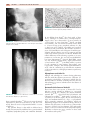
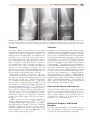
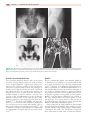
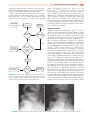
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
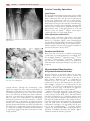
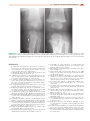
Download Chapter 37 - INFECTIOUS ARTHRITIS AND OSTEOMYELITIS
Traveler's diarrhea wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Anaerobic infection wikipedia , lookup
Onchocerciasis wikipedia , lookup
Clostridium difficile infection wikipedia , lookup
Chagas disease wikipedia , lookup
Gastroenteritis wikipedia , lookup
Dirofilaria immitis wikipedia , lookup
West Nile fever wikipedia , lookup
Trichinosis wikipedia , lookup
Sarcocystis wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Leptospirosis wikipedia , lookup
Marburg virus disease wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Neonatal infection wikipedia , lookup
Hepatitis C wikipedia , lookup
Schistosomiasis wikipedia , lookup
Oesophagostomum wikipedia , lookup
Hepatitis B wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
SECTION 5 Arthritis Related to Infection Chapter 37 INFECTIOUS ARTHRITIS AND OSTEOMYELITIS Ronald M. Laxer and Carol B. Lindsley The relation of infectious agents to arthritis is an area of great interest to the rheumatologist. Important discoveries have led to an understanding of the origin, pathogenesis, treatment, and cure of at least one infection-related arthritis—Lyme disease—and have given impetus to investigations of other possible arthrogenic infectious agents. Arthritis related to infection can be regarded as septic, reactive, or postinfectious.1 Septic arthritis occurs when a viable infectious agent is present or has been present in the synovial space. Although direct bacterial infection of the joint constitutes the most widely recognized form of septic arthritis, direct infection with viruses, spirochetes, or fungi also occurs. Reactive arthritis is a response to an infectious agent that is or has been present in some other part of the body, usually the upper airway, gastrointestinal tract, or genitourinary tract. By definition, viable infectious agents are not recoverable from the synovial space in patients with reactive arthritis, which may be regarded as an autoimmune disorder resulting from immunological crossreactivity between articular structures and infectious antigens. The reactive arthritis group merges pathogenically with diseases such as the spondylarthritides. Postinfectious arthritis may be considered a special type of reactive arthritis in which immune complexes containing nonviable components of an initiating infectious agent may be present in the inflamed joint. Lyme disease is discussed in Chapter 38, the reactive arthritides in Chapter 40, and poststreptococcal arthritis in Chapter 41. The precise relation of infection to arthritis is complex and by no means completely understood. As techniques for the demonstration of infectious organisms improve, the frequency with which they are detected in synovial fluid or membrane is increasing, lending authority to the suspicion that some or many of the chronic arthritides of children are related to infectious diseases. Perhaps many of the so-called reactive arthritides will be found to represent diseases in which living pathogens are present in the joint and are, by definition, septic. In some of the viral arthritides that fit the concept of reactive arthritis (i.e., joint disease follows the onset of the acute illness by days or weeks), viral antigen or living virus can be isolated from synovial fluid lymphocytes or membrane when appropriate techniques are used. The same has been true for Lyme disease, in which early attempts to demonstrate Borrelia were unsuccessful, although the organisms have since been demonstrated by silver stain in several different laboratories. The lesson implicit in all of these observations is that in chronic arthritides, which we currently consider aseptic, concerted investigations for infectious agents using the most powerful techniques of molecular biology may yet demonstrate the causative agent in the joint space. Although study of infectious agents, such as viruses, as possible initiators of some forms of arthritis in children has attracted much attention, it is important to remember that intraarticular and systemic bacterial infections remain the most important curable causes of arthritis in childhood. Septic Arthritis Epidemiology Septic arthritis of bacterial origin accounts for approximately 6.5% of all childhood arthritides.2 It has been suggested that its frequency might be increasing,3,4 although the retrospective reviews from Dallas5 and Memphis6 did not document an increase in incidence. In a 1997 report, 12.7% of 1158 patients with septic arthritis were children younger than 10 years old.7 559 560 SECTION 5 — ARTHRITIS RELATED TO INFECTION Sex Ratio and Age at Onset Septic arthritis is slightly less common in girls than in boys, who account for 55% to 62% of patients in reported series.8-10 Septic arthritis is found most often in the very young11 and the very old; it may occur in the neonate, is most common in children younger than 2-years-old, and diminishes in frequency throughout childhood.5,12 Familial and Geographical Clustering There does not appear to be a genetic predisposition to septic arthritis. Typical cases of presumed septic arthritis in which no pathogen is identified tend to occur in the summer and fall,4 but geographical clustering has not been reported. In spirochetal arthritis, such as Lyme disease, there are marked geographic and seasonal outbreaks. Etiology and Pathogenesis A wide range of microorganisms can cause septic arthritis in children; Staphylococcus aureus and nongroup A and B streptococci are most common overall.7,12-14 However, different organisms are more common at some ages and in certain circumstances (Table 37–1). Haemophilus influenzae type B had been the most common infection identified in children younger than 2-years-old, but vaccination of infants for H. influenzae has significantly decreased the frequency of infection with this organism.13,15-18 Streptococcus pneumoniae is a frequent cause of infection in children younger than 2 years old and is common in the older child.19,20 After a child reaches 2 years old, S. aureus is the most frequently occurring organism.13 Group A streptococci and enterococci account for a small proportion of all cases of septic arthritis in childhood and are most prevalent in the 6- to 10-year age group. Salmonella arthritis constitutes approximately 1% of all cases of septic arthritis, and it is commonly associated with sickle cell disease.21 Infection with Mycobacterium Table 37–1 Common microorganisms involved in septic arthritis and osteomyelitis Age Organisms Neonate Group B Streptococcus Staphylococcus aureus Gram-negative bacilli Staphylococcus aureus Streptococcus species Haemophilus influenzae Staphylococcus aureus Streptococcus pneumoniae Group A Streptococcus Kingella kingae (some areas) Staphylococcus aureus Streptococcus pneumoniae Group A Streptococcus Neisseria gonorrhoeae Infant Child Adolescent tuberculosis is an unusual cause of septic monarthritis in childhood. Other rare causes of infectious arthritis in children include Streptobacillus moniliformis (rat-bite fever), Pseudomonas aeruginosa, Bacteroides species, Campylobacter fetus, Serratia species, Corynebacterium pyogenes, Neisseria meningitis, Pasteurella multocida, and Propionibacterium acnes. Kingella kingae is emerging as an important pathogen in children with septic arthritis22-24 and may account for a significant portion of culture negative cases.25 Most infections with this organism occur in children younger than 5 years old, and 60% occur in children younger than 2 years old.26 In the neonate, S. aureus (40% to 50%) or group B Streptococcus (20% to 25%) are the most common causative organisms.5,9,27 Enterobacteriaceae, gonococcus, and Candida species are also significant pathogens in the neonate. Septic arthritis usually results from hematogenous spread from a focus of infection elsewhere in the body.28 Direct extension of an infection from overlying soft tissues (e.g., cellulitis, abscess) or bone (e.g., osteomyelitis)29 or traumatic invasion of the joint accounts for only 15% to 20% of cases. In the hips, shoulders, ankles, and elbows, the joint capsule overlies a portion of the metaphysis. As a result, if a focus of underlying osteomyelitis breaks through the metaphysis, it may enter the joint and result in septic arthritis. Joint damage results from several mechanisms. Proliferation of bacteria in the synovial membrane results in accumulation of polymorphonuclear (PMN) leukocytes and the inflammatory effects outlined in Chapter 4. Synovial fluid contains high levels of proinflammatory cytokines (tumor necrosis factor-α, interleukin-1β)30 that mediate cartilage damage by metalloproteinases.31 The ensuing damage to cartilaginous surfaces of the bone and the supporting structures of the joint may be severe and permanent if treatment is not urgently initiated. Although trauma or extraarticular infection preceding onset of septic arthritis is common in case histories, knowledge of the etiological significance of these factors is incomplete.32 In one series, upper respiratory tract infections preceded septic arthritis in approximately 50% of patients, and approximately one-third had received antibiotics within one week of onset.4 A history of a mild, nonpenetrating injury to the affected extremity was elicited in approximately one-third of patients. Intravenous (IV) drug users are at particular risk for septic arthritis of the sacroiliac and sternoclavicular joints, usually caused by gram-negative organisms.33 Other recognized risk factors include prosthetic joints, diabetes, alcoholism, recent intraarticular steroids, and cutaneous ulcers.34 Chronic inflammatory arthritis, such as juvenile idiopathic arthritis (JIA), may predispose to joint infection.35 Clinical Manifestations Septic arthritis is usually accompanied by systemic signs of illness (e.g., fever, vomiting, headache)36 and may be a component of a more generalized infection that might include meningitis, cellulitis, osteomyelitis, or pharyngitis.37 Joint pain is usually severe, and the infected joint and periarticular tissues are swollen, hot, and sometimes erythematous. Passive and active motion 37 — INFECTIOUS ARTHRITIS AND OSTEOMYELITIS 561 of the joint is severely, often completely, restricted (i.e., pseudoparalysis). Osteomyelitis frequently accompanies bacterial arthritis, and the presence of bone pain (as opposed to joint pain) should alert the examiner to this possibility. Other sites of hematogenous spread, although less common, are nonetheless important (Table 37–2).4,8,9 Geographical variation exists with up to 24% multisite involvement reported in some studies.39 Certain immune deficiencies, such as chronic granulomatous disease or acquired immunodeficiency syndrome (AIDS), may predispose to septic arthritis in multiple joints. Affected Joints Diagnosis The joints of the lower extremity are most commonly the sites of infection. Knees, hips, ankles, and elbows account for 90% of infected joints in children. Septic arthritis affecting the small joints of the hands or feet is rare (Table 37–3).5,9,10 Pyogenic sacroiliac joint disease can occur.38 A recent systematic review concluded that in the absence of positive cultures in either the synovial