* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download period ____ due date
Survey
Document related concepts
Transcript
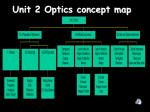
Fourth Quarter Notes Light & Optics Unit light electromagnetic spectrum electromagnetic radiation Huygens principle Planck’s proposal Einstein’s idea A range (frequencies) of the electromagnetic spectrum which can be detected by the human eye. Phrase used to describe electromagnetic radiation of all wavelengths and frequencies. This includes radio waves, microwaves, infrared, visible light, ultraviolet, x-rays, gamma rays, etc. Waves which can move through a vacuum because they are self propagating (causing their own continuation). They self propagate by using changing electric fields which generate changing magnetic fields which generate changing electric fields and so on Light must be made of waves this explains diffraction. Proposed energy is made-up of packets of energy (particles) called quanta. He did this to explain blackbody radiation. Einstein described the photoelectric effect as particles of light called photons bumping the electrons off. This led to the dual nature of light principle. wave or particle (duality of light) Light is both a particle and a wave. luminous source An object that emits light. illuminated source Object that is visible as a result of light reflecting off of it. transparent Media which transmits light. translucent Media which transmits light but does not allow objects to be seen. opaque Media which does not allow light to transmit through it. additive colors Colors which when combined optically form white. (luminous sources) This process is used for film and television. additive primary colors red, green, and blue (RGB) additive secondary colors cyan, magenta, yellow subtractive colors Colors which combined, usually by mixing pigments, form black. (illuminated sources) This process is used in printing and art. subtractive primaries (or primary pigment) cyan, magenta, and yellow (CMY) or blue, red, and yellow photometry Measuring light. luminous intensity Amount of light used to define the base metric unit, candela (cd). One candela was defined as the light candela, cd metric unit of light intensity luminous flux The rate of energy (energy per time or power) in the form of visible light is emitted by a luminous source. Note: Most luminous sources have an output consisting of a lot of infrared light, this is not included in this measurement. lumen, lm metric unit of luminous flux illuminance The measurement of the amount of light that strikes a surface. lux, lx speed of light FORMULA: wave speed for electromagnetic waves metric unit on illuminance, 1 lux = 1 lumen per meter squared 300,000 kilometers per second or 186,000 miles per second symbolized by c (lower case) c=λ·f E= P 4π r 2 ray model of light The use of rays (lines with arrows on one end as in geometry) to represent light waves. polarization The use of filters to limit waves to only one plane of travel. Filters may be naturally occurring or man-made. reflection When a waves bounces off of a surface. specular reflection Mirror-like reflection, a high degree of reflection with little scattering. diffuse reflection Reflection with a lot of scattering. diffusion The scattering of light in many directions. incident ray Incoming light ray. “I” in the diagram. reflected ray Outgoing light ray. “R” in the diagram. normal line A line perpendicular to the surface of reflection or refraction. “N” in the diagram angle of incidence Angle between the incident ray and the normal line. angle of reflection Angle between the reflected ray and the normal line. law of reflection angle of incidence = angle of reflection plane mirror A flat mirror. Images formed by plane mirrors are virtual, upright, left-right reversed, the same distance from the mirror as the object's distance, and the same size as the object. object The person or thing reflected in the mirror. real image An image formed in front of a mirror. Light converges to form real images. Real images can be projected. virtual image An image formed behind a mirror. Virtual images are formed where light does not reach. concave mirror A spherical mirror which is curved towards the object. principal axis focal point focal length A line from the center of curvature of a mirror to the object. The point of focus of a mirror. “F” in the diagram. The distance from the focal point to the mirror. 1 1 1 = + f do di do, distance to object di, distance to image f, distance to focus FORMULA: mirror equations h d M= i = − i ho do spherical aberration ho, height of object hi, height of image M, magnification Image defect of spherical mirrors which does not allow parallel light rays to converge at the focal point. Produces a fuzzy image. magnification The factor of enlargement. convex mirror A spherical mirror which is curved away from the object. refraction The bending of a wave when it passes from one medium to another. optical density optical refraction The property of a transparent material that is an inverse measure of the speed of light through the material. The bending of light rays as they pass obliquely from one medium into another of different optical density. index of refraction FORMULA: Snell’s law The ratio of the speed of light in a vacuum to the speed of light of the medium in question. n1·sinθ1 = n2·sinθ2 n1, index of refraction of one material n2, index of refraction of second material θ1, angle of incidence θ2, angle of refraction lenses Transparent material with two nonparallel surfaces. Surfaces can be spherical, parabolic, cylindrical, or flat. diverging lens A lens which causes light rays to spread out from the focal point. converging lens A lens which causes light rays to spread in at the focal point. 