* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Introduction Geological setting and host rocks
Survey
Document related concepts
Transcript
HARMOTOME AND ANALCIME FROM ZLATOLIST AREA,
THE EASTHERN RHODOPES, BULGARIA
SVETLANA ENTCHEVA1, PETKO PETROV1, DENKA YANAKIEVA1,
STELA ATANASOVA2, DIMITAR STOYANOV3
1
Earth and Man National Museum, Sofia; 2Institute of Physical Chemistry, BAS, Sofia; 3Institute of
Mineralogy and Crystallography, BAS, Sofia
Abstract. The aim of this work is to present harmotome and analcime from Zlatolist area, Krumovgrad district,
the Eastern Rhodopes. It is the second discovery of harmotome in Bulgaria. Harmotome samples from 5
outcrops in andesites, latite-andesites and tuffs are analyzed. They show some differences in chemistry, X-Ray
patterns and the associated minerals. Three types of twinning law are described. All analyses fall in the field of
Na harmotome. Some XRDs show traits typical of the harmotome spectrum, but lower than the standard dspacing. In others there is a splitting of the peaks in the entire spectrum of the diffractograms.
X-Ray data has confirmed analcime with cubic as well as orthorhombic symmetry.
The source of zeolite-forming elements is the highly altered volcanic rocks in the area.
Резюме. Целью данной работы является характеристика гармотома и анальцима из района Златолиста,
Крумовград, Восточные Родопы. Это вторая находка гармотома в Болгарии. Охарактеризованы
гармотомы из 5 обнажений в андезитах, латит-андезитах и туфах. Они показывают некоторые различия в
химии, рентгенограммах и сопутствующих минералах. Описаны три типа законы двойникования. Все
анализы гармотома попадают в области натрия. Некоторые рентгенограммы показывают характерный
для гармотома спектр, но более низкие, чем стандарты, межплоскостные расстояния. При другим есть
расщепление пиков весь спектра дифрактограмм.
Рентгеновские данные доказали анальцим как с кубической, так и с ромбической симметрией.
Источником цеолит-формирующих элементов можно считать сильно измененные вулканические породы
в этом районе.
Introduction
The proposed work is based on a large collection gathered as a result of many years’
trips in the area of Zlatolist neighborhood, Krumovgrad district, The Eastern Rhodopes,
Bulgaria. These are the best zeolites for mineral collection so far in Bulgaria: analcime,
heulandite, chabazite (phacolite), and mordenite. It is the second discovery of a rare barium
zeolite harmotome in Bulgaria. In fact, it is the only one because the first locality, near the
village of Iskra, Haskovo district, as described by Kostov (1960/61), is completely depleted.
The harmotome samples from Zlatolist area are of world-class quality - with dimensions of
individual crystals over 2 cm (the largest specimen of our collection is 3,5x2,5x1,7 cm!).
Crystals of analcime reach up to 5-6 cm in size. Although the area is already well known to
collectors of minerals, there are not many publications with a complete description. For the
first time, heulandite and mordenite were mentioned by Pozharevski (1988) in a publication
on an agate prospective for the region. During the V International Symposium „Mineral
diversity – research and preservation” Lovskaya and Kononkova (2009) presented cationexchange properties of harmotome from the region. Same data on crystallography, chemistry
and structure of harmotome were provided by Atanassova et al. (2011).
Geological setting and host rocks
The zeolites outcrops are situated about 5 km NW of the town of Krumovgrad and E of
Zlatolist neighborhoods. The host rocks belong to the horizon of the Second middle acid
volcanism of the Lower Oligocene (Kozhuharov et al., 1995аб) or to the Rabovo volcanic sub
complex of Rupellian age (Yordanov et al., 2008). These are basaltic andesites, andesites and
latites and their tuffs. They are heavily altered, greenish in colour rocks. The porphyry
generation is represented mainly by zoned plagioclase and monoclinic pyroxene. Altered
orthopyroxene, amphibole and biotite are rare. The groundmass has microlitic texture with
completely argilizated glass. The tuffs are of crystallo-vitroclastic type. The rocks fall within
the field of medium - and high-potassium volcanic rocks. The varying in size amygdales are
233
filled with smectites and celadonite in the periphery and chalcedony or zeolites in the centre.