fluid or the blood, the overall clinical judgment of an experienced clinician is superior to laboratory or radiological investigations for the diagnosis of septic arthritis.40 Guidelines for the management of suspected septic arthritis have been published for adults and may be applicable to the older child as well.41 It is essential that every child with acute unexplained monarthritis undergo aspiration of the affected joint immediately, because septic arthritis continues to be associated with considerable morbidity and mortality.18,42,43 Synovial fluid examination is outlined in Table 37–4. If an anaerobic organism or mycobacterium is suspected, enriched culture medium and special anaerobic culture conditions are necessary. Children in whom septic arthritis is considered should also have cultures of blood and of any potential source of infection (e.g., cellulitis, abscess, and cerebrospinal fluid) performed. Rapid antigen latex agglutination tests for H. influenzae, group B and C streptococci, Neisseria meningitidis, and S. pneumoniae are available in most clinics. The polymerase chain reaction (PCR) has proved useful in detecting evidence of infectious agents in synovial fluid,44-47 and realtime PCR may be even more beneficial in the investigation of culture-negative septic arthritis.23,48 In a group of children with septic arthritis in whom the bacterial agent was identified,5 Fink and Nelson reported that synovial fluid was culture positive in 307 (79%) of 389 patients (Table 37–5).4,9,10 The remaining 21% had positive cultures from sites other than the joint: blood (10%), cerebrospinal fluid (3.8%), blood and cerebrospinal fluid (2.3%), and vagina (1.3%). One of five children with culture-positive septic arthritis had a negative synovial fluid culture but a positive culture from elsewhere, most often the blood. Initial inoculation of synovial fluid Multiple Infected Joints Although septic arthritis is most often a monarthritis, two or more joints are infected simultaneously or during the course of the same illness in a few children. In the large clinical experience reported by Fink and Nelson,5 septic arthritis was monarticular in 93.4% but affected two joints in 4.4%, three in 1.7%, and four in 0.5% of patients. Table 37–2 Extraarticular sites of infection in children with septic arthritis Nelson and Koontz8 Welkon et al4 Speiser et al9 Sites of Infection n = 117 (%) n = 95 (%) n = 86 (%) Osteomyelitis Meningitis Cellulitis, abscess Respiratory tract Middle ear Urine Genital tract Pericardium Pleura 12 4 19 4 - 12 4 20 - 26 11 9 9 3 1 1 1 1 Table 37–3 Frequency of infected joints in septic arthritis Fink and Nelson5 Infected Joint Knee Hip Ankle Elbow Shoulder Wrist PIP, MCP, MTP Other n = 591 (%) 40 23 13 14 4 4 1 1 Welkon et al4 Speiser et al9 Wilson and DiPaola10 Overall n = 95 (%) n = 86 (%) n = 61 (%) n = 833 (%) 46 25 15 5 4 5 30 29 17 11 2 1 10 - 29 40 21 3 3 1 1 39 25 14 12 4 3 2 1 MCP, metacarpophalangeal; MTP, metatarsophalangeal; PIP, proximal interphalangeal. 562 SECTION 5 — ARTHRITIS RELATED TO INFECTION into blood culture bottles may increase the yield of some organisms, especially Kingella kingae.24 Although an organism can be identified in one-third to two-thirds or more of patients by the culturing of all appropriate sites, no causative organisms are ever identified in approximately one-third of children with pyogenic arthritis.49 In these patients, the diagnosis of septic arthritis is based on a typical history and the demonstration of frank pus by arthrocentesis. Synovial Fluid Analysis The characteristics of the synovial fluid depend somewhat on the duration and severity of the disease and previous administration of antibiotics. Synovial fluid may appear normal, turbid, or grayish green with bloody streaks (see Table 37–4). The synovial fluid white blood cell (WBC) count is often markedly elevated, with 90% PMNs. Speiser and colleagues9 reported that synovial WBC counts in septic arthritis were less than 50,000/mm3 (50 × 109/L) in 15% of children, 50,000 to 100,000/mm3 (50-100 × 109/L) in 34%, and more than 100,000/mm3 (100 × 109/L) in 51%. Fink and Nelson5 found a relatively low WBC count (less than 25,000/mm3 or 25 × 109) in one-third of their patients. The protein content is high (more than 2.5 g/dL), and the glucose concentration compared with plasma glucose is usually low in septic arthritis, although it may be normal. A Gram stain identifies the organism in one-half of untreated patients but in only one-fifth of those who Table 37–4 Synovial fluid examination in a child with suspected septic arthritis Synovial fluid aspiration must be done under strictly aseptic conditions to minimize the risk of bacterial contamination. Septic arthritis is strongly suggested if: •Visual inspection finds cloudy, serosanguineous, or greenish fluid. •Cell count reveals elevated numbers of neutrophils (50,000 to 300,000/mm3) (50–300 ´ 109/L). •Synovial fluid viscosity is low. •Synovial fluid glucose is low (<30 mg/dL). •Lactate dehydrogenase level is high (>500 IU). •Gram stain is positive. Culture is positive for about 70% of those tested. have received antibiotics. A Gram stain provides rapid c onfirmation of bacterial infection and tentative identification of the organism (if the findings are positive), permitting rational antibiotic therapy. Special procedures such as counterimmunoelectrophoresis, latex agglutination, or evaluation by PCR may sometimes identify bacterial antigens in a culture-negative fluid (i.e., blood, urine, or cerebrospinal fluid). These techniques have the advantage of providing antigenic identification much more rapidly than cultures, but they do not provide antibiotic sensitivities. Blood Studies At least two blood cultures should always be performed for a child suspected of having septic arthritis. An elevated WBC count with a predominance of PMNs and bands and a markedly elevated erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) level—although of limited help in specific diagnosis—provide a baseline whereby the efficacy of subsequent treatment can be judged. CRP is a better predictor than ESR. If the CRP value is less than 1 mg/dL, the likelihood that the patient does not have septic arthritis is 87%.50 Concentrations of other acute phase reactants are usually increased, but provide no additional useful information. Radiological Examination A number of imaging techniques may be helpful in evaluating a child with septic arthritis (Fig. 37–1).51-55 Plain radiographs are not diagnostic but might be helpful in excluding other disorders. They may show an underlying osteomyelitis as the etiology of the septic arthritis and may demonstrate only increased soft tissue and capsular swelling. Juxtaarticular osteoporosis reflects inflammatory hyperemia and is evident within several days after onset of infection. Cartilage loss and narrowing of the joint space develop as the disease progresses. These changes are followed by marginal erosions and eventually by ankylosis (Fig. 37–2).54 Computerized tomography (CT) and especially magnetic resonance imaging (MRI) are additional confirmatory techniques. In the hip, accumulation of fluid within the joint displaces the gluteal fat lines laterally, or the obturator sign (i.e., displacement of the margins of this muscle medially) may be present. Traction applied to the leg during the radiographic procedure normally induces a radiolucent outline of the femoral head; this is referred to as the Table 37–5 Laboratory confirmation of septic arthritis in children Laboratory Confirmation Confirmed diagnosis (culture positive) Positive synovial fluid Gram stain Positive synovial fluid culture Positive blood culture *Depends †Depends Fink and Nelson5 Welkon et al5 Speiser et al9 Wilson and DiPaola10 n = 591 (%) n = 95 (%) n = 86 (%) n = 61 (%) 66 33 79 33 64 − 84 46 84 19 to 54* 36 to 70* 46 92 71 to 80† 41 on prior administration of antibiotics. on procedure (aspiration = 80, arthrotomy = 71). 37 — INFECTIOUS ARTHRITIS AND OSTEOMYELITIS vacuum” phenomenon. This lucency does not occur in “ the presence of increased intraarticular fluid.54 Computerized tomography As MRI is much better for the evaluation of soft tissues, the role of CT is limited, except for the evaluation of sacroiliac and sternoclavicular joints. It is also helpful to guide aspirations and biopsies. Plain radiographs Suspected osteomyelitis Yes Osseous abnormality No Reassess; evaluate for osteomyelitis Hip sonography Joint effusion No Yes Aspiration, therapy MR imaging for possible osteomyelitis and abscess No Response in 48 hr Ultrasonography Detection of joint fluid by ultrasound helps guide fluid aspiration. The ultrasonic detection of an effusion in the hip of a child treated for osteomyelitis of the femur indicates the presence of septic arthritis of the joint.54 Color Doppler may show increased capsular vascularity. Radionuclide Scans During the first few days of disease, when plain radiographs show only soft tissue changes, 99mTc-MDP scans reflect hyperemia of the infected area on blood flow studies and increased uptake of the isotope on both sides of the joint.55 Occasionally, decreased uptake may occur if significant accumulation of intraarticular fluid impedes local blood flow. This technique is useful in the early detection of joint or bone inflammation or infection, but it does not differentiate the two with certainty and cannot differentiate septic arthritis from synovitis from other causes (e.g., JIA). It is helpful in differentiating septic arthritis from osteomyelitis and soft tissue infection and in the detection of multifocal joint infections.56 Radionuclide scans with gallium 67 or the patient’s indium 111-labeled granulocytes or monoclonal antibodies may be helpful but are not routinely needed. 2-deoxy-2 [13F] fluoro-D-glucose (FDG)-positron emission tomography (PET)/CT may have an important role in the diagnosis of difficult cases owing to the fact that PET is a relatively fast, whole-body imaging modality and can be used to find infectious foci outside of the bone or joint.56 It may be particularly helpful for infections of the vertebrae. Magnetic Resonance Imaging Yes Follow-up radiographs FIGURE 37–1 Flowchart of recommendations for evaluating septic arthritis in children. (Adapted from Jaramillo D, Treves ST, Kasser JR et al: Osteomyelitis and septic arthritis in children: appropriate use of imaging to guide treatment, AJR Am J Roentgenol 165:399-403, 1995.) A 563 Delineation of soft tissue structures by MRI is superior to that provided by CT.53,54 Changes may be seen as soon as 24 hours following infection. Synovial enhancement is detected in virtually all patients. Signal-intensity alterations in the bone marrow are characteristic but not diagnostic of septic arthritis (i.e., low intensity on fat-suppressed, gadolinium-enhanced, T1-weighted spinecho images, and high signal intensity on fat-suppressed, T2-weighted, fast spin-echo images).57 Articular cartilage and growth cartilage are depicted along with other B FIGURE 37–2 A, Questionable joint space widening of the right hip of a 10-year-old girl with fever and an irritable hip. B, Repeat x-ray film taken 20 days later demonstrated epiphyseal demineralization and erosion (arrow). 