1 1 1 = + f do di FORMULA: lenses chromatic aberration total internal reflection h d M= i = − i ho do do, distance to object di, distance to image f, distance to focus ho, height of object hi, height of image signs: hi is negative for an inverted image di is negative for a virtual image (same side of lens as the object) f is negative for diverging lens, positive for converging lens. Color distortion in an image produced by a lens, caused by the inability of the lens to bring the various colors of light to focus at a single point. M, magnification A refraction event that causes a “perfect” reflection inside of a material. critical angle interference diffraction An angle of incidence that will produce an angle of refraction of 90º. Any angle greater than the critical angle will cause total internal reflection. The interaction of two or more waves with each other interaction of two or more waves with each other. The bending of waves through an opening or around small objects. Since diffracted light interferes with itself, it was considered proof that light travels in waves. scattering A beam of light cannot be seen unless it is scattered by particles. lasers A light which produces a coherent beam of light in which all of the waves have identical frequency and wavelength and are in phase with each other. Nuclear Physics Unit The three subatomic parts of an atom, their charges and where they are located. a) proton b) neutron c) electron elements isotopes The positively charged atomic particle which is located in the center or nucleus of the atom. The number of protons designates the atomic number which determines the unique nature of the The atomic particle of no charge which is located in the center or nucleus of the atom. The neutron is believed serve a function of holding the nucleus together. The negatively charged particle which can be found in regions of high probability outside of the nucleus. A substance composed of atoms having an identical number of protons in each nucleus. There are 92 naturally occurring elements and 23 artificially, laboratory created elements. Atoms of the same atomic number or element but different numbers of neutrons. Some isotopes are stable and last indefinitely. Some are unstable and decay. ions Atoms which do not have the same number of protons and electrons and, therefore, have a charge. atomic number The number of protons in an atom. Also the number of the element. symbol: Z mass number The number of protons and neutrons in an atom. symbol: A atomic mass Weighed average of all the isotopes of an element. Blackbody Radiation. Objects will glow at colors which depend on their temperatures. The objects start to glow at the long wavelength colors (red) and continue to shorter wavelengths (ultraviolet) as the temperature increases. Objects stop moving to shorter wavelengths once they reach UV even if the temperatures increase. This is referred to as the “UV catastrophe”. Photoelectric effect. A metal surface emits electrons when light of certain frequencies shines on it. Planck’s formula energy = Planck’s constant * frequency E=n·h·f n can be 1, 2, 3, 4, …. Planck’s constant h = 6.63 x 10-34 J·s Historic Summary a) J.J. Thompson’s idea b) Rutherford’s idea c) Planck’s idea d) Einstein’s idea Discovered the electron and proposed the “plum pudding” model of the atom. Think of blue berry muffins with the electrons as blue berries. Rutherford shot alpha particles at gold foil. Most of the alpha particles went through but some bounced back which indicated a dense nucleus surrounded by empty space. This led him to propose the “planetary model” of the atom. Proposed energy is made-up of packets of energy (particles) called quanta. He did this to explain blackbody radiation. Einstein described the photoelectric effect as particles of light called photons bumping the electrons off. This led to the dual nature of light principle. e) Bohr’s idea Explained the spectra of atoms by using Planck’s quanta. Also proposed Bohr’s model of the atom. f) De Broglie’s idea All particles have wave properties. g) Schrödinger & Heisenberg’s idea Introduced uncertainty and probability to the study of the atom and the quantum mechanical model. strong nuclear force The force that holds the nucleus together. One of four forces of nature. mass deficit/binding energy The mass of the nucleus is less than the sum of its parts. This difference is the mass deficit. It is held as energy that holds the nucleus together. This is the binding energy. atomic mass unit to megaelectron volt conversion 931.494 mega-electron volts = 1 atomic mass unit Radioactivity Why do isotopes decay? Because they are unstable. parent Unstable isotopes before they decay. daughter Unstable isotopes after they decay and transmute into something else. half-life The time it takes for half of the original amount to decay. It is different for different isotopes. half-life curve No is the original amount of atoms. N is the current amount of atoms. k is the radioactive decay constant which is substance dependent. t is time. half-life formula N = N 0e radioactivity Spontaneous emission of energy or radiation. kt three types of radiation a) alpha particle, α 4 +2 2 He b) beta particle, β An electron or positron. A sheet of aluminum foil offers protection. c) gamma particle, ɣ A high energy photon. No mass and no charge. One inch of lead offers protection. , a helium nuclei. A sheet of paper offers protection. units of radioactive decay becquerel Unit of radioactive decay. 1 becquerel (Bq) = 1 decay/second curie Unit of radioactive decay. 1 currie (Ci) = 3.7 x 1010 decays/second nuclear decay series If the daughter nucleus is stable the decay process ends, if it is unstable the process continues. Nuclear Reactions nuclear fission Breaking an atom apart. chain reaction Neutrons released by fission can then cause more nuclei to fission. controlled chain reaction The process that is used in a nuclear power plant. uncontrolled chain reaction The process that is used in a nuclear bomb. nuclear fusion Smashing atoms together. radiometric dating The use of radioactive isotopes to determine the age of archeological and geological samples.