* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Adhesive diffusive controlled systems
Survey
Document related concepts
Transcript
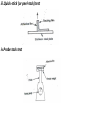
TRANSDERMAL DRUG DELIVERY SYSTEMS Department of Pharmaceutics TRANS DERMAL DRUG DELIVERY SYSTEMS DEFINITION: TDDS are adhesive-drug containing device of defined surface area that deliver a predetermined amount of drug to the surface of intact skin at programmed rate to reach the systemic circulation Advantages • Self administration is possible and Continuous, sustained release of drug • multiday dosing interval • Avoids first-pass hepatic metabolism and enzymatic degradation by gastrointestinal tract • Less frequent dosing improves patient Compliance • Alternate route for patients who are unable to take oral medications • Dose delivery unaffected by vomiting or Diarrihoea • Drug administration stops with patch removal Disadvantages • Only small, lipophillic drugs can be delivered currently through the skin • Not suitable for high drug doses • Adhesion may vary with patch type and environmental conditions • Skin irritation and hypersensitivity reactions may occur • The barrier function of the skin changes from one site to another on the same person, from person to person and with age The skin is one the most extensive organs of the human body covering an area of about 2m2 in an average human adult. The skin separates the underlying blood circulation network from the outside environment Anatomically, the skin has many histologic layers but in general, it is described in terms of three major tissue layers: The epidermis, The dermis and The hypodermis . There are critically three ways in which a drug molecule can cross the intact stratum corneum: (a)through the walls of the hair follicles, (b) through the sweat glands or the sebaceous glands, (c) or between the cells of the horny layer BASIC COMPONENTS OF TDDS The components of transdermal devices include: Polymer matrix Drug Drug permeation enhancers Other excipients 1. POLYMER MATRIX The polymer controls the release of the drug from the device. Materials used for polymer matrix: Natural polymers Cellulose derivatives, Zein, Gelatin, Shellac, Waxes, Proteins, Gums and their derivatives, Natural rubber, starch etc. Synthetic elastomers Polybutadiene, Hydrin rubber, Polysiloxane, Silicone rubber, Nitrile, Acrylonitrile, Butyl rubber, Styrenebutadiene rubber, Neoprene etc. Synthetic polymers Polyvinyl alcohol, Poly vinyl chloride, Polyethylene, Polypropylene, Polyacrylate, Polyamide, Polyurea, Polyvinylpyrrolidone, Polymethylmethacrylate, etc. 2.DRUG Ideal properties of drug for TDDS Dose Should be low (less than 20mg/day) Half-life 10/less (hrs) Molecular weight < 400 Da Partition co-efficient (octanol-water) between 1.0-4.0 Skin permeability coefficient > 0.5×10-3 cm/h Skin reaction Non irritating and nonsensitizing Oral bioavailability Low Therapeutic index Low Melting Point < 2000F pH Between 5.0-9.0 3. PERMEATION ENHANCERS These are compounds which promote skin permeability by altering the skin as a Barrier to the flux of a desired penetrant surfactants, azone, dimethylsulfoxide (DMSO), dimethylacetamide, dimethylformamide, alcohol, acetone, propylene glycol, and polyethylene glycol. APPROACHES FOR DEVELOPMENT OF TDDS Membrane moderated systems Adhesive diffusive controlled systems Matrix dispersion type systems Micro reservoir systems Membrane moderated systems The intrinsic rate of drug release from this type of drug delivery system is defined by Cr dq/dt = 1/Pm + 1/Pa Pm=Km/r(Dm/δm) pa =Ka/m(Da/ δa) where Cr- is drug concentrate in the reservoir compartment. Pa & Pm- are permeability coefficients of the adhesive layer and the rate controlling membrane respectively Adhesive diffusive controlled systems The rate of drug release is defined release is defined by dq /dt = Ka/r Da Cr δa where Ka/r is partition coefficient of drug from the reservoir layer to the adhesive layer. Matrix dispersion type systems Rate of drug release from this matrix dispersion type TTS is defined as dq/dt = (ACp Dp/ 2t)1/2 where A -is initial drug loading dose dispersed in the polymer matrix. Cp and Dp- solubility and diffusivity of the drug in the polymer respectively. Micro reservoir systems The rate of release of drugs from the microreservoir system is defined by dQ/dt = Dp.Dd.m.kp/Dp.hd+Dd.hp.m.kp EVALUATION OF TRANSDERMAL DRUG DELIVERY SYSTEMS 1.Evaluation of adhesive Pressure sensitive adhesives are evaluated for the following properties a.Peel adhesion properties b.Tack properties Tack is the ability of a polymer to adhere to a substrate with little contact pressure. It is important transdermal devices which are applied with finger pressure. Tests for tack include i.Thumb tack test This is a subjective test evaluation is done by pressing the thumb briefly into adhesives. Experience is required for using this test. ii.Rolling ball tack test iii.Quick-stick (or peel-tack) test iv.Probe tack test c.Shear Strength Properties 2.In-vitro drug release evaluation 8 – Cell Trans Diffusion Cell Keshary Chein Diffusion Cell Franz Diffusion Cell Franz Diffusion Cell 3.In-vivo evaluation In vivo evaluation of transdermal drug delivery systems can be carried out using: a. Animal models b. Human volunteers c. Biophysical models Marketed Products of Transdermal Patches Brand Name NicotinellR NuPatch 100 Alora Androderm Drug Nicotine Diclofenac diethylamine Estradiol Manufacturer Novartis Zydus Cadila TheraTech/Proctol and Gamble Testosterone Nitroglycerin Catapres TTSR Clonidine OxytrolR oxybutynin Pharmacological smoking cessation Anti Inflammatory Postmenstrual syndrome Hypogonadism in TheraTech/GlaxoSmithKl ine Nitrodisc Indications Roberts Pharmaceuticals Alza Watson Pharma males Angina pectoris Hypertension Overactive bladder REFERENCES •Chein YW. Transdermal Controlled Systemic Medication. New York and Basel, Marcel Dekker Inc. 1987; 159 – 176 •http://www.pharmainfo.net •http://en.wikipedia.org/wiki/Transdermal_patch •http://www.bentham.org/cdd/sample/cdd2-1/003AP.pdf •http://molinterv.aspetjournals.org/content/4/6/308.full •http://www.docoop.com/drug-delivery/drug-deliverysystems.asp