564 SECTION 5 — ARTHRITIS RELATED TO INFECTION fibrous structures, muscle, blood vessels, and synovial fluid. An abnormal collection of fluid or debris, often displacing the joint capsule, eroding into other tissues, or in children, leading to subluxation, supports the possibility of septic arthritis. Fat suppressed, gadolinium-enhanced MRI is 100% sensitive and 79% specific for the diagnosis of septic arthritis in adults.58 Treatment The child with septic arthritis requires hospitalization and consultation with an orthopaedic surgeon and specialist in infectious diseases. Nonsteroidal antiinflammatory drugs (NSAIDs) may be used to help minimize the effects of inflammation, to control fever, and to contribute to pain relief. IV dexamethasone was shown to reduce duration of symptoms and minimize joint damage in a randomized, double-blind study, but is rarely, if ever used.59 A clinical practice guideline for the management of septic arthritis in children has been proposed.60 It was demonstrated that this approach was effective in minimizing bone scans, in minimizing the rate of joint drainage, in accelerating the change to oral antibiotic administration, and in shortening the duration of hospital stay. There were no differences in outcomes such as readmission to the hospital, recurrence of infection, or the development of residual joint damage. Antibiotics In a child with septic arthritis, IV antibiotics should be administered as promptly as possible. The choice of antibiotic depends on the presence of predisposing factors, the age of the child, and the organisms suspected because of the Gram stain or rapid antigen detection tests (although it is hazardous to narrow initial treatment based solely on these results because either can be wrong). If the Gram stain and results of rapid antigen detection are negative or not available, an approach based on age outlined in Table 37–6 is suggested.38 The demonstration of an organism or antigen may support or contradict the generalizations outlined in this table and should influence the physician in selection of the initial antibiotic treatment.42,61 It is prudent to monitor intravenous antibiotic efficacy with serum bactericidal titer determinations at frequent intervals initially and at follow-up for compliance, especially if home therapy is instituted. After satisfactory control of the infectious process with IV antibiotic a dministration is achieved (including resolution of fever, significant decrease in pain, improvement in range of motion, and falling laboratory measures of inflammation), treatment by the oral route in the hospital or on an outpatient basis may be appropriate.62-64 Home IV antibiotic programs may also be effective in reducing the hospital stay. Such programs should be undertaken only after careful consideration and consultation with an expert in pediatric infectious disease. If the cultures are negative, IV antibiotics should be continued for a minimum of 21 days.1 If the child’s clinical state is improving (i.e., temperature returning to normal, pain diminishing, range of motion improving) and the WBC count and ESR are falling, the initial antibiotics should be maintained. If the patient does not appear to be responding, additional IV antibiotic coverage should be instituted. Because of various patterns of antibiotic susceptibility and resistance, guidelines regarding antibiotic choice and duration of treatment are constantly changing, and the physician is urged to review the most current recommendations. This is especially important with the rise in the incidence of community-acquired Methicillin-resistant Staphylococcus aureus (CA-MRSA), which requires specific antibiotic management. Aspiration and Drainage The usefulness of repeated aspiration and drainage of an infected joint has been hotly debated. There is no dispute that an initial diagnostic arthrocentesis must be performed. Any joint that appears to be under pressure from an effusion can probably benefit from aspiration, if only for pain relief. Studies of the importance of repeated aspirations under other circumstances, however, have failed to show a consistent benefit. Similarly, open drainage is worse than closed needle aspiration (except for specific joints such as the hip and shoulder) and is attended by significantly increased morbidity. Irrigation of the joint at the time of aspiration has no demonstrated additional benefit. Occasionally, arthroscopic examination is indicated. Intraarticular administration of antibiotic is unnecessary because therapeutic synovial fluid antibiotic levels are readily achieved,65 and it may induce chemical synovitis in the infected joint. Special Cases Neonatal Septic Arthritis Table 37–6 Recommended empirical antibiotic therapy by age Age Recommended Empirical Antibiotic Therapy Neonate Cloxacillin + gentamicin OR cloxacillin + cefotaxime Cefotaxime + cloxacillin OR cefuroxime Cefazolin OR cloxacillin OR clindamycin Ceftriaxone OR cefixime + azithromycin Infant Child Adolescent Alternatives appropriate to local epidemiology and patient comorbidities (e.g., immunocompromise) may be needed. Vancomycin should be included in patients with high risk for MRSA. In addition to S. aureus, group B Streptococcus and gramnegative bacteria can be the offending organisms in the neonate.66 They are rare but potentially very serious infections in this age group, may have a subtle presentation, and can occasionally be bilateral. They are much more likely to occur in association with osteomyelitis than in older children. Most affected newborns show no fever, toxemia, or leukocytosis.67,68 Any infant who has swelling in the region of the thigh or holds the leg flexed, abducted, or externally rotated must be investigated promptly. Problems in early recognition of disease undoubtedly contribute to the often disastrous outcome of this involvement.69 37 — INFECTIOUS ARTHRITIS AND OSTEOMYELITIS Septic Hip Joint Septic arthritis of the hip is such an important problem that it merits special attention.70,71 Because the risks of missing this diagnosis are so high, there must a very low threshold for hip aspiration to establish the diagnosis.72 The femoral head is intracapsular, and the arterial supply passes through the ligamentum teres through the intracapsular space. Increased intracapsular pressure can therefore interrupt the blood supply to the femoral head, with disastrous consequences to its viability and to the subsequent development of avascular necrosis.73 Meta physeal osteomyelitis readily leads to septic arthritis of the hip joint in the infant because nutrient blood vessels pass from the metaphysis through the epiphyseal growth plate and terminate in the distal ossification center. Septic arthritis of the hip joint is most common in infants and very young children; 70% of patients are 4-years-old or younger.74 The typical clinical picture is that of an infant or young child who may have an unexplained fever; is irritable; and refuses to move an extremity, bear weight, or walk. Any movement of the hip is extremely painful; and the affected leg is held in a position of partial flexion, abduction, and external rotation at the hip. Occasionally, the child has lower abdominal pain or tenderness, sometimes with paralytic ileus. In very young or premature infants, a number of risk factors predispose to septic hip joint arthritis.75 In a study of septic arthritis of the hip of 16 infants younger than 4 weeks old, 11 were premature, 7 had an umbilical catheter, and 12 had septicemia. In contrast, of 13 children with septic arthritis between the ages of 1 month and 3 years, none was premature or had an umbilical catheter, and only five were septicemic. A high frequency of preceding or accompanying osteomyelitis of the femur or pelvis has been observed. The association of septic arthritis of the hip and femoral venipuncture has been recorded76 and may account in part for the high frequency of arthritis of this site in the premature neonate. Management of septic arthritis of the hip requires open drainage to minimize intraarticular pressure.77,78 Traction and immobilization for the first two to three days of treatment provide pain relief, but should be followed by passive and then active physiotherapy to prevent loss of range of motion. Prognosis is guarded even with the best treatment, especially in the neonate. The anatomy of the shoulder joint is not unlike that of the hip with respect to vascular supply. Septic arthritis of this joint, although rare, should be treated similarly.79 Gonococcal Arthritis In reported series of septic arthritis in children and adolescents, disease caused by N. gonorrhoeae appears to be uncommon. When it occurs, it is most common in the adolescent, although it occasionally occurs in the neonate in association with disseminated infection.80 It is more common in girls than in boys and is particularly likely just after menstruation or with pregnancy.81 Gonococcal arthritis usually develops in patients with primary asymptomatic genitourinary gonorrhea or with a gonococcal infection of the throat or rectum. The patient presents with a systemic illness characterized by fever and 565 chills.82 A vesiculopustular rash, sparsely distributed on the extremities, commonly yields organisms on culture or Gram stain of the smear. Gonococcal arthritis may have an initial migratory phase and may be accompanied by tenosynovitis. In contrast to most patients with septic arthritis, those with gonococcal arthritis may present with a purulent arthritis of several joints. For a patient with suspected gonococcal arthritis, it is important to culture samples from the genital tract, throat, rectum, and any vesicles in addition to the affected joint. Special culture media with chocolate agar will help in isolating the organism. The possibility of sexual abuse should be considered and appropriately investigated. Tuberculous Arthritis Tuberculous arthritis is seldom encountered in North America or Europe, although its frequency may be increasing because of immunosuppressive therapy, drugresistant strains of tuberculosis, and the human immunodeficiency virus (HIV) epidemic.83-85 Tuberculous arthritis is by no means rare in other parts of the world. Typically, arthritis arises on a background of pulmonary tuberculosis as indolent, chronic monarthritis, often of the knee or wrist, that eventually results in extreme destruction of the joint and surrounding bones. Rarely, it manifests as acute arthritis.86 Al Matar and colleagues87 have observed two young children with tuberculous monarthritis that mimicked oligoarticular JIA. They were unresponsive to NSAIDs and intraarticular corticosteroids. One had a history of exposure, but the other child had no identifiable contact with tuberculosis. Joint infection occurs by hematogenous dissemination of the organism from adjoining osteomyelitis. Pott disease is a consequence of vertebral osteomyelitis (Fig. 37–3). Tuberculous dactylitis may occur with cystic expansion and destruction of bone (i.e., spina ventosa) (Fig. 37–4).88 A family or environmental history of pulmonary tuberculosis and a positive purified protein derivative (Mantoux) skin test result suggest the possibility of tuberculous arthritis. Although synovial fluid cultures are positive in approximately three-fourths of patients, synovial membrane biopsy and culture are preferred and confirm the diagnosis in almost all patients (Fig. 37–5). The synovial WBC count is classically less than 50,000/mm3 (50 × 109/L), with a high proportion of mononuclear cells. Genus-specific PCR is invaluable in the diagnosis.46 Rarely, a polyarthritis accompanies tuberculosis (i.e., Poncet disease); it probably represents a reactive arthritis because culture of the inflamed joints fails to demonstrate tubercle