There are microprobe analysis of ferrisaponite in the periphery and ferroceladonite in the
centre of a small amygdale.
Characteristics of the harmotome
Harmotomes from 5 outcrops are examined. Their places have been marked with GPS.
The GPS point numbers are used in this paper. Harmotome morphology does not differ
significantly, but there are some differences in the chemistry, X-Ray patterns and the
associated minerals.
Intermediate tuffs
Latite-andesites
P. 386 - Harmotome in amygdales in an andesite block (20 x 4 m) among intermediate
tuffs. Main associated minerals: celadonite, smectite, calcite, barite, chalcedony, mordenite;
very rare: quartz, heulandite, chabasite (phacolite), and analcime.
P. 388 - Harmotome in small veins and nests among intermediate tuffs. Main associated
minerals: celadonite, smectite, calcite, barite, pyrite; very rare – analcime, heulandite.
P. 391 - Harmotome in amygdales in several andesite blocks (30x10 m) among
intermediate tuffs. Main associated minerals: celadonite, smectite, calcite, barite, mordenite;
vary rare: chabasite (phacolite), analcime.
P. 392 – Harmotome in amygdales in latite-andesite lava flow (basement – intermediate
tuffs). Main associated minerals: celadonite, smectite, calcite, chalcedony, mordenite,
heulandite; relatively rare - chabasite (phacolite), analcime.
P. 393 - Harmotome in amygdales in a latite-andesite lava flow (basement –
intermediate tuffs). Main associated minerals: celadonite, smectite, calcite, chalcedony,
quartz, mordenite; relatively rare – analcime, heulandite, chabasite (phacolite).
Chemical composition
Data from microprobe analyses often show significant deviations in stoichiometry in the
direction of deficit of extra-framework cations. According to the criteria of Passaglia (1970),
the recalculation of many of the chemical analyses gives an error of more than 10% by the
formula:
Al Fe 3 Al theor .
E=
(where Al theor. = Na+K+2(Ca+Mg+Sr+Ba)
100
Al theor.
The reasons for these deviations are discussed in detail by Pekov et al. (2004).
Considering their arguments about this problem, all analyses (including those with deviations)
have been presented in the following diagrams.
234
235
X-Ray Diffraction Powder Data. Common diffractograms and enlarged areas
Unit cell parameters
P. 386 P. 391 P. 388 P. 392 P. 393
a
b
c
β
V
9.69
14.03
8.61
124.7
962
9.72
13.89
8.59
125.7
941
9.99
14.05
8.76
125.5
1002
9.83
14.19
8.62
124.8
987
236
9.82
14.11
8.65
124.9
983
After Rinaldi
et al., 1974
9.88
14.13
8.69
124.8
997
Crystal morphology
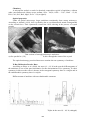
The larger crystals are white, opaque, invariably twined. {001} and {010} faces show a
mosaic-block structure. Stock-like aggregates are common. The microscopic crystals are
transparent with a prismatic pseudo tetragonal habit. They very often represent penetrating
twins.
