* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download WHO Staging System for HIV Infection and Disease in Adults and
Survey
Document related concepts
Transmission (medicine) wikipedia , lookup
Neonatal infection wikipedia , lookup
Infection control wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Globalization and disease wikipedia , lookup
Transcript
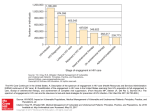
Antiretroviral Therapy Perspectives for Developing Countries Christopher Mathews, MD University of California, San Diego Survival Time from 1st Owen Clinic Visit, n=4854 yrentry 1999 1.00 yrentry 1998 yrentry 1997 yrentry 1996 0.75 yrentry 1995 0.50 yrentry 1994 yrentry 1993 yrentry 1992 0.25 yrentry 1991 yrentry 1990 0.00 1 2 3 4 5 Years 6 7 8 9 10 Time to Undetectable VL, by Year of Entry, Owen Clinic, n=701 Proportion with pVL>400 copies 1.00 0.75 0.50 1995-96 1999 1998 0.25 1997 0.00 0 1 2 Follow-up time (years) 3 4 (Perrin & Telenti. Science 1998;280:1871-1873) Licensure of Antiretroviral Agents by Year 1987: 1988: 1989: 1990: 1991: 1992: 1993: 1994: 1995: zidovudine didanosine zalcitabine stavudine lamivudine saquinavir 1996: ritonavir indinavir nevirapine 1997: nelfinavir delavirdine 1998: efavirenz abacavir 1999: amprenavir 2000: lopinavir/ritonavir 2001: tenofovir WHO Antiretroviral Guidelines Primary targets are national treatment advisory boards and senior-level policymakers Outline a public health approach to enable treatment of 3 million individuals in the next 3 years WHO placed ARVs on Essential Drug List in April 2002 – “they should be available at all times in adequate amounts, appropriate dosage forms, at a price individuals and communities can afford…” (http://www.who.int/HIV_AIDS/first.html) Feasibility and Will to Use ARVs UN General Assembly Special Session (article 15) recognized that access to medication is integral to the human right to health Global Fund to Fight AIDS, Tuberculosis, and Malaria” – target $7-10 billion/year Of $174 million approved for coming year, 67% assigned to HIV 21 of 28 countries awarded Global Fund grants specifiy purchase of ARVs as central aim Factors affecting success Drug acquisition costs Facility and personnel infrastructure Minimizing side effects Preventing rapid evolution of drug resistance Recognizing indirect benefit of treatment on transmission probability – “Therapy as Prevention” Affordable prices Annual cost per person for triple therapy in Africa (US$) $12,000 $10,000 Drug Access Initiative $8,000 $6,000 Domestic production $4,000 Accelerated access initiative February-April 2001 offers $2,000 $0 1991 1993 1995 1997 1999 2001 2003 WHO Staging System for HIV Infection and Disease in Adults and Adolescents Clinical Stage I: 1. Asymptomatic 2. Persistent generalized lymphadenopathy (PGL). Performance scale 1: Asymptomatic, normal activity. WHO Staging System for HIV Infection and Disease in Adults and Adolescents Clinical Stage II: 3. Weight loss, < 10 % of body weight. 4. Minor mucocutaneous manifestations (seborrheic dermatitis, prurigo, fungal nail infections, recurrent oral ulcerations, angular cheilitis). 5. Herpes Zoster, within the last 5 years. 6. Recurrent upper respiratory tract infections (i.e., bacterial sinusitis). And/or Performance scale 2: symptomatic, normal activity. WHO Staging System for HIV Infection and Disease in Adults and Adolescents: Clinical Stage III 7. Weight loss, > 10 % of body weight. 8. Unexplained chronic diarrhoea, > 1 month. 9. Unexplained prolonged fever (intermittent or consant), > 1 month. 10. Oral candidiasis (thrush). 11. Oral hairy leukoplakia. 12. Pulmonary tuberculosis, within the past year. 13. Severe bacterial infections (i.e., pneumonia, pyomyositis). And/or Performance scale 3: bed-ridden, < 50% of the day during the last month. WHO Staging System for HIV Infection and Disease in Adults and Adolescents: Clinical Stage IV 14. HIV wasting syndrome, as defined by CDC1. 15. Pneumocystis carinii pneumonia. 16. Toxoplasmosis of the brain. 21. Any disseminated endemic 17. Cryptosporidiosis with 24. 18. 19. 20. 21. diarrhoea, > 1 month. Cryptococcosis, extrapulmonary Cytomegalovirus (CMV) disease of an organ other than liver, spleen or lymph nodes. Herpes simplex virus (HSV) infection, mucocutaneous > 1 month, or visceral any duration. Progressive multifocal leukoencephalopathy (PML). 23. 25. 26. 27. 28. 29. mycosis (i.e. histoplasmosis, coccidioidomycosis). Candidiasis of the oesophagus, trachea, bronchi or lungs. Atypical mycobacteriosis, disseminated. Non-typhoid Salmonella septicaemia. Extrapulmonary tuberculosis. Lymphoma. Kaposi’s sarcoma (KS HIV encephalopathy, as defined by CDC2. And/or Performance scale 4: bed-ridden, > 50 % of the day during the last month. CDC Classification of HIV Infection (1993) Clinical Categories A B C CD4 Categories >500 A-1 B-1 C-1 200-500 A-2 B-2 C-2 <200 A-3 B-3 C-3 Table A Recommendations for Initiating Antiretroviral Therapy in Adults and Adolescents with Documented HIV Infection If CD4 Testing Available: WHO Stage IV disease irrespective of CD4 cell count WHO Stage I, II or III3 with CD4 cell counts <200/mm3 (1) If CD4 Testing Unavailable: WHO Stage IV disease irrespective of total lymphocyte count WHO Stage II or III (3) disease with a total lymphocyte count <1000-1200/mm3 – (2) 1The precise CD4 level above 200/mm3 at which to start ARV treatment has not been established but the presence of symptoms and the rate of CD4 cell decline (if measurement available) should be factored into the decision making. 