bacilli.89,90 Leprosy can result in articular changes and inflammatory disease, including polyarthritis and subcutaneous nodules.91 Clinical differentiation from JIA may be difficult, especially if the possibility of leprosy is not considered in a nonendemic area.92 Mycobacterium leprae is not always easily identified in synovial biopsies.93,94 Arthritis Associated with Brucellosis Human Brucella infections are reported primarily from European,95,96 Israeli,97 and South American98,99 clini cians with a substantial number of patients having arthritis. Cases in North America are more likely to have 566 SECTION 5 — ARTHRITIS RELATED TO INFECTION FIGURE 37–5 Synovial biopsy specimen of chronic inflammation in tuberculous joint disease in which a giant cell (arrow) is indicated. FIGURE 37–3 Pott disease of the vertebral column in an adolescent boy with pulmonary tuberculosis produced destruction of the disk space and vertebral end-plate erosion (arrow). In 88 children from Israel,97 the classic triad of fever (91%), arthralgia or arthritis (83%), and hepatosplenomegaly (63%) was characteristic of most patients. In a large series of cases from Peru,98 almost one-third were children, and one third had arthritis. In the birth to 15-year-old age group, peripheral arthritis of a hip or knee was most common. Spondylitis and sacroiliac arthritis became predominant after children reached 15 years old. Gomez-Reino and colleagues96 found that periarthritis without effusion was most common and that small joints and the spine were not affected. Whether this reflects differences in the infecting organism or in ascertainment is not known. No association with HLA-B27 has been demonstrated.95 Synovial fluid WBC counts are only modestly elevated with a slight predominance of mononuclear cells.98,102 Joint fluid culture is positive for the organism in some patients. Tetracyclines, aminoglycosides, rifampin, and trimethoprim-sulfamethoxazole, often in combination, provide effective treatment of the acute infection, although permanent sequelae may result.96,99 Mycoplasma and Arthritis Myalgia and arthralgia are common during pulmonary infection with Mycoplasma pneumoniae. Objective oligoarticular, polyarticular, or migratory arthritis has also been described.103 Sensitive screening tests may uncover Mycoplasma as a cause of arthritis even in the absence of pneumonia.104,105 Bartonella Infection and Arthritis FIGURE 37–4 Advanced osseous destruction occurred in the foot of a child with tuberculous dactylitis (i.e., spinal ventosa). been acquired elsewhere.100 The species most frequently implicated are Brucella melitensis96,98,99 and, less commonly, Brucella canis.101 Unpasteurized milk is a source of infection. The systemic illness is often mild in children but is usually characterized by undulant fever, gastrointestinal complaints, lymphadenopathy, and sometimes dermatitis. There have been rare reports of Bartonella henselae infection causing arthritis in children (i.e., cat-scratch disease).106-108 In two children, the disease mimicked systemic JIA107,108; a third child had polyarthritis and subcutaneous nodules.106 Arthropathy occurred in 3% of patients in an Israeli registry. It was characterized by large- and medium-sized mono-, oligo-, or polyarthritis (most commonly symmetric oligoarthritis), which was debilitating. Despite cat-scratch disease being a disease of children and adolescents, in this series no patient under 20 years old had joint involvement. The arthropathy usually occurred concurrent with the lymphadenopathy. Erythema nodosum was more common in those with than without arthropathy.109,110 37 — INFECTIOUS ARTHRITIS AND OSTEOMYELITIS Arthritis in Immunocompromised Patients Chronic inflammatory arthritis in patients with a primary immunodeficiency is discussed in Chapter 42. Typical septic arthritis has been reported infrequently in immunodeficient children.111 Mycoplasma is the most common cause of severe chronic erosive arthritis in patients with congenital immunodeficiency syndromes112 and has been recovered from joints of patients with AIDS.113 Ureaplasma urealyticum has been identified in patients with agammaglobulinemia.114 Candida albicans is occasionally responsible for arthritis in immunosuppressed patients.114,115 Course of the Disease and Prognosis The outcome in septic arthritis is somewhat guarded because, even with early and appropriate antibiotic treatment, permanent damage is common. The child usually recovers from the acute illness, but with the passage of time, reduction in range of motion, pain, and eventually degeneration of the surfaces of the affected joint may require surgical intervention. It is estimated that residual dysfunction occurs in 10% to 25% of children, although the changes (e.g., limited joint mobility, joint instability of chronic subluxation) may not be apparent until years later.4 Recently, a simplified radiographic classification system has been developed to determine prognosis and to guide surgical management of the sequelae of septic arthritis of the hip.116 Osteomyelitis Although osteomyelitis, like septic arthritis, is most often encountered and treated by specialists in orthopaedics and infectious diseases, its frequent association with septic arthritis and the diagnostic problems that it presents require that it be included in this discussion.3,63,117-122 Definition and Classification Osteomyelitis is an intraosseous infection with bacteria or, rarely, fungi. It is classified as acute, subacute, or chronic. Acute osteomyelitis is of recent onset and short duration. It is most often hematogenous in origin but may result from trauma such as a compound fracture or puncture wound. It can be metaphyseal, epiphyseal, or diaphyseal in location. Subacute osteomyelitis is of longer duration and is usually caused by less virulent organisms. Chronic osteomyelitis results from ineffective treatment of acute osteomyelitis and is characterized by necrosis and sequestration of bone. Epidemiology Acute osteomyelitis is somewhat less common than acute septic arthritis. An incidence of 16.7 cases per year was reported from an institution at which acute septic arthritis occurred at a rate of 28.4 cases per year.5 However, it may be more common than septic arthritis in developing countries of the world.123 Although its incidence may be 567 declining in many parts of the world, that is not necessarily the case everywhere.124 It occurs twice as often in boys as in girls5,123 and is more common in younger children. It can occur in the neonate.66 Etiology and Pathogenesis S. aureus (50% to 80%) and the group A streptococci (5% to 10%) are the predominant organisms at all ages.5,125 Recently, CA-MRSA has emerged as an important pathogen.6,126,127 Up to 15% of children with CAMRSA that carry the genes encoding Panton-Valentin leukocidin (pvl) have multiple sites of infection.128 These organisms are also associated with chronic osteomyelitis. Even before specific immunization, H. influenzae seldom caused osteomyelitis (2% to 10%) and should now be even less common.16,129 In certain circumstances, specific or unusual organisms (15%) are found. For example, infection of the calcaneus or other bone in the foot associated with a puncture wound through athletic footwear is likely caused by P. aeruginosa.28,32,130,131 Osteomyelitis caused by S. pneumoniae19,132 usually occurs in children with associated diseases such as sickle cell anemia,133-135 asplenia,119,136 or hypogammaglobulinemia,120,137 although it has been observed in young infants without underlying disease.138 Salmonella osteomyelitis is a complication of sickle cell anemia but also occurs in normal children.139 In the neonate, group B streptococci,140,141 gram-negative organisms,142-144 and Candida in addition to S. aureus are all potential causes of osteomyelitis. B. melitensis uncommonly results in osteomyelitis, but when it does, it has a predilection for the vertebral bodies.101 Tuberculous osteomyelitis may take various forms and may mimic chronic pyogenic disease, Brodie abscess, tumor, or other types of granuloma.145-147 B. henselae (the organism of cat-scratch disease) has been identified as the causative agent in a few patients with osteomyelitis.148-150 Clinical Manifestations Fever, severe bone pain, and tenderness with or without local swelling should suggest the possibility of acute osteomyelitis. Although a history of prior trauma is elicited in approximately one-third of young patients, its significance is uncertain. In the infant, fever may be minimal, and localization of the pain may be difficult on physical examination.151 Pseudoparalysis of a limb is often evident. The examiner may find clinical evidence of a preceding systemic infection. The site of the bone infection is usually metaphyseal, and bony tenderness is elicited by pressure near or over the infected area. There may also be an area of overlying cellulitis, especially in the infant, in whom the thin cortex allows pus to erode into the periosteal structures. The presence of a joint effusion adjacent to the site of bone infection may reflect septic arthritis or a sterile noninflammatory “sympathetic” effusion.152 Osteomyelitis in children has a predilection for the metaphysis of rapidly growing bone. Many explanations have been suggested for this tendency. The anatomical differences in vasculature in this area in children and its easily compromised blood supply may in part explain the clinical observation (see Chapter 2). In one anatomical model, 568 SECTION 5 — ARTHRITIS RELATED TO INFECTION Table 37–8 Table 37–7 Brodie abscess Affected sites in septic osteomyelitis Bone Tibia Clavicle Fibula Spine Femur Metatarsus, metacarpus, phalanx Radius Pelvis Humerus Ulna Sternum Mandible Scapula Rib Talus Calcaneus Osteomyelitis* (%) 25 <1 6 1 27 4 4 6 11 2 <1 <1 <1 <1 1 6 *Data from Fink CW, Nelson JD: Septic arthritis and osteomyelitis in children, Clin Rheum Dis 12:423, 1986, and from Cole WG, Dalziel RE, Leitl S: Treatment of acute osteomyelitis in childhood, J Bone Joint Surg Br 64: 218, 1982. bacteremia and, in some cases, preceding microtrauma were sufficient to initiate disease.153 The bones of the lower extremity are affected in two-thirds of patients; those of the upper extremity account for approximately 25%, but those of the skull, face, spine, and pelvis are the sites of infection in fewer than 10% (Table 37–7).5,123,154-156 Less than 10% of children have two or more simultaneously infected bones; in some cases, five or more bones are involved as part of a severe septicemic illness, usually caused by staphylococci. This type of involvement must be distinguished from chronic recurrent multifocal osteomyelitis (see Chapter 44). Acute osteomyelitis can be associated with the development of deep-vein thromboses (DVT).157 In a series of 35 patients from Dallas who had osteomyelitis involving the proximal humerus, femur, proximal tibia or fibula, pelvis, or vertebrae, 10 had evidence of DVT based on imaging studies, although it was only symptomatic in one patient. In two patients, the DVT was related to a central catheter placed for long-term antibiotic therapy, where as in the remaining eight, the DVT occurred in veins adjacent to the site of infection. The authors attributed the DVTs to the inflammatory response leading to localized endothelial damage and activation of the coagulation cascade. Compounding factors include the local edema, venous compression and immobility in patients with lower extremity involvement. Organisms