Well expressed mosaic-block structure on the larger crystals (SEM photographs)
Microscopic crystals with pseudo tetragonal habit, penetrating twins and splitting
(SEM photographs)
Twins
(The names and the drawings are after Ralf Scheinpflug, 2011 and Hochleitner, Weiβ,
2011)
Morvenit-twin law (fourling) – each crystal is composed of four sectors that are
arranged crosswise to the twin planes {001} and {201}.
a
b
Morvenit-twin: a) macro crystal; b) thin section
237
Perier-twin law (eightling) - penetration of two flattened on {010} Morvenit fourlings,
one of which is rotated by 90 ° around the a-axis.
with re-entrant edges and {100}
a
b
Perier-twin: a) micro photo; b) SEM photo
Marburg-twin law (eightling) - penetration of two flattened on {001} Morvenit twins,
one of which is rotated by 90 ° around the a-axis
without re-entrant edges
a
b
Marburg-twin law: a) striation among {110} faces; b) striation on {010} face
238
Characteristics of the analcime
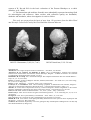
Crystal morphology
The crystals reach up to 5-6 cm in size. The large ones are white, opaque to translucent;
the microscopic ones – transparent colorless. Crystals are commonly trapezohedra {211},
rarely modified with cube {100} faces. Radial fiber analcime is a rarity, proved by X-Ray.
Analcimes with inclusions
Quite often there are inclusions of other minerals, which give a variety of colors to the
analcimes:
- milky white analcimes – with many inclusions of mordenite
- red analcimes - with inclusions of hematite
- greenish to black analcimes – with inclusions of celadonite and smectite
Inclusions of mordenite – thin section
Inclusions of celadonite and smectite
239
Chemistry
A microprobe analysis revealed a chemical composition typical of analcimes, without
other extra-framework cations except sodium: SiO2 – 64,30; Al2O3 – 21,87; Na2O – 13,84;
K2O, CaO, SrO, BaO, MgO, Fe2O3 – 0,0 (weight%).
Optical properties
Under an optical microscope, larger analcimes consistently show strong anisotropy.
According to Akizuki (1981), this is probably due to two-dimensional atomic arrangements
on the vicinal faces. Their symmetries control the Al/Si ordering in the process of crystal
growth.
a
b
Thin sections of strongly anisotropic analcimes
a) slice parallel to {211}
b) slice through the center of the crystal
The optical anisotropy provoked interest to examine the true symmetry of analcime.
X-Ray Diffraction Powder Data
According to Pekov et al. (2004), the area 6,8 – 6,9 Å in the powder diffractograms of
the analcimes is one of the most informative for the determination of its symmetry. In cubic
symmetry in this area the reflex is absent; in the tetragonal symmetry there is a singlet and in
the orthorhombic symmetry there is a triplet.
Diffractogram of analcime with an orthorhombic symmetry
Triplet in the area 6,8 – 6,9 Å (enlarged)
240
Diffractogram of analcime with a cubic symmetry
without reflex in the area 6,8 – 6,9 Å
Conclusions and discussion
1. All analyses of the harmotomes fall in the field of Na. The content of Ba and Na
varies considerably not only for the different outcrops but also within a single crystal. It can
be assumed that phillipsite-Na was formed first, and it later turned into harmotome by ion
exchange.
2. The formation namely of phillipsite/harmotome-Na (not-K) can be explained with the
abundant presence of celadonite in the host rocks („exhaustion” of all potassium), while the
lack of magnesium and iron in the harmotomes – with abundant formation of smectite.
3. The harmotomes from p. 392 have constant presence of Ca, with calcite being most
abundant there. The very presence of calcite in all points suggests a high activity of CO2 – a
necessary condition for the formation of zeolites (Kostov, 1993).
4. In the places with higher sulphur content (visible presence of pyrite, e.g. p. 388)
harmotome associates also with barite.
5. A direct relationship is not established between the chemistry of harmotome and the
type of host rock. The harmotomes from p. 392 and p. 393 (latite-andesite) are with very high
and very low Ba content, respectively.
6. The outcrops in the andesite blocks among tuffs and in the tuffs themselves are the
richest in harmotome. Harmotome is less in the latite-andesites and associates there with a
larger amount of analcime, heulandite, chabazite, chalcedony and quartz. Obviously, the
variable pH values of the solution play a greater role than the type of host rock.
