* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Two Novel Bi-Based Borate Photocatalysts: Crystal Structure
Survey
Document related concepts
X-ray crystallography wikipedia , lookup
Nanochemistry wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Geometrical frustration wikipedia , lookup
Transparency and translucency wikipedia , lookup
Colloidal crystal wikipedia , lookup
Crystallographic defects in diamond wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Crystal structure wikipedia , lookup
Heat transfer physics wikipedia , lookup
Tight binding wikipedia , lookup
Transcript
Article
pubs.acs.org/JPCC
Two Novel Bi-Based Borate Photocatalysts: Crystal Structure,
Electronic Structure, Photoelectrochemical Properties, and
Photocatalytic Activity under Simulated Solar Light Irradiation
Hongwei Huang,*,† Ying He,† Zheshuai Lin,‡ Lei Kang,‡ and Yihe Zhang†
†
National Laboratory of Mineral Materials, School of Materials Science and Technology, China University of Geosciences, Beijing,
100083, PR China
‡
Beijing Center for Crystal R&D, Key Lab of Functional Crystals and Laser Technology of Chinese Academy of Sciences, Technical
Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, PR China
S Supporting Information
*
ABSTRACT: Through the combination of Bi3+ and a large
negative charge ion (BO3)3−, two novel Bi-based borate
photocatalysts Bi4B2O9 and Bi2O2[BO2(OH)] with layered
structure have been successfully developed. For the first time,
the borates were investigated as photocatalysts. They were
synthesized by solid-state reaction and hydrothermal method,
respectively, and further characterized by XRD, SEM, TEM,
HRTEM, and DRS. Bi4B2O9 and Bi2O2[BO2(OH)] possess
direct and indirect transition optical band gaps of 3.02 and
2.85 eV, respectively. Density functional calculations revealed
that the valence band (VB) and conduction band (CB) of both
borates were composed of hybridized states of the O 2p and Bi 6p or 6s orbitals, and a large dispersion was observed in the
energy band of Bi2O2[BO2(OH)]. The photodecomposition experiments demonstrated that Bi4B2O9 and Bi2O2[BO2(OH)] can
be used as effective photocatalysts under simulated solar irradiation, and Bi2O2[BO2(OH)] exhibits the high photocatalytic
activity, which is 2.5 and 3.2 times compared with that of P25 and Bi2O2CO3, respectively. Moreover, the photocurrent
conversion further confirmed that Bi4B2O9 and Bi2O2[BO2(OH)] were potential photofunctional materials. The layered
structure with (Bi2O2)2+ layer, hybridized and dispersion energy band, and large negative charge of (BO3)3− ion should be
responsible for the high photocatalytic activity of Bi2O2[BO2(OH)].
■
INTRODUCTION
Photocatalysts have attracted much attention for solving the
severe problems of energy shortages and environment crises as
a potential solution over the past decades.1−3 It is because that
they can be used to decompose organic contaminants for
environmental purification and split water into hydrogen and
oxygen gases for clean energy production and under UV and
visible-light irradiation. Besides the focused work on TiO2 and
its modifications,4−6 many efforts were made to develop other
novel efficient photocatalysts, which can be generally classified
as oxides,7 sulfides,8 oxysalts,9 and polymers.10
Among these, Bi-based compounds have drawn a lot of
attention for their potential application as novel photocatalysts.
Because of the lone pair electrons of Bi3+, the Bi-based
compounds were often found to possess hybridized band
structures. The hybridized states of band structures can not
only decrease the effective masses of holes and electrons, to
favor a longer traveling distance for excited carriers,11 but also
effectively decrease the band gaps and increase the light
absorption in the long-wavelength region, enhancing their high
photocatalytic activities. Though bismuth-based photocatalysts
crystallize in different structure types, they all exhibit high
© 2013 American Chemical Society
efficiency in the degradation of organic pollutants, including
perovskite-structured NaBiO312 and BiFeO3,13 Scheelitestructure BiVO4,14 pyrochlore-structure Bi2MNbO7 (M = Al,
Ga, In, Fe, and Sm),15 Aurivillius structure Bi2MoO6,16
Bi2WO6,17 and Bi2SiO5,18 Sillén structure BiOX (X = Cl, Br,
and I),19 and Sillén−Aurivillius structure Bi4NbO8Cl.20 Among
these, the compounds with Aurivillius and Sillén structures
display more excellent photooxidation ability and interesting
structure−property relationships due to the existence of an
active (Bi2O2)2+ layer. The Aurivillius structure is built up from
alternate layers of (Bi2O2)2+ cations and perovskite-like
(Am−1BmO3m+1)2‑ anionic blocks, with m being an integer
corresponding to the number of cornershared octahedra
forming the perovskite blocks. In the Sillén family expressed
by [Bi2O2][Xm] (m = 1−3), the bismuth oxide-based fluoritelike layers, (Bi2O2)2+, are intergrown with single, double, or
triple halogen layers to construct such compositions. These
layered structure compounds were considered to promote the
Received: August 22, 2013
Revised: October 10, 2013
Published: October 11, 2013
22986
dx.doi.org/10.1021/jp4084184 | J. Phys. Chem. C 2013, 117, 22986−22994
The Journal of Physical Chemistry C
Article
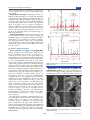
Figure 1. Crystal structure of (a) Bi4B2O9, (b) one-dimensional Bi2O2 chain in Bi4B2O9, and (c) Bi2O2[BO2(OH)].
raw materials of Bi2O3 and H3BO3 were mixed in stoichiometric
proportions and then gradually elevated to sintering temperatures of 600 °C and kept at this temperature in air for 10 h.