with the pvl gene were more likely to be associated with DVTs.158 Subacute Osteomyelitis Compared with acute osteomyelitis, patients with subacute osteomyelitis usually have a longer duration of illness (more than two weeks), experience less pain, often have no fever, and have frequently taken a trial of antibiotics. Characteristic Findings or Procedures Symptoms Pain may be severe; child awakens at night. Evidence of a penetrating injury of hematogenous spread First week: soft tissue swelling Second week: metaphyseal osteolytic lesion with surrounding sclerosis Sterile joint effusion; curettage samples and cultures may be negative. Immobilization, nonsteroidal antiinflammatory drugs, antibiotics Signs Investigations Treatment Laboratory changes are less common, but radiographs are usually abnormal and may be confused with Ewing sarcoma or osteoid osteoma.124 Brodie abscess, a unique form of subacute osteomyelitis, is usually of staphylococcal origin and may develop after a penetrating injury or by hematogenous spread of an infection to the metaphysis. It is characterized clinically by localized soft tissue swelling and tenderness with marked pain that may awaken the child at night (Table 37–8). Radiographs demonstrate only soft tissue swelling in the first week, but metaphyseal osteolytic lesions are evident by the second week of the illness. They are most common in the proximal or distal ends of the tibia (Fig. 37–6).159,160 Culture of the abscess may be negative. Treatment includes IV antibiotics, an NSAID, and immobilization. Chronic Osteomyelitis Chronic osteomyelitis take places when symptoms occur for longer than three months and develops in states of impaired host or antibiotic resistance. Reduced blood flow leads to the formation of a sequestrum, which may be surrounded by a sleeve of periosteal new bone (involucrum) and represents inadequate treatment. This in turn requires surgical excision. Complications of chronic osteomyelitis include growth plate arrest or stimulation, avascular necrosis, pathological fracture, septicemia, and amyloidosis.124 Neonatal Osteomyelitis Neonatal osteomyelitis merits special consideration.67 Up until a child reaches the age of approximately 18-months-old, metaphyseal blood vessels can cross over the open physis into the epiphysis, permitting infection to move across the growth plate. The thin cortical bone of newborns allows infection to spread rapidly into the subperiosteal region, and the relative immune deficient state of the newborn allows for rapid spread. The lack of a systemic inflammatory response often leads to a delay in diagnosis, and involvement of more than one site is common. Although S. aureus remains the most common organism responsible for neonatal osteomyelitis, especially in newborns with central lines, group B streptococci, gram-negative bacteria, and Candida albicans may also be responsible. 37 — INFECTIOUS ARTHRITIS AND OSTEOMYELITIS A 569 C B FIGURE 37–6 Brodie abscess is revealed in radiographs of the knee of a 16-month-old boy with acute hematogenous osteomyelitis inadequately treated one month earlier. A, Central sequestration with a surrounding, ill-defined lytic margin. The patient was appropriately treated with antibiotics at this stage. B, One month later, the sequestrum has been removed by osteoclasts. C, One month later, the radiograph shows a well-defined lesion with sclerotic borders. (A to C, courtesy of Dr. B. Wood.) Diagnosis Treatment As in septic arthritis, it is essential that every reasonable attempt be made to identify the organism and determine its antibiotic susceptibility.119 A high index of suspicion for this diagnosis must be maintained in any child with unexplained pain, fever, or lack of use of an extremity. Aspiration of subperiosteal pus is the diagnostic procedure of choice and, together with cultures of the blood, synovial fluid, or an infected wound, should yield an organism in approximately 70% to 80% of cases. Blood cultures alone are positive in 30% to 50% of infants and children.161 A bone biopsy may be desirable or necessary if other sites of culture prove negative. The elevated WBC count and ESR are nonspecific and provide little help with diagnosis; they are useful in assessing effectiveness of therapy. Radiographic evaluation may delineate soft tissue swelling very early, but osteoporosis is not evident until days 10 to 14, and diagnostic findings may not be clear until days 10 to 21 (Fig. 37–7).52 Radionuclide scanning (i.e., technetium 99m polyphosphonate or diphosphonate) provides a sensitive if nonspecific method for the early detection of increased blood flow and uptake in the infected bone (see Fig. 37–7C).28,55,162 A bone scan is particularly helpful in localizing osteomyelitis in the neonate or infection of the axial skeleton and in searching for subclinical areas of infection in multifocal osteomyelitis. A positive result is not necessarily diagnostic of osteomyelitis, but a negative scan is unlikely for a child with bacterial osteomyelitis, except in the very early stages of the illness. MRI is superior to other modalities in identifying changes in the marrow (see Fig. 37–7D).38,163-165 T1- or T2-weighted MR images, fat suppression, and gadolinium enhancement can confirm a focal area of increased inflammatory exudate (i.e., protons or water). A major advantage of MRI in early disease over plain radiographs, ultrasonography,166-168 or CT is the delineation of soft tissue or subperiosteal pus (Fig. 37–8).169 In the absence of specific indications to the contrary,64 the initial antibiotic choices in the treatment of acute osteomyelitis should be effective against MRSA (see Table 37–6). Screening of S. aureus for inducible clindamycin resistance with the D-test helps determine whether the patient should be treated with clindamycin or vancomycin.170 Linezolid may become an important oral agent in the initial treatment and in the treatment of clindamycin-resistant MRSA, although studies to date are limited.171 IV antibiotics for four to six weeks have been traditionally recommended with subsequent oral coverage if appropriate. Because of the complications that may occur from central venous catheters required to maintain IV access174 recent recommendations have included a shortened course of IV treatment.169,172,173 Surgical treatment, which should be kept to a minimum,175 includes drainage of subperiosteal and soft tissue abscesses and débridement of associated lesions. Surgery is often needed for subacute and chronic osteomyelitis. Immobilization of the extremity for relief of pain is often necessary; otherwise, weight-bearing may be permitted as tolerated by the patient. Course of the Disease and Prognosis The most dreaded complications of acute osteomyelitis are chronic osteomyelitis and impaired bone growth.175 Chronic osteomyelitis should be suspected in a child whose systemic symptoms have responded slowly or incompletely to antibiotics or in whom there is a late recurrence of pain at the affected site. Differential Diagnosis and Related Disorders Both chronic recurrent multifocal osteomyelitis and synovitis, acne, pustulosis, hyperostosis, and osteomyelitis syndrome are discussed extensively in Chapter 44. 570 SECTION 5 — ARTHRITIS RELATED TO INFECTION A C B D FIGURE 37–7 A, Anteroposterior radiograph of the pelvis of a 14-year-old body with fever and an irritable left hip. This x-ray film (A) and close-up film of the left hip (B) are normal. C, Bone scan documents increased uptake of technetium 99m in the area of the left proximal femur (arrow). D, Magnetic resonance imaging demonstrates an increased marrow signal in the same area of the left hip (arrow), which indicates acute osteomyelitis. Arthritis Associated with Acne Diskitis The association between arthritis and acne has been noted for decades. Most patients are male and have onset of musculoskeletal complications during adolescence. The syndrome includes severe truncal acne followed in several months by fever and arthralgia or arthritis, most often involving hips, knees, and shoulders. Myopathy may also accompany the disorder (see Chapter 24).176 It is possible that this syndrome is another example of reactive arthritis, or it may even be part of the autoinflammatory group of diseases. Although arthritis lasts for only a few months in some patients, recurrences over many years have been documented.177,178 Treatment with NSAIDs and with antibiotics for control of the acne is indicated. Treatments for acne may also be associated with arthritis, such as minocycline-induced autoimmune phenomena179 and isotretinoin causing an acute arthritis.180-183 Infliximab for the treatment of arthritis has been reported to cause acne.184 There is considerable dispute about whether diskitis is an infectious process. Infection of an intervertebral disk space from osteomyelitis of an adjoining vertebral body is rare.185 However, acute diskitis unassociated with vertebral osteomyelitis is a self-limited inflammation of an intervertebral disk that may be caused by pathogens of low virulence, although bacteria or viruses are seldom recovered by aspiration. S. aureus and Enterobacteriaceae or Kingella organisms are responsible in some patients. Diskitis occurs throughout childhood, but one-half of the cases manifest before the patient reaches 4 years old (peak age, 1- to 3-years-old).186,187 The sex ratio is approximately equal, although one review observed that diskitis occurred more frequently in girls.188 Clinical signs may be subtle. Diskitis is characterized by vague back pain and stiffness, often resulting in a characteristic tripod position during sitting or other unusual posturing.189 The child, who usually has a low-grade fever, often refuses to walk, stand, or bend over; and may 37 — INFECTIOUS ARTHRITIS AND OSTEOMYELITIS complain of abdominal pain. Palpation of the spine produces well-localized tenderness, usually in the lower lumbar region. The ESR is usually moderately elevated. Plain radiographs of the affected area often appear normal until late in the disease (Fig. 37–9). A technetium 99m bone scan is valuable diagnostically (Fig. 37–10). The L4-L5 interspace is most often affected Suspected osteomyelitis Plain radiographs (44%), followed by L3-L4 (37%), L2-L3 (7%), and L5-S1 (6%).186-191 The cervical spine may also be involved. In one study, disk space narrowing occurred in 82% of children, and a bone scan was positive in 72%.192 MRI may be valuable in differentiating infection from other conditions, including idiopathic disk calcification (Fig. 37–11).193-195 Aspiration of the disk space or disk biopsy should not be routinely necessary. Immobilization provides symptomatic relief. If bacterial infection is suspected, IV antibiotics should be instituted until results of blood cultures are available. Reassess Whipple Disease Negative Osseous abnormality No Whipple disease, first described in 1907,196 is rare in childhood; approximately five afflicted children younger than 15 years old have been reported.197 It is caused by the bacterium Tropheryma whipplei, a ubiquitous organism present in the environment, and is characterized by abdominal pain, weight loss, diarrhea, and, in 65% to 90% of patients, arthralgias or arthritis.198-200 Whipple disease occurs 10 times more frequently in males than in females and is most common in middle age, although it has been identified in a 3 month old boy201 and a 7 year old boy.202 There is also one report of central nervous system disease in a young boy.197 Migratory, peripheral joint pain and inflammation lasting hours to months occur over a period of many years, often in association with fatigue, weight loss, and anemia. Joint swelling with increased synovial fluid and restriction of range of motion may occur,203 although residual deformity does not.204 The joints most frequently affected are the ankles, knees, shoulders, and wrists,203 and spondylitis has been reported in 20%.204 Occasionally, arthritis or spondylodiscitis may be present in the absence of gastrointestinal symptoms, and Whipple disease should be considered in patients who are resistant to antirheumatic treatment.205 A small percentage of the population, especially those who work with soil or in sewage plants, may be asymptomatic carriers. Periodic acid-Schiff-positive material and bacteria are detectable in macrophages infiltrating the upper small intestine and abdominal lymph nodes. Other diagnostic tools include PCR and immunohistochemistry. Because it takes several months to culture the organism, cultures 99mTc-MDP bone scan Yes Positive Contrastenhanced MR imaging Preoperative evaluation of spine (always) pelvis physis Yes No Therapy MR imaging for possible abscess No Response in 48 hr Yes Follow-up radiograph FIGURE 37–8 Flowchart of the recommendations for evaluating acute hematogenous osteomyelitis in children. (Adapted from Jaramillo D, Treves ST, Kasser JR et al: Osteomyelitis and septic arthritis in children: appropriate use of imaging to guide treatment, AJR Am J Roentgenol 165:399-403, 1995.) A 571 B FIGURE 37–9 A, Normal disk space is demonstrated on a lateral view of the lumbar spine. B, Diskitis has caused collapse of the disk space (arrow). 