7. Some XRDs show characteristics typical of the harmotome spectrum, but lower than
the standard d-spacing. By others there is a splitting of the peaks in the entire spectrum of the
diffractograms. It can be assumed that there are two phases with similar composition. So far,
the chemical data does not provide a satisfactory explanation for that.
8. Favorable conditions for the formation of harmotome: high alkalinity of a solution
with presence of Ba, high activity of CO2, and low sulfur content (otherwise all barium would
be deposited as a barite).
9. The area with analcime is more widespread than that of harmotome. X-Ray data has
proved analcime with cubic as well as orthorhombic symmetry.
10. The highly altered volcanic rocks in the area are the source of zeolite-forming
elements. Data from the geological mapping of a sub volcanic body, which is about 1 km
from the area, revealed Ba content twice higher than the content of Ba in the other lava rocks
in the area (Yordanov et al., 2008). Furthermore, geochemical data revealed an increased
241
content of K, Ba and H2O in the basic volcanism of the Eastern Rhodopes as a whole
(Marchev et al., 2004).
11. The harmotome and analcime from this area undoubtedly represent interesting finds
for mineralogists and collectors. Other zeolites with collection quality are heulandite,
chabasite and mordenite, whose investigation is a task to follow.
This work was prepared on the basis of more than 150 specimens from the Main Fund
and Scientific Collections Fund of the Earth and Man National Museum.
№22523 Harmotome (3,5х2,5х1,7 см!)
№23052 Analcime (3.5х3.5х2 сm)
REFERENCES
Akizuki, M. 1981. Origin of optical variation in analcime. – Am. Miner., 66, 403-409
Atanassova R., R. Vassileva, M. Kadiyski, Z. Zlatev. 2011. Crystallographic, chemical and structural
characteristics of harmotome from Zlatolist, Eastern Rhodopes, Bulgaria. – In Book of abstracts: III-rd National
Crystallographic Symposium Sofia, October 3-5, 2011
Hochleitner, R., St. Weiβ. 2011. Steckbrief Phillipsit: Die komplette Information über das Zeolith-Mineral. –
Lapis, 36, 2, 9-15 (in German)
Kostov, I. 1960/61. Zeolites in Bulgaria: analcime, chabasite, harmotome.– Ann. Sofia Univ., Fac. Geol. Geogr.,
55, 2, 159-173 (in Bulgarian)
Kostov, I. 1993. Mineralogy. - „Technica”, Sofia, 734 p. (in Bulgarian)
Lovskaya, E., N. Kononkova. 2009. Cation-exchange properties of harmotome from Zlatolist, Bulgaria –
Abstracts//V International Symposium „Mineral diversity – research and preservation”, Sofia, p. 31
Marchev, P. et al. 2004. Compositional diversity of Eocene-Oligocene basaltic magmatism in the Eastern
Phodopes, SE Bulgaria: implications foe genesis and tectonic setting – Tectonophysics 393, 301-328
Pekov, I. et al. 2004. Zeolites of alkaline massifs. – М., Assoc. „Ecost”, 168 p.. (in Russian)
Pozharevsky, I. 1988. New occurrence of agates in the Krumovgrad area. – C. R. Acad. Bulg. Sci, 41, 10, 73-75
(in Russian)
Passaglia, E. 1970. The Crystal Chemistry of Chabazites. - Amer. Miner., 55, 1278-1301
Ralf Scheinpflug. 2011. Internet site: http://scheinpflug.privat.t-online.de (in German)
Rinaldi, R. et al. 1974. Zeolites of the phillipsite family. Refinements of the crystal structure of phillipsite and
harmotome. – Acta Cryst., B30, 2426-2433.
Yordanov, B. et al. 2008. Explanatory note to the geological map of the Republic of Bulgaria М 1:50 000, map
sheet Studen Kladenets (in Bulgarian)
242