The calcination procedure was repeated another three times
after grinding to ensure a complete reaction. Bi2O2[BO2(OH)]
nanosheets were prepared via a hydrothermal method using
Bi4B2O9 as the precursor. Typically, a powder of 1 g of Bi4B2O9
and 5 mL of water was added in a 15 mL Teflon autoclave and
maintained at 200 °C for 24 h. After being cooled to room
temperature, the yellowish products were washed with ethanol
and distilled water several times and dried at 80 °C for 4 h.
The Bi2O2CO3 sample as a reference was synthesized by a
hydrothermal method.24
Characterization. The crystal structures of the obtained
samples were examined by X-ray diffraction (XRD) using a D8
Advance X-ray diffractometer (Bruker AXS, Germany) with Cu
Kα radiation (λ = 1.5418 Å). The scanning step width of 0.02°
and the scanning rate of 0.2° S−1 were applied to record the
patterns in the 2θ range of 1070°. The morphology and
microstructure were obtained by a S-4800 scanning electron
microscope (SEM) and a transmission electron microscope
(TEM and HRTEM; JEM-2100F). UV−vis spectra were
performed with sample powder from a PerkinElmer Lambda
35 UV−vis spectrometer. The spectra were collected at 200−
1000 nm referenced to BaSO4. Room temperature excitation
and emission spectra were measured on a JOBIN 10 YVON
FluoroMax-3 fluorescence spectrophotometer with a photomultiplier tube 11 operating at 400 V, and a 150 W Xe lamp
was used as the excitation lamp. Specific surface areas of
samples were characterized by the nitrogen adsorption BET
method with a Micromeritics 3020 instrument. Electrochemical
and photoelectrochemical measurements were performed in
three-electrode quartz cells with a 0.1 M Na2SO4 electrolyte
solution. Platinum wire was used as the counter electrode, and
saturated calomel electrodes (SCE) were used as the reference
electrodes. Bi4B2O9 and Bi2O2[BO2(OH)] film electrodes on
ITO served as the working electrode. The photoelectrochemical experiment results were recorded using an electrochemical
system (CHI-660B, China). The intensity of light was 1 mW/
cm2. Potentials are given with reference to the SCE. The
photoresponses of the photocatalysts as UV light on and off
were measured at 0.0 V. Electrochemical impedance spectra
generation and separation of the charge carriers, and to exhibit
high photocatalytic activity for water splitting and degradation
of pollutants under light irradiation.
Recently, bismuth subcarbonate (Bi2O2CO3) with Sillénrelated structure has been found to be an efficient photocatalyst
for decomposing organic contaminants under UV−vis light
irradiation.21 Different from the Aurivillius and Sillén structures,
the crystal structure of Bi2O2CO3 is composed of alternate
(Bi2O2)2+ and (CO3)2− layers, presenting a new structural type
of photocatalyst. Intrigued by the synergistic effects of layer
structure and hybridized energy band, it is of great interest and
importance to develop new Bi-based, especially Sillén-related,
compounds for photocatalysis application.
In addition, the newly found phosphate photocatalysts
BiPO422 and Ag3PO423 were reported to possess excellent
photooxidation properties. It is mainly because that (PO4)3−
ions have a large negative charge which maintains a large dipole
in these phosphates preferring the photogenerated charge
separation. This effect is called an inductive effect described as
the action of one group to affect electrostatically the electron
distribution in another group.
Herein, through the combination of Bi3+ and another large
negative charge ion (BO3)3−, we successfully developed two
novel Bi-based borate photocatalysts Bi 4 B 2 O 9 and
Bi2O2[BO2(OH)]. So far, to our best knowledge, there is no
report on the borates used as photocatalysts. In this paper,
Bi4B2O9 and Bi2O2[BO2(OH)] were synthesized by solid-state
reaction and hydrothermal method, respectively.
Bi2O2[BO2(OH)] was found possessing a Sillén-related
structure similar to Bi2O2CO3. The photocatalytic performances of Bi4B2O9 and Bi2O2[BO2(OH)] were investigated
systematically. The photochemical properties of Bi4B2O9 and
Bi2O2[BO2(OH)] were demonstrated by the photocatalytic
decomposition of MB under simulated solar irradiation and
photocurrent measurements under UV light. The origin of high
photocatylytic activities was also suggested on the basis of the
understanding of the structure−property relationship.
■
EXPERIMENTAL SECTION
Synthesis. Bi2O3 and H3BO3 are all in analytic grade purity
and used as received, without further purification. Bi4B2O9
microparticles were synthesized by a solid-state reaction. The
22987
dx.doi.org/10.1021/jp4084184 | J. Phys. Chem. C 2013, 117, 22986−22994
The Journal of Physical Chemistry C
Article
(EIS) were measured at 0.0 V. A sinusoidal ac perturbation of 5
mV was applied to the electrode over the frequency range
0.05−105 Hz.
Photocatalytic Evaluation. Photocatalytic activities of
Bi4B2O9 and Bi2O2[BO2(OH)] were evaluated by degradation
of methylene blue under simulated solar light irradiation of a
1000 W Xe lamp. Powder photocatalyst (50 mg) was dispersed
into 100 mL of dye solution (10−5 mol/L). Before illumination,
the photocatalyst powder and dye solution were vigorously
stirred in the dark for 0.5 h to achieve the adsorption−
desorption equilibrium of suspensions. After that, the light was
turned on, and 2 mL of the suspension was taken at certain
intervals and separated through centrifugation. The UV−vis
spectra of the centrifuged solution were recorded using a U3010 spectrophotometer.