572 SECTION 5 — ARTHRITIS RELATED TO INFECTION A FIGURE 37–10 Technetium 99m bone scan of a 2.5-year-old girl with back pain demonstrates increased uptake of the isotope in the inferior end plate of L2 and superior end plate of L3 (arrow), which is characteristic of diskitis. (courtesy of Dr. H. Y. Nadel.) are not recommended as a diagnostic tool, A genetic predisposition has been suggested based on the ubiquitous nature of the organism, but causative genes have not been identified. Antimicrobial therapy (e.g., ceftriaxone, penicillin, doxycycline, cotrimoxazole, streptomycin) has greatly improved the outcome in this disease. Arthritis Caused by Viruses A classification of viruses known to be associated with arthritis in humans is shown in Table 37–9.206 The togaviruses account for most of the viral arthritides. In general, viral arthritides occur much more often in adults than in children.207 Arthralgia is more common than objective arthritis, and both are usually migratory and of short duration (one to two weeks), disappearing without residual joint disease. There are a number of potential mechanisms by which viruses may lead to arthritis, including targeting the cells of innate immunity and adaptive immunity, inducing the production of autoantibodies and T cell mediated autoimmunity, or directly infecting synovial cells.208 As arthritis is seldom the main symptom of viral disease, clues as to the etiology should be sought in the history, including travel to areas where alphaviruses and Human T-cell leukemia virus type-1 (HTLV-1) may be common, a history of blood transfusions, IV drug abuse, recent vaccinations, or ongoing epidemics or exposures. Small joints are most often affected by rubella, hepatitis B, and members of the alphavirus group (e.g., Ross River, chikungunya), whereas one or two large joints (usually the knees) are most often affected by mumps, varicella, and other viruses. In some viral arthritides, virus (e.g., rubella, varicella, herpes simplex, cytomegalovirus) can be isolated from the joint space; in others, only virus-containing immune complexes (e.g., hepatitis B, adenovirus 7) are found; and in still others, neither virus nor viral antigen can be recovered from the joint.209 Whether this represents limitations of recovery and culture techniques B C FIGURE 37–11 A 10 year old male with back pain and diskitis. A, Axial T1 weighted fat-suppressed MR image postcontrast showing bone enhancement. B, Sagittal T1-weighted fat-suppressed MR image following contrast administration with increased signal on the inferior aspect of the vertebral body surrounding an area of decreased enhancement. C, Sagittal T2 weighted MR image showing increased signal intensity within the T7 vertebral body and narrowing of the adjacent inferior disk (courtesy of Dr. P. Babyn). or the fact that culture-negative viral arthritis is “reactive” rather than “septic” is unknown. Rubella Virus Rubella-associated arthropathy was recognized by Osler210 and was one of the most commonly identified virus-associated arthritides in North America. Musculoskeletal symptoms after natural rubella infection are relatively common in young women. These symptoms are unusual in preadolescent children and in males, however, and are much less frequent after rubella immunization than after natural infection. Arthritis is more common after natural infection, and it is more severe and lasts longer.211 Arthralgia usually begins within seven days of the appearance of the rash or 10 to 28 days after immunization. The joints of the fingers and, later, the knees are most frequently affected. Joint pain may be accompanied by warmth, erythema, and effusion, and tenosynovitis is common. Carpal tunnel syndrome has also been reported. These findings usually disappear within three to four weeks but occasionally persist for months or even years. In a study of natural rubella infection in 37 teenage students, 52% of girls and 8% of boys developed objective arthritis,211 and an additional 13% and 48%, respectively, experienced arthralgia. In a group of young 37 — INFECTIOUS ARTHRITIS AND OSTEOMYELITIS Table 37–9 Viruses that cause arthritis in humans Virus Togaviruses Rubivirus Rubella Alphaviruses Ross River Chikungunya O’nyong-nyong Mayaro Sindbis Ockelbo Pogosta Parvoviruses Hepadnaviruses Hepatitis B Hepatitis C Adenoviruses Adenovirus 7 Herpesviruses Epstein-Barr Cytomegalovirus Varicella-zoster Herpes-simplex Paramyxoviruses Mumps Enteroviruses Echovirus Coxsackievirus B Orthopoxvirus Variola virus (smallpox) Vaccinia virus Comment Global; most reports from North America and Europe Australasia Africa, Asia Africa South America Africa, Asia, Australia Sweden Finland B19 associated with fifth disease, aplastic crises Global Global Rare Rare; suggested role in rheumatoid arthritis Rare Rare Rare Rare Rare Rare Nonexistent today Rare Adapted from Petty RE, Tingle AJ: Arthritis and viral infection, J Pediatr 113:948, 1998. women who received RA 27/3 rubella vaccine, 14% developed an acute polyarthritis. Arthralgia and arthritis are most common in adults, although approximately 25% of prepubertal females develop arthralgia, and 10% arthritis.212,213 Chronic arthritis in adult women has been reported after vaccination in 5% to 11%.214,215 It has been suggested that reinfection contributes to arthritis in susceptible hosts.216,217 Rubella virus has been recovered from the synovial fluid of patients with rubella arthritis in many218,219 but not all instances.207,220 The virus was isolated from synovial or peripheral blood mononuclear cells in seven of 19 children with juvenile rheumatoid arthritis but from no control subjects.221 Other investigations have shown no association.222 Parvovirus Parvoviruses are the latest candidates in the list of viruses putatively involved in the cause of rheumatoid arthritis in adults.223,224 Parvovirus RA-1 has been isolated from the 573 synovial membrane of one patient with classic rheumatoid arthritis.225 A second parvovirus, B19, is the agent responsible for erythema infectiosum (i.e., fifth disease or slapped cheek syndrome).226,227 This illness is sometimes accompanied by an arthritis not unlike that of rubella infection.227-230 Arthralgia, symmetrical joint swelling, and morning stiffness have been described in adults, especially women, after parvovirus B19 infection.230 Carpal tunnel syndrome, hepatitis, and angioedema have been described. This syndrome may be more widespread than previously thought and may be considerably underdiagnosed. In some patients, symptoms have persisted for years. An early study of erythema infectiosum in 364 patients indicated that joint pain, most often affecting knees and wrists, was present during the first week in 77% of adults and 8% of patients younger than 20-years-old.231 Subsequently, it was determined that arthritis was most common in patients who were HLA-DR4 positive.232 Whether or not a chronic arthritis results from parvovirus B19 infection is still controversial.209,233 Parvovirus infection is common and widespread. Parvovirus B19 genome consists of a linear, 5.6-kD, single-stranded deoxyribonucleic acid (DNA). There is only one serotype of parvovirus B19. Human parvovirus B19 has been implicated as the causative agent in erythema infectiosum, aplastic crises, some cases of hemophagocytic syndrome, and hydrops fetalis.234 Erythema infectiosum, is a common exanthem of older children that lasts for a few days to a week and presents with a low-grade fever, an erythematous facial rash (“slapped cheeks”), and a lacy, reticular rash on the extremities. These manifestations often recur with malaise, irritability, and arthralgia. Only a few cases of children with documented B19associated arthritis have been reported.228,235-237 Joint symptoms tend to be mild and transient. In the children reported by Reid and coworkers,238 there was symmetrical involvement of the small joints of the hands and feet. In one child, the arthritis preceded development of the rash. In another, arthritis persisted for three months. Antinuclear antibodies and rheumatoid factors (RFs) were absent in all of the patients in this series. Rivier and colleagues236 described a 5-year-old boy with arthritis of one knee that lasted for six weeks after typical erythema infectiosum. Nocton and associates235 described acute arthritis in 20 children with parvovirus B19 infections. The arthritis was associated with constitutional symptoms in one-half of the children and was of brief duration (less than four months) in 14. Six children had persistent arthritis lasting up to 13 months; criteria for a diagnosis of JIA would have been met in this group. Laboratory results were generally normal, except for serological evidence of the B19 infection. Usually, NSAID treatment will suffice, but for patients with persistent symptoms, intravenous immunoglobulin (IVIG) may be a treatment option.239 A recent study on seroprevalence of parvovirus B19 did not show an increase in patients with JIA compared with diseased or healthy controls.240 The precise relation of the viral infection to arthritis has not been clarified. The virus has not been grown from synovial fluid or blood from patients with joint 574 SECTION 5 — ARTHRITIS RELATED TO INFECTION s ymptoms, although B19-specific DNA has been identified by hybridization in the synovial fluid of adults238 and by PCR amplification in synovial tissue.241 However, Soderlund and colleagues242 demonstrated genomic B19 DNA in the synovium of joints that had suffered trauma even more frequently than in those of children with chronic arthritis. Inflammatory synovitis may not be identifiable by arthroscopy. Although infection gives rise first to IgM antibodies243 and then to IgG antibodies, there is no evidence that the arthropathy represents an immune complex disease. Demonstration of IgM