* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download to presentation
HIV and pregnancy wikipedia , lookup
Forensic epidemiology wikipedia , lookup
Birth control wikipedia , lookup
Neonatal intensive care unit wikipedia , lookup
Prenatal development wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Women's medicine in antiquity wikipedia , lookup
Prenatal nutrition wikipedia , lookup
Prenatal testing wikipedia , lookup
Maternal health wikipedia , lookup
Maternal physiological changes in pregnancy wikipedia , lookup
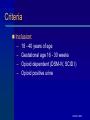
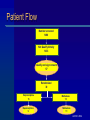
Treating Pregnant Opioid Dependent Women: Examining Buprenorphine and Methadone Hendrée E. Jones, Ph.D. Associate Professor Department of Psychiatry and Behavioral Sciences Johns Hopkins University School of Medicine Baltimore, Maryland Presentation Goals Use of medication to treat opioid dependence during pregnancy Review of published prenatal buprenorphine exposure data Randomized double-blind study AATOD 2004 Studies of Medication During Pregnancy Controversial Some say unethical Stigma associated with medication treatment for pregnant women is severe AATOD 2004 Goals of Opioid Agonist Treatment Cessation of opioid use Stabilize intrauterine environment Increased prenatal care compliance Enhanced pregnancy outcomes AATOD 2004 Methadone is effective during pregnancy Methadone is recommended for the treatment of opioid dependent pregnant women Over 30 years of experience and research Does not appear to have teratogenic potential AATOD 2004 Methadone is not a “Magic Bullet” Medication Neonatal Abstinence Syndrome (NAS) – Neuralgic excitability (hyperactivity, irritability, sleep disturbance) – Gastrointestinal dysfunction (uncoordinated sucking/swallowing, vomiting) – Autonomic Signs (fever, sweating, nasal stuffiness) AATOD 2004 The NAS of Opioid Exposed Neonates 55-90% exhibit NAS Methadone dose relationship to NAS severity is inconsistent Onset within 48 to 72 hours after birth Subacute signs for a year AATOD 2004 Buprenorphine Subutex or Suboxone Buprenorphine reported to produce less physical dependence in adults Full Agonist Heroin Morphine Methadone Full Antagonist Buprenorphine Nalmefene Naloxone Naltrexone AATOD 2004 Case Reports and Open-Label Studies Since 1995, 23 reports of prenatal exposure to buprenorphine Approximately 338 babies and number of cases ranged from 1 to 153 (median=6) 61% NAS with 48% requiring treatment – NAS appears in 12-48 hrs, – peaks 72-96 hrs – Duration 120-168 hrs AATOD 2004 Purpose Compare methadone and buprenorphine in pregnant opioid-dependent women and to provide preliminary safety and efficacy data for a larger multi-center trial AATOD 2004 Randomized Controlled Study – Double-blind (staff and patient) – Double-dummy (two medications) – Two groups: Methadone or Buprenorphine – Flexible dosing Methadone 20-100 mg Buprenorphine 4-24 mg AATOD 2004 Setting: Center for Addiction & Pregnancy Interdisciplinary Approach – Psychiatry – Obstetrics – Pediatrics – Nursing AATOD 2004 Criteria Inclusion: – 18 - 40 years of age – Gestational age 16 - 30 weeks – Opioid dependent (DSM-IV, SCID I) – Opioid positive urine AATOD 2004 Criteria Exclusion: – Methadone positive urine at admission – DSM IV axis I current diagnosis other than psychoactive substance use – Serious medical or psychiatric illness – Diagnosis of preterm labor – Congenital fetal malformation – Current alcohol abuse/dependence – Benzodiazepine use (8 or more times/month and/or 2 or more times /week) AATOD 2004 Primary Outcome Measures Infant Neonatal Abstinence Syndrome (NAS) Length of Hospital Stay (LOS) AATOD 2004 Selected Secondary Outcome Measures Maternal – Days of treatment – Prenatal care visits – Illicit drug use Infant – Physical birth parameters AATOD 2004 Patient Flow Number screened 1490 Not Qualify Initially 1433 Qualify and sign consent 57 Randomized 30 Buprenorphine 15 Buprenorphine 9 Methadone 15 Methadone 11 AATOD 2004 Induction Patients stabilized on immediate release morphine (IRM) prior to randomization Is transition from IRM to methadone or buprenorphine similar? Withdrawal scores over first 3 days appeared mild for both medications AATOD 2004 Induction Methadone Mean ( 95% CL) IRM transition Dose (95% C L) Range Ind uction Dose (95% CL) Range Ind uction Untransformed Total Withd rawal sc ore Ind uction L og transformed Total Withd rawal sc ore Ind uction L og transformed Total Withd rawal sc ore with co-variates Buprenorphine Mean ( 95% CL) Levene’s Test of Equality of Error Variance F (df); p value 268.0 (214.0-322.0) 207.5 (161.0-253.9) 100-390 mg 140-300 mg 53.5(48.6-58.4) 20-70 mg 10.9 (10.2-11.7) 8-14 mg 3.1 (1.42-4.85) 1.5 (-0.37-3.46) 3.27 (1,16); .089 .43 (.25-.62) .42 (.21-.63) 1.70 (1,16); .211 .43 (.25-.62) .42 (.21-.63) .67 (1,16); . 426 Adapted from Jones,H.E. et al., In press. Drug and Alcohol Dependence AATOD 2004 Maternal Outcome Drug Use During Pregnancy Methadone % + Urine Samples N=11 Buprenorphine N=9 opioid 15.6 16.7 cocaine 11.2 15.2 amphetamine 0.0 0.0 barbiturates 0.0 0.0 benzodiazepine 0.4 2.5 THC 7.5 0.0 AATOD 2004 Maternal Characteristics Methadone N=11 % African-American Buprenorphine N=9 63.6 88.9 Gestation (weeks) 23.6 22.8 Education (yrs) 10.0 10.3 0.0 0.0 % Employed Age (yrs) 30.3 Smoked Cigarettes 30.0 81.8 77.8 AATOD 2004 Maternal Outcomes Days in Treatment Methadone N=11 99.9 Buprenorphine N=9 115.6 Prenatal care visits 3.4 3.6 LOS mom 2.2 2.2 C section % 9.1 11.1 Tox. + delivery (mom)% 9.1 0.0 Normal presentation % 100 100 Preterm birth % 9.1 0.0 Gestational age delivery 38.8 38.8 Ave. dose at delivery (mg) 79.1 18.7 AATOD 2004 Birth Outcomes Methadone N=11 Buprenorphine N=9 deliveries (10 babies) % Treated for NAS 45.5 20.0 Morphine Drops 93.1 23.6 3001.8 3530.4 8.1 6.8 18.0 10.0 APGAR 1 8.3 8.1 APGAR 5 8.9 8.7 Length (cm)* 49.6 52.8 Head Cir. (cm)* 33.2 34.9 Birth Weight (gm)* LOS baby % NICU treatment * data safety monitoring board recommended removing twin data from these variables AATOD 2004 NAS Time Course AATOD 2004 Limitations of Study Small sample size I/E criteria limits generalizability Nicotine exposure and effect on NAS needs more study Long-term outcomes beyond scope of study AATOD 2004 Conclusions Both methadone and buprenorphine provide positive benefits to mothers 100% of infants had NAS signs/symptoms Tendency for fewer buprenorphine-exposed babies to be treated for NAS Significantly fewer days of hospitalization with buprenorphine exposure AATOD 2004 Bottom Line Both medications have strong support to document safety and efficacy for mother and infant NAS is only part of the complete risk:benefit ratio A greater range of medication options will improve the treatment of pregnant women AATOD 2004 Future Directions Multi-center trial comparing methadone and buprenorphine 8 sites submitted applications May provide data needed to change FDA labeling for methadone and buprenorphine Develop infrastructure for studying other medications and women’s health issues during pregnancy AATOD 2004 Acknowledgements Patients and infants Rolley “Ed” Johnson NIDA R01 DA12220 (P.I.Johnson/Jones) Co-Investigators Staff at Center for Addiction and Pregnancy Staff at BPRU AATOD 2004