Theoretical Calculation. The electronic structures, as well
as total and partial densities of states (DOS), of Bi4B2O9 and
Bi2O2[BO2(OH)] were obtained by the plane-wave pseudopotential method.25 The calculations were carried out using the
local density approximation (LDA) with a very high kinetic
energy cutoff of 500 eV adopted. The Monkhorst−Pack k-point
with a density of (2 × 2 × 2) points in the Brillouin zone of the
unit cell is chosen.26
■
RESULTS AND DISCUSSION
Crystal Structures of Bi4B2O9 and Bi2O2[BO2(OH)].
Bi4B2O9 crystallizes in the monoclinic space group P2/c with
the unit cell parameters a = 11.127(9) Å, b = 6.641(5) Å, c =
11.058(9) Å, and β = 90.91(9)°.27 The crystal structure was
shown in Figure 1a. It was constructed by BO3 triangles, BiO4
tetrahedron, and BiO5 polyhedra. In the asymmetric units, there
are four crystallographically independent Bi atoms, among
which two Bi atoms form a BiO4 tetrahedron and another two
constitute BiO5 polyhedra with the Bi−O bond length ranging
from 2.123 to 2.4794 Å. In this structure, two neighboring BiO5
polyhedra connect each other through edge sharing to form a
Bi2O2 chain, as displayed in Figure 1b. Figure 1c illustrates the
crystal structure of Bi2O2[BO2(OH)], which crystallizes in the
monoclinic space group Cm with the lattice parameters a =
5.4676 Å, b = 14.6643 Å, c = 3.9058 Å, and β = 135.59°.28 In
the asymmetric units, there is only one crystallographically
independent Bi atom, one independent B atom, and three
independent O atoms. Bi2O2[BO2(OH)] possesses the Sillénrelated crystal structure composed of (Bi2O2)2+ layers and
(BO3)3− layers, as shown in Figure 1c. In Bi2O2[BO2(OH)],
the Bi3+ cation links to oxygen atoms by five short and three
long Bi−O bonds, and then, the lone electron pair of Bi3+ faces
toward the open space around Bi3+, that is, the upper and lower
sides of the (Bi2O2)2+ layers. This is very similar to that of
bismuth oxycarbonates, Bi2O2CO3, and hydroxynitrates,
Bi2O2(OH)(NO3),29 which were also reported to have Sillénrelated structures, built by (Bi2O2)2+, (CO3)2−, and (NO3)−
layers, respectively.
The XRD patterns of Bi4B2O9 and Bi2O2[BO2(OH)] were
presented in Figure 2a and b, respectively. Obviously, all of the
observed peaks of the patterns can be indexed to pure
monoclinic Bi4B2O9 (ICSD#2796) and Bi2O2[BO2(OH)]
phases (simulated from the single crystal model). No other
peaks are found, suggesting the high purity and crystallinity of
the two samples. Moreover, the strongest peaks of Bi4B2O9 and
Bi2O2[BO2(OH)] are attributed to (−302) and (130) planes,
respectively, which are in good agreement with the following
HRTEM analyses.
Figure 2. XRD pattern of as-prepared samples of (a) Bi4B2O9 and (b)
Bi2O2[BO2(OH)].
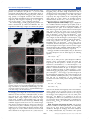
Morphologies and microstructure of Bi4B2O9 and
Bi2O2[BO2(OH)] Products. The surface morphologies and
particle sizes of Bi4B2O9 and Bi2O2[BO2(OH)] were observed
by SEM. Figure 3a and b showed that the Bi4B2O9 products are
Figure 3. SEM images of as-prepared samples of (a, b) Bi4B2O9 and (c,
d) Bi2O2[BO2(OH)].
22988
dx.doi.org/10.1021/jp4084184 | J. Phys. Chem. C 2013, 117, 22986−22994
The Journal of Physical Chemistry C
Article
facets, as shown in Figure S1 (Supporting Information). For
Bi2O2[BO2(OH)], the lattice fringe measurements with an
interplanar spacing of 0.194 and 0.305 nm (Figure 4j and l) can
be assigned to the corresponding (−222) and (130) planes,
respectively. The angle indicated in the corresponding FFT
image (Figure 4i) is 80.9°, which is in accordance with the
theoretical values between the {−222} and {130} facets, as
displayed in Figure S2 (Supporting Information).
Optical Properties and Band Gaps. Figure 5 displays the
UV−vis diffuse reflectance absorption spectra (DRS) of the asprepared nanocrystals of Bi4B2O9 and Bi2O2[BO2(OH)]. In
semiconductors, the square of the absorption coefficient is
linear with energy for direct optical transitions in the absorption
edge region, whereas the square root of the absorption
coefficient is linear with energy for indirect transitions.22 Data
plots of absorption2 versus energy and absorption1/2 versus
energy in the absorption edge region are shown in the upper
inset of Figure 5a and b. From Figure 5a, the absorption2 versus
energy plot is nearly linear for Bi4B2O9, while the absorption1/2
versus energy deviates from the fitted straight line. For
Bi2O2[BO2(OH)], the absorption1/2 versus energy plot is
nearly linear, while the absorption2 versus energy deviates from
the fitted straight line from Figure 5b. These features suggest
that the absorption edges of Bi4B2O9 and Bi2O2[BO2(OH)] are
caused by direct and indirect transitions, respectively.