antibodies, however, is essential to diagnosis. The prevalence of IgG antibodies in the general population is too high to be diagnostically helpful, unless a fourfold increase concurrent with the clinical symptoms is demonstrated.244-246 Hepatitis B Arthritis–Dermatitis Syndrome and Asia.255 These viruses are transmitted by arthropods, usually the mosquito, and incite an illness characterized by arthritis and a rash that may be macular, papular, vesicular, or purpuric. Although there are some virusspecific differences in these illnesses, they usually are mild in children and occur with equal frequency in males and in females. In Ross River virus disease, the wrist is most commonly affected and is often accompanied by tenosynovitis and enthesitis at the insertion of the plantar fascia into the calcaneus.255,256 The synovial fluid is said to be highly characteristic, with a predominance of vacuolated macrophages and very few PMNs. In chikungunya, the knee is the most commonly involved joint, and back pain and myalgia are prominent. The arthritis lasts one to two weeks and is followed by complete recovery. Diagnosis rests on the clinical presentation and an elevated level of antibodies to the specific virus. Viral antigen has not been recovered from synovial fluid. Herpesviruses In adults, up to 20% of infections with hepatitis B virus are characterized by a period of rash and arthritis that resembles serum sickness.247 The arthritis is often explosive in onset and often occurs in the preicteric phase of hepatitis B infections. In a review of reported cases of arthritis associated with hepatitis B infection,248 the age of the patients ranged from 14 to 56 years, and the male-to-female ratio was 1.5:1. The dermatitis is characterized by a maculopapular rash, sometimes with petechiae, or urticaria, and is most prominent on the lower extremities. The arthritis usually begins abruptly and symmetrically and affects the interphalangeal joints in 82%, knees in 30%, and ankles in 24% of patients. Although erythema and warmth are present, synovial effusions are uncommon. Joint symptoms last for four weeks on average, respond well to NSAIDs, and disappear without sequelae. The ESR is usually normal, although serum and synovial fluid complement levels are low in the early stages of the illness.249 Synovial fluid has been reported to show a mononuclear cell predominance.250 Electron microscopic evidence of hepatitis antigen in the synovial membrane has been reported.251 Four of the herpesviruses have been associated with arthritis. Herpes simplex virus type 1 has been isolated from the synovial fluid of one patient with arthritis and disseminated herpes simplex infection.257 Epstein-Barr virus has long been thought by some investigators to have a primary role in the cause or pathogenesis of rheumatoid arthritis, although direct evidence is lacking.258-260 Arthritis is a rare complication of infectious mononucleosis.261-263 Cytomegalovirus is occasionally associated with arthritis and has been isolated from synovial fluid in one instance.257 Varicella-zoster infection is uncommonly complicated by arthritis.264-271 However, there have been instances of bacterial septic arthritis complicating chickenpox.269,272,273 In one instance, varicella-zoster virus was grown from synovial fluid of an 8-year-old girl with acute, painless monarthritis occurring three days after the onset of chickenpox.264 The synovial fluid cells were predominantly lymphocytes.264,271,274 Occasionally, chickenpox is associated with the emergence of psoriatic arthritis.275 Acute monarthritis has been reported in association with herpes zoster in two adults.276,277 Hepatitis C Mumps Virus Hepatitis C virus is lymphotrophic and is associated with rheumatic symptoms resulting from mixed cryoglobulinemia207 and producing an intermittent monoarticular or oligoarticular nondestructive arthritis affecting large- and medium-sized joints.252 A rheumatoid arthritis-like picture may also occur.253 As many of these patients may be rheumatoid factor positive, the presence of anti-CCP has been proposed as a useful tool in diagnosing true RA in the hepatitis-C infected individual.254 The paramyxovirus (mumps) rarely causes arthritis. In a 1984 review,278 only 32 cases were well documented. Since then, two additional patients have been reported.279,280 The male-to-female ratio is 3.6:1, and the peak age of occurrence is 21- to 30-years-old. Four patients younger than 11 years old and seven between the ages of 11 and 20 years have been described. Arthritis occasionally preceded parotitis but usually followed by one to three weeks. In children, the arthritis was mild, affected few joints, and lasted one to two weeks. In postadolescent males, arthritis was often accompanied by orchitis and pancreatitis.278 It is reported that the arthritis responds to ibuprofen or prednisone but not to aspirin.278 The pathogenesis is unknown, and no attempts at recovery of mumps virus from synovium Alphaviruses Epidemic polyarthritis caused by infection with one of the alphaviruses is the most common virus-associated arthritis in Australia, the islands of the South Pacific, Africa, 37 — INFECTIOUS ARTHRITIS AND OSTEOMYELITIS or synovial fluid have been reported. Arthritis has not occurred after mumps immunization. Human Immunodeficiency Virus The spectrum of rheumatic diseases that can accompany HIV infection is extremely large and has changed with the introduction of highly active antiretroviral (HAART) treatment. AIDS resulting from HIV infection may be complicated by septic arthritis, although this is now considered rare.281 Virtually any organism can be responsible; S. aureus and Streptococcus species are the most common. Although the precise nature of rheumatic syndromes associated with HIV infection remains controversial, a number of stereotypical presentations have been documented in adults.282 These include reactive arthritis, psoriasiform arthritis, and an undifferentiated spondyloarthropathy,283-287 often more severe than in patients without HIV infection. A similar picture has been observed in children infected with HIV.287a Arthralgias occur initially with viremia; lower extremity oligoarthritis or persistent polyarthritis can supervene later. A study of 270 patients concluded that the most frequent pattern of joint involvement was one of an acute onset, short duration, few if any recurrences, and no erosive sequelae.288 The authors have observed chronic oligoarthritis in two young children with HIV infection (one transplacental and one related to blood products). Osteomyelitis may also occur; tuberculosis infection is a relatively common cause.289 In the post-HAART era, there has been a decline in reactive, psoriatic, and infectious causes of musculoskeletal syndromes, but disorders such as osteoporosis, osteomalacia, and osteonecrosis have become more prevalent.281 Other Viruses There are reports of arthritis associated with adenovirus type 7 infections, although the virus was not isolated from the synovial fluid, and diagnosis was confirmed only on clinical and serological grounds.288,290,291 Echoviruses292-294 and coxsackie B viruses291,295 have been rarely implicated as the cause of arthritis. Smallpox (variola virus infection), now eradicated from the world, was often accompanied by arthritis, especially in children younger than 10 years old. Arthritis also followed cowpox vaccination.296 Human T cell leukemia virus type 1 HTLV-1 has been associated with a number of rheumatic disorders in adults, including arthritis and Sjögren’s syndrome.297 A 1998 report298 outlined an outbreak of Sindbis virusinduced Pogosta disease (e.g., fever, rash, joint symptoms) in Finland. Syndromes Presumably Related to Viral Infection Transient or toxic synovitis of the hip is an idiopathic disorder often preceded by a nonspecific upper respiratory tract infection. It occurs most commonly in boys (70%) between 3- and 10-years-old.299 Pain in the hip, thigh, or knee may be of sudden or gradual onset and lasts for an average of six days. Bilateral involvement 575 occurs in approximately 4% of cases. There is loss of internal rotation of the hip, and the hip may be held in the flexed, abducted position. The ESR and WBC count are usually normal.299,300 Radiographs often appear normal or may document widening of the joint space with lateral displacement of the femoral head because of effusion. These findings can be confirmed by CT or ultrasound studies.299 Radionuclide scanning may demonstrate a transient decrease in uptake of technetium 99m phosphate. Signal intensity is normal with MRI and differentiates toxic synovitis of the hip from a septic process,57 which is often the principal differential diagnosis.301 After a diagnostic ultrasound scan to confirm the presence of fluid, the hip joint should be aspirated to exclude bacterial sepsis.302 The synovial fluid has a normal or minimally increased cell count but may be under high pressure.299 After aspiration, the pain and range of motion are dramatically improved, at least temporarily. Treatment includes the use of analgesics or NSAIDs, bed rest, and skin traction with the hip in 45 degrees of flexion to minimize intracapsular pressure.299 Long-term sequelae include Legg-Calvé-Perthes disease in about 1.5% of cases,303,304 coxa magna, and osteoarthritis. Recurrences are often accompanied by low-grade fever. Arthritis Associated with Other Infections Fungal Arthritis Arthritis caused by fungal infection is rare305 and is almost unknown in children beyond the neonatal period. Fungi that have been reported as causing arthritis or osteomyelitis are C. albicans,306 Sporothrix schencii,307,308 Actinomyces israelii,309 Aspergillus fumigatus,310 Histoplasma capsulatum,311 Cryptococcus neoformans,311 Blastomyces dermatitidis,312 Coccidioides immitis,313 Paracoccidioides brasiliensis,314 Nocardia asteroides,315 and Pseudallescheria boydii.316 Candidal arthritis,306 often with accompanying osteomyelitis, is a recognized entity in the newborn317,318 and occurs occasionally in immunocompromised patients319,320 and in patients with prosthetic joints.321 Sporotrichosis and Plant Thorn Synovitis Infection with Sporothrix schenckii is a rare but significant occupational hazard of gardeners, night-crawler farmers, and field workers.307,308 Monarthritis or, less commonly, polyarthritis resembling rheumatoid arthritis, has been reported (Fig. 37–12). Synovial biopsy is often necessary to make the diagnosis (Fig. 37–13). Other fungal infections are even less common causes of bone or joint infection in children. The interested reader is referred to the review by Goldenberg and Cohen308 and to other select references.310-316,318,321 Synovitis caused by the penetration of a plant thorn into the joint space or surrounding structures is probably a reaction to the foreign material rather than an 576 SECTION 5 — ARTHRITIS RELATED TO INFECTION Arthritis Caused by Spirochetes Lyme Disease The geographical and temporal clustering of cases of what was thought to be juvenile rheumatoid arthritis in Old Lyme, Connecticut, led to the discovery and description of the cause, pathogenesis, and cure of Lyme disease. This epidemiological work is one of the most important developments of the past three decades in rheumatology and provides a model for approaching the question of the infectious cause of other chronic arthritides of childhood. Unfortunately, work on the development of a safe and effective vaccine has stalled.323 Chapter 38 provides