeruginosa susceptibility: – Combined %S of meropenem and imipenem used for analysis • Evaluation of drug use: – Total grams & patient days extracted from database – DDDs as determined by WHO – Use Density Ratio (UDR) calculated for ertapenem plus each antimicrobial class • Statistical Analysis: – GLM using repeated measures ANOVA – Dependent variable: 6-year repeated carbapenem %S – Explanatory variable: ertapenem UDR in each year – Controlled for UDR of other carbapenems or other classes Eagye KJ, Nicolau DP. Presented at 49th ICAAC Meeting, San Francisco, CA, September 2009. Use Density Ratio 25 20 15 10 5 0 1 2 Ertapenem 3 4 Study Year Other Carbapenems 5 6 Susceptibility Eagye KJ, Nicolau DP. Presented at 49th ICAAC Meeting, San Francisco, CA, September 2009. 100 95 90 85 80 75 70 Percent Mean Carbapenem Use and P. aeruginosa Susceptibility at 25 Hospitals during 6 Years of EURECA Association Between Antibiotic Use and Resistance • Decreasing susceptibility trends over time were not statistically associated with the primary drug – eg, organism susceptibility rate to imipenem with imipenem usage • Secondary drug use was associated with susceptibility rates – eg, susceptibility of E. coli to cefepime with pip/tazo usage • Conclusions: These results suggest that antibiotic use resistance relationships are influenced by the use of secondary antibiotics. Thus, a resistance problem may not be adequately addressed by simply altering the utilization of the primary antibiotic. Bosso J, et al. Presented at the 48th Annual ICAAC/IDSA 46th Annual Meeting. Washington, DC, October 25-28, 2008. Beyond the Target Pathogen Collateral Benefits vs. Collateral Damage • Initial aggressive therapy • De-escalation • Tailored therapy lowers mortality lowers resistance lowers resistance • Fecal Flora Changes and Colonization – Resistant organism proliferation – Transmission/outbreak due to inadvertent contact • C. difficile infection • Effects on non-target organisms – Collateral resistance – Integrons – ESBLs – P. aeruginosa