Band gaps of Bi4B2O9 and Bi2O2[BO2(OH)] are determined
by optical absorption near the band edge by the following
equation:
composed of irregular microparticles, and the dimension of the
particles was estimated to be 500 nm ∼ 2 μm. The SEM
micrographs of Bi2O2[BO2(OH)] were illustrated in Figure 3c
and d. It reveals that products of Bi2O2[BO2(OH)] consist of a
large quantity of rectangular nanosheets with uniform cut
edges. The length and width are about 1 μm and 200−400 nm,
respectively. This nanosheet morphology is very similar to
those of other (Bi2O2)2+ layers containing compounds.30
The obtained Bi4B2O9 and Bi2O2[BO2(OH)] products were
further characterized by TEM and HRTEM. The low
magnification TEM image in Figure 4a confirmed the particle
αhν = A(hν − Eg )n /2
(1)
where α, hν, A, and Eg are the optical absorption coefficient,
photonic energy, proportionality constant, and band gap,
respectively.31 In this equation, n decides the type of the
transition in a semiconductor (n = 1, direct absorption; n = 4,
indirect absorption). By applying n = 1, the direct band gap of
Bi4B2O9 is determined from the plot of absorption2 versus
energy, as indicated in Figure 5c, and by applying n = 4, the
indirect band gap of Bi2O2[BO2(OH)] is determined from the
plot of absorption1/2 versus energy, as presented in Figure 5d.
By extrapolating the straight line to the x-axis in this plot, the Eg
values of Bi4B2O9 and Bi2O2[BO2(OH)] were estimated to be
3.02 and 2.85 eV. Furthermore, we can calculate their
conduction and valence band positions through the following
equations:
Figure 4. (a) TEM and (c) HRTEM images, (e, g) FFT (fast Fourier
transition) patterns, and (f, h) magnified HRTEM images of the lattice
fringe of the Bi4B2O9. (b) TEM and (d) HRTEM images, (i, k) FFT
(fast Fourier transition) patterns, and (j, l) magnified HRTEM images
of the lattice fringe of the Bi2O2[BO2(OH)].
E VB = X − E e + 0.5Eg
(2)
ECB = E VB − Eg
(3)
where X is the absolute electronegativity of the semiconductors,
which is defined as the geometric average of the absolute
electronegativity of the constituent atoms, Ee is the energy of
free electrons on the hydrogen scale (≈4.5 eV), and Eg is the
band gap.32 For Bi4B2O9, X is calculated to be 6.16 eV,
consequently. ECB and EVB are estimated to be 0.15 and 3.17
eV, respectively. The X of Bi2O2[BO2(OH)] is calculated to be
6.34 eV, and ECB and EVB are estimated to be 0.41 and 3.26 eV,
respectively.
Band Structures and Density of States. The electronic
structures of Bi4B2O9 and Bi2O2[BO2(OH)] were calculated by
using the ab initio density functional theory (DFT)
calculations. Although the bandgaps from DFT calculations
are usually underestimated, they nonetheless often provide
size of Bi4B2O9. From Figure 4b, it can be clearly seen that the
thicknesses of these nanosheets of Bi2O2[BO2(OH)] are in the
range 40−50 nm. The HRTEM image and fast Fourier
transform (FFT) images (Figure 4c−l) confirm the single
crystal nature of Bi4B2O9 and Bi2O2[BO2(OH)]. The highresolution transmission electron microscopy (HRTEM) image
of Bi4B2O9 (Figure 4f and h) shows two sets of lattice fringes
with spacings of 0.309 and 0.302 nm, which can be indexed to
(−302) and (121) planes of Bi4B2O9, respectively. The angle
indicated in the corresponding fast-Fourier transform (FFT)
image (Figure 4e) is 85.6°, which is identical to the theoretical
values obtained for the angles between the {−302} and {121}
22989
dx.doi.org/10.1021/jp4084184 | J. Phys. Chem. C 2013, 117, 22986−22994
The Journal of Physical Chemistry C
Article
Figure 5. UV−vis diffuse reflectance spectra of (a) Bi4B2O9 and (b) Bi2O2[BO2(OH)]. The upper inset shows the plots of absorption2 vs energy and
absorption1/2 vs energy in the absorption edge region (circles for experimental data and the line for a linear fit). (c) The absorption2 vs energy in the
absorption edge region of Bi4B2O9. (d) The absorption1/2 vs energy in the absorption edge region of Bi2O2[BO2(OH)].