a complete discussion of classic Lyme disease. Other Spirochetes and Arthritis FIGURE 37–12 Destruction of the first metatarsophalangeal joint was caused by sporotrichosis. Arthritis rarely complicates leptospirosis (Leptospira icterohemorrhagica)324 and syphilis (Treponema pallidum)325,326 Congenital syphilis causes juxtaepiphyseal osteochondritis and periarthritis in infancy and syphilitic dactylitis in early childhood (Fig. 37–14). Clutton joints— relatively painless, recurrent, nonprogressive, symmetrical synovitis of the knees—develop later.327 Parasites and Arthritis There have been case reports of arthritis accompanying a wide range of parasitic infestations,328 including Giardia intestinalis (lamblia),329 Endolimax nana,330 Toxocara canis,331 schistosomiasis,332 and others.333 In general, the joint disease is presumably reactive or postinfectious rather than septic and pursues a benign course with a good prognosis. Musculoskeletal Manifestations of Systemic Bacterial Infections FIGURE 37–13 Sporothrix schenckii is identified in a Gram-stained preparation of the synovial fluid aspirate. utright infection, although the circumstances of the o injury may suggest the latter. The synovial effusion is inflammatory, and culture occasionally yields a relatively nonvirulent organism. In the case of rose thorn penetration, S. schenckii is the probable cause. More commonly, the thorn of the palm tree or blackthorn is implicated. There are signs of local inflammation, and radiographs demonstrate periosteal new bone formation, a radiolucent defect in bone, or the presence of radiopaque foreign material. CT is indicated if plain radiographic results are negative. Because this disorder may develop months after the initial injury, foreign-body synovitis may be ignored as a diagnostic possibility. Treatment should be directed at appropriate surgical exploration and removal of the foreign material.322 Bacterial infection of ventricular shunts for the management of hydrocephalus may result in arthritis and nephritis.334 RFs may be demonstrable in the sera of these patients. Meningococcemia is complicated by arthritis in up to 10% of cases.335 It is usually oligoarticular and occurs most often during the recovery phase, when immune complexes can be demonstrated in the synovium.336 It can also be complicated by acute septic arthritis in the early stage of the disease. H. influenzae type B meningitis may lead to a sterile arthritis.337 Infective endocarditis frequently causes arthralgia or arthritis338,339 and signs suggesting vasculitis (e.g., Osler nodes, Janeway lesions, Roth spots). The musculoskeletal signs and symptoms (e.g., arthralgia, arthritis, myalgia, low back pain) may precede other manifestations of infective endocarditis by weeks.339 The arthritis is characteristically polyarticular and symmetrical, affecting both large and small joints. An immune complex-mediated pathogenesis is thought to be responsible, and the presence of hypocomplementemia,340 circulating immune complexes,313 and sometimes RFs340 support this theory. Specificity of the RFs is directed to the patient’s IgG in combination with the infecting organism. 37 — INFECTIOUS ARTHRITIS AND OSTEOMYELITIS A 577 B FIGURE 37–14 Lesions of congenital syphilis were identified in a 6-month-old girl brought to the child abuse clinic because of multiple fractures that occurred during the previous 10 days. Her rapid plasma reagin test result was 1:256. A, Bilateral, symmetrical, destructive metaphysitis lesions of the proximal ends of the tibiae (Wimberger sign) (solid arrow) and periosteal new bone apposition (open arrow) can be seen. B, With penicillin therapy, the lesions have almost healed two months later. REFERENCES 3. P. Christiansen, B. Frederiksen, J. Glazowski, et al., Epidemiologic, bacteriologic, and long-term follow-up data of children with acute hematogenous osteomyelitis and septic arthritis: a ten-year review, J. Pediatr. Orthop. B 8 (1999) 302–305. 5. C.W. Fink, J.D. Nelson, Septic arthritis and osteomyelitis in children, Clin. Rheum. Dis. 12 (1986) 423–435. 16. A.W. Howard, D. Viskontas, C. Sabbagh, Reduction in osteomyelitis and septic arthritis related to Haemophilus influenzae type B vaccination, J. Pediatr. Orthop. 19 (1999) 705–709. 23. S. Chometon, Y. Benito, M. Chaker, et al., Specific real-time polymerase chain reaction places Kingella kingae as the most common cause of osteoarticular infections in young children, Pediatr. Infect. Dis. J. 26 (2007) 377–381. 24. P. Yagupsky, Kingella kingae: from medical rarity to an emerging paediatric pathogen, Lancet Infect. Dis. 4 (2004) 358–367. 25. K.M. Kiang, F. Ogunmodede, B.A. Juni, et al., Outbreak of osteomyelitis/septic arthritis caused by Kingella kingae among child care center attendees, Pediatrics 116 (2005) e206–e213. 34. C.J. Mathews, G. Coakley, Septic arthritis: current diagnostic and therapeutic algorithm, Curr. Opin. Rheumatol. 20 (2008) 457–462. 39. T.R. Nunn, W.Y. Cheung, P.D. Rollinson, A prospective study of pyogenic sepsis of the hip in childhood, J. Bone Joint Surg. Br. 89 (2007) 100–106. 40. C.J. Mathews, G. Kingsley, M. Field, et al., Management of septic arthritis: a systematic review, Ann. Rheum. Dis. 66 (2007) 440–445. 46. I.M. van der Heijden, B. Wilbrink, L.M. Schouls, et al., Detection of mycobacteria in joint samples from patients with arthritis using a genus-specific polymerase chain reaction and sequence analysis, Rheumatology (Oxford) 38 (1999) 547–553. 49. R.M. Lyon, J.D. Evanich, Culture-negative septic arthritis in children, J. Pediatr. Orthop. 19 (1999) 655–659. 51. R.F. Buchmann, D. Jaramillo, Imaging of articular disorders in children, Radiol. Clin. North Am. 42 (2004) 151–168. vii. 52. D. Jaramillo, S.T. Treves, J.R. Kasser, et al., Osteomyelitis and septic arthritis in children: appropriate use of imaging to guide treatment, AJR 165 (1995) 399–403. 56. K.D. Stumpe, K. Strobel, Osteomyelitis and arthritis, Semin. Nucl. Med. 39 (2009) 27–35. 58. M. Karchevsky, M.E. Schweitzer, W.B. Morrison, et al., MRI findings of septic arthritis and associated osteomyelitis in adults, AJR 182 (2004) 119–122. 60. M.S. Kocher, R. Mandiga, J.M. Murphy, et al., A clinical practice guideline for treatment of septic arthritis in children: efficacy in improving process of care and effect on outcome of septic arthritis of the hip, J. Bone Joint Surg. Am. 85-A (2003) 994–999. 62. P.O. Newton, R.T. Ballock, J.S. Bradley, Oral antibiotic therapy of bacterial arthritis, Pediatr. Infect. Dis. J. 18 (1999) 1102–1103. 68. A.C. Offiah, Acute osteomyelitis, septic arthritis and discitis: differences between neonates and older children, Eur. J. Radiol. 60 (2006) 221–232. 69. R.R. Betz, D.R. Cooperman, J.M. Wopperer, et al., Late sequelae of septic arthritis of the hip in infancy and childhood, J. Pediatr. Orthop. 10 (1990) 365–372. 72. M.S. Kocher, How do you best diagnose septic arthritis of the hip? in: J.G. Wright (Ed.), Evidence-based orthopedics, Saunders, Philadelphia, 2009. 81. S.P. Brogadir, B.M. Schimmer, A.R. Myers, Spectrum of the gonococcal arthritis-dermatitis syndrome, Semin. Arthritis Rheum. 8 (1979) 177–183. 100. M.W. Shen, Diagnostic and therapeutic challenges of childhood brucellosis in a nonendemic country, Pediatrics 121 (2008) e1178–e1183. 110. E. Maman, J. Bickels, M. Ephros, et al., Musculoskeletal manifestations of cat scratch disease, Clin. Infect. Dis. 45 (2007) 1535–1540. 113. M.S. Gilbert, L.M. Aledort, S. Seremetis, et al., Long term evaluation of septic arthritis in hemophilic patients, Clin. Orthop. Relat. Res. (1996) 54–59. 578 SECTION 5 — ARTHRITIS RELATED TO INFECTION 114. B.I. Asmar, J. Andresen, W.J. Brown, Ureaplasma urealyticum arthritis and bacteremia in agammaglobulinemia, Pediatr. Infect. Dis. J. 17 (1998) 73–76. 116. E. Forlin, C. Milani, Sequelae of septic arthritis of the hip in children: a new classification and a review of 41 hips, J. Pediatr. Orthop. 28 (2008) 524–528. 121. R.J. Scott, M.R. Christofersen, W.W. Robertson Jr., et al., Acute osteomyelitis in children: a review of 116 cases, J. Pediatr. Orthop. 10 (1990) 649–652. 126. R.J. Gorwitz, A review of community-associated methicillin- resistant Staphylococcus aureus skin and soft tissue infections, Pediatr. Infect. Dis. J. 27 (2008) 1–7. 127. J. Saavedra-Lozano, A. Mejias, N. Ahmad, et al., Changing trends in acute osteomyelitis in children: impact of methicillin-resistant Staphylococcus aureus infections, J. Pediatr. Orthop. 28 (2008) 569–575. 143. L.T. Gutman, Acute, subacute, and chronic osteomyelitis and pyogenic arthritis in children, Curr. Probl. Pediatr. 15 (1985) 1–72. 147. M.N. Wang, W.M. Chen, K.S. Lee, et al., Tuberculous osteomyelitis in young children, J. Pediatr. Orthop. 19 (1999) 151–155. 152. M.H. Perlman, M.J. Patzakis, P.J. Kumar, et al., The incidence of joint involvement with adjacent osteomyelitis in pediatric patients,, J. Pediatr. Orthop. 20 (2000) 40–43. 156. P.N. Tyrrell, V.N. Cassar-Pullicino, S.M. Eisenstein, et al., Back pain in childhood, Ann. Rheum. Dis. 55 (1996) 789–793. 160. N.E. Green, R.D. Beauchamp, P.P. Griffin, Primary subacute epiphyseal osteomyelitis, J. Bone Joint Surg. Am. 63 (1981) 107–114. 161. J.J. McCarthy, J.P. Dormans, S.H. Kozin, et al., Musculoskeletal infections in children. Basic treatment principles and recent advancements, J. Bone Joint Surg. Am. 86-A (2004) 850–863. 164. G.A. Mandell, Imaging in the diagnosis of musculoskeletal infections in children, Curr. Probl. Pediatr. 26 (1996) 218–237. 172. A. Karwowska, H.D. Davies, T. Jadavji, Epidemiology and outcome of osteomyelitis in the era of sequential intravenous-oral therapy, Pediatr. Infect. Dis. J. 17 (1998) 1021–1026. 174. R. Ruebner, R. Keren, S. Coffin, et al., Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis, Pediatrics 117 (2006) 1210–1215. 185. F.L. Sapico, J.Z. Montgomerie, Pyogenic vertebral osteomyelitis: report of nine cases and review of the literature, Rev. Infect. Dis. 1 (1979) 754–776. 193. M. Fernandez, C.L. Carrol, C.J. Baker, Discitis and vertebral osteomyelitis in children: an 18-year review, Pediatrics 105 (2000) 1299–1304. 205. T. Schneider, V. Moos, C. Loddenkemper, et al., Whipple’s disease: new aspects of pathogenesis and treatment, Lancet Infect. Dis. 8 (2008) 179–190. 206. R.E. Petty, A.J. Tingle, Arthritis and viral infection, J. Pediatr. 113 (1988) 948–949. 208. R. Franssila, K. Hedman, Infection and musculoskeletal conditions: Viral causes of arthritis, Best Pract. Res. Clin. Rheumatol. 20 (2006) 1139–1157. 235. J.J. Nocton, L.C. Miller, L.B. Tucker, et al., Human parvovirus B19-associated arthritis in children, J. Pediatr. 122 (1993) 186–190. 248. R.D. Inman, Rheumatic manifestations of hepatitis B virus infection, Semin. Arthritis Rheum. 11 (1982) 406–420. 253. I. Rosner, M. Rozenbaum, E. Toubi, et al., The case for hepatitis C arthritis, Semin. Arthritis Rheum. 33 (2004) 375–387. 269. P. Schreck, P. Schreck, J. Bradley, et al., Musculoskeletal complications of varicella, J. Bone Joint Surg. Am. 78 (1996) 1713–1719. 281. N. Patel, N. Patel, L.R. Espinoza, HIV infection and rheumatic diseases: the changing spectrum of clinical enigma, Rheum. Dis. Clin. North Am. 35 (2009) 139–161. 299. H. Wingstrand, Transient synovitis of the hip in the child, Acta. Orthop. Scand. (Suppl.) 219 (1986) 1–61. 301. M.S. Kocher, D. Zurakowski, J.R. Kasser, Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm, J. Bone Joint Surg. Am. 81 (1999) 1662–1670. Entire reference list is available online at www.expertconsult.com.