2A total lymphocyte count of <1000-1200/mm3 can be substituted for the CD4 count when the latter is unavailable and HIV-related symptoms exist. It is less useful in the asymptomatic patient. Thus, in the absence of CD4 cell testing, asymptomatic HIV infected patients (WHO Stage I) should not be treated because there is currently no other reliable marker available in severely resource constrained settings. 3Treatment is also recommended for patients with advanced WHO Stage III disease including recurrent or persistent oral thrush and recurrent invasive bacterial infections irrespective of CD4 cell or total lymphocyte count. When to change therapy? Intolerance leading to poor adherence Drug toxicity Occurrence of active tuberculosis Pregnancy Treatment failure – Clinical Disease progression on therapy, not due to immunologic reconsitution syndrome – Immunologic: Drop >30% CD4 from peak Return to baseline or below – Virologic Continued detectable viremia Cytochrome P-450, HIV-1 Protease Inhibitors and NNRTIs Enzyme Substrates Inducers Inhibitors Ritonavir 1A2 Ritonavir Delavirdine Efavirenz Ritonavir Delavirdine Efavirenz Ritonavir 2C9 2C19 2D6 Delavirdine 3A4 Saquinavir Indinavir Ritonavir Nelfinavir Nevirapine Delavirdine Efavirenz Lopinavir Nevirapine Efavirenz Indinavir Ritonavir Nelfinavir Lopinavir Delavirdine Efavirenz Abacavir Hypersensitivity Syndrome Incidence about 5% Onset within 6 weeks of initiation Definition: – A fever > 100.9 F and one of the following: Nausea greater than baseline Malaise greater than baseline With or without rash Stop the drug and do not re-challenge Metabolic Abnormalities Associated with HIV Protease Inhibitor Therapy Peripheral lipodystrophy (Carr et al. AIDS 1998;12:F51-8) Hypertriglyceridemia Hypercholesterolemia & decreased HDL Hyperinsulinemia & glucose intolerance Accelerated atherosclerosis (Henry et al. Lancet 1998;351:1328) Dorsal fat pads (“Buffalo humps”) Peripheral Lipodystrophy Variable definitions and prevalence (5-60%) Characteristics – Change in body habitus – Increase abdominal girth with visceral fat deposition (Miller et al. Lancet 1998;351:871-5) – Loss of appendicular fat, with thinning of legs and prominent veins – Breast hypertrophy in women Treatment Adherence (Altice & Friedland. Ann Intern Med 1998;129:503-505) “…compared with therapies for other chronic diseases, which are often forgiving of lapses in adherence, HIV therapy is unforgiving.” Nonadherence may mean: – Not taking medication at all – Taking reduced amounts – Not taking doses at prescribed frequencies or intervals – Not matching medication to food requirements SS % Patients with HIV RNA <400 c/mL Correlation Between Optimal Therapeutic Response and Adherence to Protease Inhibitor Therapy 100% 80% 60% 40% 20% 0% >95 90-94.9 80-89.9 70-79.9 <70 % Adherence Paterson D, et al. Ann Inter Med. 2000;133:21-30. Definitions Genotype Virus nucleotide sequence from which a protein’s amino acids can be deduced – Mutations reported as change in the deduced amino acid sequence, e.g., Met184Val – Specific mutations confer phenotypic resistance – The phenotype is always derived from the genotype Phenotype Relative growth of the virus in the presence of different drug concentrations – Usually reported as the drug concentration that inhibits virus replication by 50% (IC50), or the fold increase in IC50 IAS-USA Recommendations for Use of HIV Drug Resistance Assays Clinical characteristics Recommendation Rationale Primary HIV infection Consider testing Established HIV infection Consider testing First regimen failure Recommend testing Multiple regimen failures Recommend testing Pregnancy Recommend testing Hirsch. JAMA 2000;283:2417. Detect transmission of drugresistant virus; modify therapy to optimize response and maintain HIV-specific immune responses Detect prior transmission of drugresistant HIV although this may not always be possible with current tests Document drug(s) to which there is resistance Optimize the number of active drugs in the next regimen; exclude drugs to which response is unlikely Optimize maternal treatment and prophylaxis for the neonate Types of Drug Resistance Assays: Strengths and Weaknesses Strengths Availability Turnaround time 2 weeks Mutations may precede phenotypic resistance Lower cost Measures susceptibility directly Results are easier to interpret Fast results (2 weeks) Moderate cost Weaknesses GENOTYPE Requires expert interpretation Measures susceptibility indirectly Insensitive for detecting minor species Does not assess interactions among mutations Does not address drug levels PHENOTYPE Restricted availability Turnaround time 2–4 weeks Insensitive for detecting minor species Clinically significant cutoff values may not be defined for some drugs More expensive VIRTUAL PHENOTYPE Measures susceptibility indirectly Insensitive for detecting interactions between mutations Phenotype Sample Report From ViroLogic