also a little of the Bi 6s and 6p orbitals, which varies from those
in other oxides or oxygen compounds.33,34 These results are
also obviously different from foreign elements creating impurity
levels in the forbidden band in doped oxides. Meanwhile, we
also found that the bottoms of the CB were mainly composed
of the hybridized Bi 6p and 6s orbitals. The contribution of O
2p to the CB seemed to be much smaller than that in the oxides
whose conduction electron was O 2p.35
Photocatalytic Activities of Bi4B2O9 and
Bi2O2[BO2(OH)] Samples. Figure 7a showed the photocatalytic performance of the as-prepared Bi4 B2O9 and
Bi2O2[BO2(OH)] samples evaluated by the degradation of
MB. For comparison purposes, the MB photodegradated by
TiO2 (P25) and Bi2O2CO3 was also performed. The XRD and
TEM images of as-prepared Bi2O2CO3 were shown in Figures
S3 and S4 (Supporting Information), respectively. It can be
obviously seen that MB was almost photodecomposed and
catalyzed by Bi2O2[BO2(OH)] and TiO2 (P25) in 80 and 160
min, and the photodegradation efficiency of Bi2O2CO3 and
Bi4B2O9 reached 91 and 88% after 180 min of reaction,
respectively. The MB photolysis without the photocatalyst can
almost be neglected. As shown in Figure 6b and c, the main
absorption peak of MB molecules at 664 nm decreases with
irradiation time, and almost disappears after about 80 min for
Bi2O2[BO2(OH)] and 180 min for Bi4B2O9. The insets in
important insight into the physicochemical behavior of the
materials investigated. Parts a and c of Figure 6 show the band
structures of Bi4B2O9 and Bi2O2[BO2(OH)]. The Fermi
energy, defined as the highest occupied energy level, has been
taken as the valence band maximum (VBM), and the lowest
unoccupied occupied state is the conduction band minimum
(CBM). For Bi4B2O9, the VBM and CBM are all situated at the
G point, indicating the direct bandgap property, whereas the
VBM and CBM of Bi2O2[BO2(OH)] are located at the point
between L and M and the G point, respectively, which confirms
the fact that Bi2O2[BO2(OH)] is an indirect bandgap
semiconductor. These results are all consistent with those
revealed from the absorption spectra. It can be found that the
energy gaps between the VB maximum and CB minimum given
from band structures are 2.8 and 2.34 eV for Bi4B2O9 and
Bi2O2[BO2(OH)], respectively, which are in good agreement
with the experimental values. Besides, dispersed energy bands
were observed in Bi2O2[BO2(OH)], as shown in Figure 6c, and
the width of dispersion of VBM and CBM is about 1.5 and 0.5
eV, respectively.
Parts b and d of Figure 6 show the total density of states
(TDOS) and main partial density of states (PDOS),
corresponding to the energy regions in Figure 6a and c. The
tops of the valence bands of Bi4B2O9 and Bi2O2[BO2(OH)]
were not only found to be composed of the O 2p orbital but
22990
dx.doi.org/10.1021/jp4084184 | J. Phys. Chem. C 2013, 117, 22986−22994
The Journal of Physical Chemistry C
Article
Figure 6. Electronic band structures of (a) Bi4B2O9 and (c)Bi2O2[BO2(OH)]. Total and partial DOS of (b) Bi4B2O9 and (d) Bi2O2[BO2(OH)].
firmed that Bi4B2O9 and Bi2O2[BO2(OH)] were two potential
photofunctional materials.
As the electrochemical impedance spectra (EIS) Nyquist
plots are supposed to indicate the charge separation and
transfer process in the electrode−electrolyte interface region,37
the EIS technology was employed to study the photocatalytic
performance. Figure 8b shows Nyquist plots of Bi4B2O9 and
Bi2O2[BO2(OH)] before and after UV light irradiation. It can
be observed that the arc radius of Bi2O2[BO2(OH)] is smaller
than that of Bi4B2O9, which indicates that Bi2O2[BO2(OH)]
exhibits a higher separation and transfer efficiency of photogenerated e−h pairs.
Structure−Property Relationship. The photocatalytic
oxidation of the organic contaminants is closely correlative to
two factors: efficient photoinduced electron−hole separation
and transfer and the structure of the band gap in the
photocatalyst.11 In the process of photocatalytic degradation,
charge separation is important and necessary to prevent
recombination of the photoinduced electrons and holes. In
the crystal structure of Bi2O2[BO2 (OH)], the layered
configuration was considered to be very beneficial for the
high photocatalytic activity. On one hand, oxidation and
reduction sites in photocatalytic reaction locate at the surface
and edge position of two-dimensional layered structure,
respectively. Thus, the photogenerated holes only travel a
very short distance (sub-nanometer) to reach the surface layer
structure, and then were trapped by the hydroxyls in the layer
gap. This rapid hole-trapping process allows more photogenerated electrons to more easily move to the edge of the
layered structure, reducing the recombination probability of
photogenerated carriers. On the other hand, the internal
Figure 7b and c showed the corresponding color changes of
MB solution from the blue starting solution gradually to
colorless with increasing light irradiation time.
In order to compare the degradation rate quantitatively, the
pseudo-first-order kinetic curves of MB photodegradation were
also plotted (Figure 7d). The experimental data obviously show
the apparent rate constant k is 0.0381, 0.0152, 0.0122, and
0.0115 min−1 for Bi2O2[BO2(OH)], P25, Bi2O2CO3, and
Bi4B2O9, respectively. In other words, Bi2O2[BO2(OH)]
exhibits the highest photocatalytic activity, which is 2.5 and
3.2 times compared with that of P25 and Bi2O2CO3,
respectively, though the surface area of Bi2O2[BO2(OH)]
(1.25 m2/g) and Bi4B2O9 (0.84 m2/g) is much smaller than P25
(48.6 m2/g) and Bi2O2CO3 (3.24 m2/g). The photocatalytic
activity of Bi4B2O9 is relatively low but very close to that of
Bi2O2CO3.
Photoelectrochemical Properties. The mobilities of the
electrons generated in the photocatalyst can be directly
monitored by the photocurrent, and the rate should directly
correlate with the photocatalytic activity of the material.36
Figure 8a shows the photocurrent of Bi 4 B 2 O 9 and
Bi2O2[BO2(OH)] samples generated in electrolyte under UV
light. When the light was on, the photocurrent of Bi4B2O9 and
Bi2O2[BO2(OH)] was generated immediately, and the photocurrent generated by Bi4B2O9 was about 1/5 times that of
Bi2O2[BO2(OH)], which is consistent with the order of their
photocatalytic activities, showing the photocurrent was
positively relevant to the photocatalytic activity. The generation
of photoelectrons was the critical initial step of the photocatalytic reaction, and the rate directly governed the photocatalytic activity. The photocurrent conversion further con22991
dx.doi.org/10.1021/jp4084184 | J. Phys. Chem. C 2013, 117, 22986−22994
The Journal of Physical Chemistry C
Article
Figure 7. (a) Photocatalytic degradation curves of MB under the irradiation of simulated solar light. Temporal absorption spectral patterns of MB
during the photodegradation process over (b) Bi4B2O9 and (c)Bi2O2[BO2(OH)]. (the insets in parts b and c demonstrate the color changes of MB).
(d) Kinetic curves for the photocatalytic degradation of MB.
Figure 8. (a) Comparison of transient photocurrent responses of Bi4B2O9 and Bi2O2[BO2(OH)] under UV light irradiation (λ = 254 nm, [Na2SO4]
= 0.1 M). (b) EIS Nynquist plots of Bi4B2O9 and Bi2O2[BO2(OH)] with light on/off cycles under the irradiation of UV light (λ = 254 nm, [Na2SO4]
= 0.1 M).
electric fields are one of the important parameters to evaluate
the ability of electron−hole separation and transport in the
crystal lattice. Generally, the presence of internal electric fields
between [Bi2O2] and [BO3] is favorable for the efficient
photoinduced electron−hole separation and transfer, which is
also propitious to a high photocatalytic efficiency of
Bi2O2[BO2(OH)].
Because of the lone pair electrons of Bi3+, the VB structures
of Bi4B2O9 and Bi2O2[BO2(OH)] are all hybridized by 0 2p, Bi
6s, and 6p orbitals, which can effectively decrease the band gaps
and increase the light absorption in the longer wavelength
region, to further enhance their photocatalytic activity under
simulated solar irradiation. Moreover, a large dispersion was
observed in the hybridized orbitals in both the CB and VB of
Bi2O2[BO2(OH)], suggesting that the photoexcited charges
have a high mobility in the VB and CB, which should be
beneficial for the transport of photoexcited electrons and
holes.23,35 This in turn is likely to suppress the recombination
of electron−hole pairs and thus account for the high
photooxidative activity.
22992
dx.doi.org/10.1021/jp4084184 | J. Phys. Chem. C 2013, 117, 22986−22994
The Journal of Physical Chemistry C
Article
In addition, the large negative charge of (BO3)3− was also
considered to be in favor of the photogenerated charge
separation like (PO4)3−,22,23 which can maintain a large dipole
in these compounds to affect electrostatically the electron
distribution in cations, enhancing the photocatalytic activity.
(5) Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. VisibleLight Photocatalysis in Nitrogen-Doped Titanium Oxides. Science
2001, 293, 269−271.
(6) Feng, N. D.; Wang, Q.; Zheng, A. M.; Zhang, Z. F.; Fan, J.; Liu, S.
B.; Amoureux, J. P.; Deng, F. Understanding the High Photocatalytic
Activity of (B, Ag)-Codoped TiO2 under Solar-Light Irradiation with
XPS, Solid-State NMR, and DFT Calculations. J. Am. Chem. Soc. 2013,
135, 1607−1616.
(7) Muruganandham, M.; Amutha, R.; Lee, G. J.; Hsieh, S. H.; Wu, J.
J.; Sillanpäa,̈ M. Facile Fabrication of Tunable Bi2O3 Self-Assembly and
Its Visible Light Photocatalytic Activity. J. Phys. Chem. C 2012, 116,
12906−12915.
(8) Huang, L.; Wang, X. L.; Yang, J. H.; Liu, G.; Han, J. F.; Li, C.
Dual Cocatalysts Loaded Type I CdS/ZnS Core/Shell Nanocrystals as
Effective and Stable Photocatalysts for H2 Evolution. J. Phys. Chem. C
2013, 117, 11584−11591.
(9) Li, S. J.; Liu, S. M.; Liu, S. X.; Liu, Y. W.; Tang, Q.; Shi, Z.;
Ouyang, S. X.; Ye, J. H. {Ta 12}/{Ta16 } Cluster-Containing
Polytantalotungstates with Remarkable Photocatalytic H2 Evolution
Activity. J. Am. Chem. Soc. 2012, 134, 19716−19721.
(10) Wen, T.; Zhang, D. X.; Zhang, J. Two-Dimensional Copper(I)
Coordination Polymer Materials as Photocatalysts for the Degradation
of Organic Dyes. Inorg. Chem. 2013, 52, 12−14.
(11) Shan, Z. C.; Wang, W. D.; Lin, X. P.; Ding, H. M.; Huang, F. Q.
Photocatalytic Degradation of Organic Dyes on Visible-light
Responsive Photocatalyst PbBiO2Br. J. Solid State Chem. 2008, 181,
1361−1366.
(12) Kako, T.; Zou, Z.; Katagiri, M.; Ye, J. Decomposition of Organic
Compounds over NaBiO3 under Visible Light Irradiation. Chem.
Mater. 2007, 19, 198−202.
(13) Joshi, U. A.; Jang, J. S.; Borse, P. H.; Lee, J. S. Microwave
Synthesis of Single-crystalline Perovskite BiFeO3 Nanocubes for
Photoelectrode and Photocatalytic Applications. Appl. Phys. Lett.
2008, 92, 242106.
(14) Dunkle, S. S.; Helmich, R. J.; Suslick, K. S. BiVO4 as a VisibleLight Photocatalyst Prepared by Ultrasonic Spray Pyrolysis. J. Phys.
Chem. C 2009, 113, 11980−11983.
(15) Zou, Z.; Ye, J.; Arakawa, H. Substitution Effects of In3+ by Fe3+
on Photocatalytic and Structural Properties of Bi2InNbO7 Photocatalysts. J. Mol. Catal. A: Chem. 2001, 168, 289−297.
(16) Bi, J.; Wu, L.; Li, J.; Li, Z.; Wang, X.; Fu, X. Simple Solvothermal
Routes to Synthesize Nanocrystalline Bi2MoO6 Photocatalysts with
Different Morphologies. Acta Mater. 2007, 55, 4699−4705.
(17) Zhang, S. C.; Zhang, C.; Man, Y.; Zhu, Y. F. Visible-light-driven
Photocatalyst of Bi2WO6 Nanoparticles Prepared via Amorphous
Complex Precursor and Photocatalytic Properties. J. Solid State Chem.
2006, 179, 62−69.
(18) Chen, R. G.; Bi, J. H.; Wu, L.; Wang, W. J.; Li, Z. H.; Fu, X. Z.
Template-Free Hydrothermal Synthesis and Photocatalytic Performances of Novel Bi2SiO5 Nanosheets. Inorg. Chem. 2009, 48, 9072−
9076.
(19) Zhang, X.; Ai, Z. H.; Jia, F. L.; Zhang, L. Z. Generalized One-Pot
Synthesis, Characterization, and Photocatalytic Activity of Hierarchical
BiOX (X = Cl, Br, I) Nanoplate Microspheres. J. Phys. Chem. C 2008,
112, 747−753.
(20) Lin, X.; Huang, T.; Huang, F.; Wang, W.; Shi, J. Photocatalytic
Activity of a Bi-based Oxychloride Bi4NbO8Cl. J. Mater. Chem. 2007,
17, 2145−2150.
(21) Zheng, Y.; Duan, F.; Chen, M.; Xie, Y. Synthetic Bi2O2CO3
nanostructures: Novel Photocatalyst with Controlled Special Surface
Exposed. J. Mol. Catal. A: Chem. 2010, 317, 34−40.
(22) Pan, C. S.; Zhu, Y. F. New Type of BiPO4 Oxy-Acid Salt
Photocatalyst with High Photocatalytic Activity on Degradation of
Dye. Environ. Sci. Technol. 2010, 44, 5570−5574.
(23) Yi, Z. G.; Ye, J. H.; Kikugawa, N.; Kako, T.; Ouyang, S. X.;
Stuart-Williams, H.; Yang, H.; Cao, J. Y.; Luo, W. J.; Li, Z. S.; Liu, Y.;
Withers, R. L. An Orthophosphate Semiconductor with Photooxidation Properties under Visible-light Irradiation. Nat. Mater.
2010, 9, 559−564.
■
CONCLUSIONS
In summary, two novel Bi-based borate phtocatalysts Bi4B2O9
and Bi2O2[BO2(OH)] have been successfully synthesized by
solid-state reaction and hydrothermal method, respectively.
The borates were investigated as photocatalysts for the first
time. Morphologies and microstructures of Bi4B2O9 and
Bi2O2[BO2(OH)] were characterized in detail, and they possess
direct and indirect transition optical band gaps of 3.02 and 2.85
eV, respectively. The calculated electronic structures of Bi4B2O9
and Bi2O2[BO2(OH)] confirmed their optical transition types
and that the VB and CB were occupied by hybridized states of
the O 2p and Bi 6p or 6s orbitals. The photodegradation
reaction revealed that Bi4B2O9 and Bi2O2[BO2(OH)] are
effective photocatalysts, which can efficiently decompose
methylene blue (MB), under simulated solar irradiation.
Meanwhile, Bi2O2[BO2(OH)] exhibits the high photocatalytic
activity, which is 2.5 and 3.2 times higher than that of P25 and
Bi2O2CO3, respectively. Moreover, they all yield photocurrent
density under ultraviolet (UV) light in the photocurrent
conversion experiments. The layered structure with the
(Bi2O2)2+ layer, hybridized and dispersion energy band, and
large negative charge of the (BO3)3− ion should be very
beneficial for the photoinduced electron−hole separation and
transfer, resulting in the high photocatalytic activity of
Bi2O2[BO2(OH)].
■
ASSOCIATED CONTENT
S Supporting Information
*
Pictures of crystal structures of Bi4B2O9 and Bi2O2[BO2(OH)]
and XRD and TEM images of as-prepared Bi2O2CO3. This
material is available free of charge via the Internet at http://
pubs.acs.org.
■
AUTHOR INFORMATION
Corresponding Author
*E-mail: [email protected]. Phone: 86-10-82332247.
Notes
The authors declare no competing financial interest.
■
ACKNOWLEDGMENTS
This work was supported by the Fundamental Research Funds
for the Central Universities (2652013052), and the special
coconstruction project of Beijing city education committee, Key
Project of Chinese Ministry of Education (No. 107023).
■
REFERENCES
(1) Kanan, M. W.; Nocera, D. G. In Situ Formation of an OxygenEvolving Catalyst in Neutral Water Containing Phosphate and Co2+.
Science 2008, 321, 1072−1075.
(2) Tong, H.; Ouyang, S. X.; Bi, Y. P.; Umezawa, N.; Oshikiri, M.; Ye,
J. H. Nano-photocatalytic Materials: Possibilities and Challenges. Adv.
Mater. 2012, 24, 229−251.
(3) Kubacka, A.; Fernández-García, M.; Colón, G. Photocatalysis on
TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem.
Rev. 2012, 112, 1555−1614.
(4) Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a
Semiconductor Electrode. Nature 1972, 238, 37−38.
22993
dx.doi.org/10.1021/jp4084184 | J. Phys. Chem. C 2013, 117, 22986−22994
The Journal of Physical Chemistry C
Article
(24) Liu, Y. Y.; Wang, Z. Y.; Huang, B. B.; Yang, K. S.; Zhang, X. Y.;
Qin, X. Y.; Dai, Y. Preparation, Electronic structure, and Photocatalytic
Properties of Bi2O2CO3 Nanosheet. Appl. Surf. Sci. 2010, 257, 172−
175.
(25) Payne, M. C.; Teter, M. P.; Allan, D. C.; Arias, T. A.;
Joannopoulos, J. D. Iterative Minimization Techniques for ab
initioTotal-energy Calculations: Molecular Dynamics and Conjugate
Gradients. Rev. Mod. Phys. 1992, 64, 1045−1097.
(26) Clark, S. J.; Segall, M. D.; Pickard, C. J.; Hasnip, P. J.; Probert,
M. J.; Refson, K.; Payne, M. C. First Principles Methods Using
CASTEP. Z. Kristallogr. 2005, 220, 567−570.
(27) Hyman, A.; Perloff, A. The Crystal Structure of Bismuth (2/1)
Borate, (Bi2O3B2O3)2. Acta Crystallogr., Sect. B 1972, 28, 2007−2011.
(28) Cong, R. H.; Sun, J. L.; Yang, T.; Li, M. R.; Liao, F. H.; Wang, Y.
X.; Lin, J. H. Syntheses and Crystal Structures of Two New Bismuth
Hydroxyl Borates Containing [Bi2O2]2+ Layers: Bi2O2[B3O5(OH)]
and Bi2O2[BO2(OH)]. Inorg. Chem. 2011, 50, 5098−5104.
(29) Cong, R. H.; Yang, T.; Liao, F. H.; Wang, Y. X.; Lin, Z. S.; Lin, J.
H. Experimental and Theoretical Studies of Second Harmonic
Generation for Bi2O2[NO3(OH)]. Mater. Res. Bull. 2012, 47, 2573−
2578.
(30) Gnayem, H.; Sasson, Y. Correction to Hierarchical Nanostructured 3D Flowerlike BiOClxBr1−x Semiconductors with Exceptional Visible Light Photocatalytic Activity. ACS Catal. 2013, 3, 186−
191.
(31) Ohko, Y.; Hashimoto, K.; Fujishima, A. Kinetics of Photocatalytic Reactions under Extremely Low-Intensity UV Illumination on
Titanium Dioxide Thin Films. J. Phys. Chem. A 1997, 101, 8057−8062.
(32) Madhusudan, P.; Ran, J. R.; Zhang, J.; Yu, J. G.; Liu, G. Novel
Urea Assisted Hydrothermal Synthesis of Hierarchical BiVO4/
Bi2O2CO3 Nanocomposites with Enhanced Visible-light Photocatalytic
Activity. Appl. Catal., B 2011, 110, 286−295.
(33) Huang, H. W.; Yao, J. Y.; Lin, Z. S.; Wang, X. Y.; He, R.; Yao,
W. J.; Zhai, N. X.; Chen, C. T. NaSr3Be3B3O9F4: A Promising DeepUltraviolet Nonlinear Optical Material Resulting from the Cooperative
Alignment of the [Be3B3O12F]10‑ Anionic Group. Angew. Chem., Int.
Ed. 2011, 50, 9141−9144.
(34) Huang, H. W.; Yao, J. Y.; Lin, Z. S.; Wang, X. Y.; He, R.; Yao,
W. J.; Zhai, N. X.; Chen, C. T. Molecular Engineering Design to
Resolve the Layering Habit and Polymorphism Problems in Deep UV
NLO Crystals: New Structures in MM′Be2B2O6F (M=Na, M′=Ca;
M= K, M′=Ca, Sr). Chem. Mater. 2011, 23, 5457−5463.
(35) Shi, R.; Xu, T. G.; Zhu, Y. F.; Zhou, J. High Photocatalytic
Activity of Oxychloride CaBiO2Cl under Visible Light Irradiation.
CrystEngComm 2012, 14, 6257−6263.
(36) Kim, H.; Borse, P.; Choi, W.; Lee, J. Photocatalytic Nanodiodes
for Visible-Light Photocatalysis. Angew. Chem., Int. Ed. 2005, 44,
4585−4589.
(37) Hosseini, Z.; Taghavinia, N.; Sharifi, N.; Chavoshi, M.; Rahman,
M. Fabrication of High Conductivity TiO2/Ag Fibrous Electrode by
the Electrophoretic Deposition Method. J. Phys. Chem. C 2008, 112,
18686−18689.
22994
dx.doi.org/10.1021/jp4084184 | J. Phys. Chem. C 2013, 117, 22986−22994