* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download here - Grene Vision Group
Survey
Document related concepts
Transcript
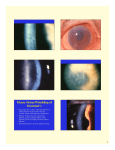
Grene Vision Group Offering FDA-Approved Cornea Strengthening Therapy for Keratoconus Patients Dr. Dasa V. Gangadhar provides Corneal Collagen Cross-Linking (CXL) to halt the progression of keratoconus and decrease the chances of needing a cornea transplant About Keratoconus: Keratoconus is a sight-threatening progressive eye condition in which the clear tissue on the front of the eye (the cornea) thins and bulges outward into a cone shape. The resulting symptoms are blurred vision, glare and halos at night, and streaking of lights. In most people who have keratoconus, both eyes are eventually affected, although not always to the same extent. It usually develops in one eye first and then later in the other eye. Who is at risk for developing Keratoconus? Keratoconus usually manifests in the late teens to early twenties and progressively worsens into middle age, when it typically stabilizes. It's possible it can occur in people 40 and older, but that is less common. The changes in the shape of the cornea can happen quickly or may occur over several years. Keratoconus appears to run in families. If you have it and have children, it’s a good idea to have their eyes checked for it starting at age 10. The condition progresses more rapidly in people with certain medical problems, including allergic conditions. It could be related to chronic eye rubbing. Treatment Options: Some individuals with keratoconus simply need glasses to see their best. Others require a hard contact lens to optimize their vision. For severe conditions, a corneal transplant may be required. However, now there is an exciting FDA-approved therapy available: corneal collagen cross-linking (CXL). The minimally invasive procedure involves applying liquid riboflavin (vitamin B2) to the surface of the eye, followed by treatment with a controlled application of ultraviolet light, to strengthen the cornea. Dasa V. Gangadhar, MD, cornea specialist at Grene Vision Group, has been involved in clinical studies of CXL and plans to initiate treatments near the end of December 2016. “This is truly an exciting treatment option and a game-changer for the management of keratoconus. Our ability to offer corneal transplants and now CXL will allow for the full gamut of treatment options for this disease afflicting an especially young population,” Dr. Gangadhar said. The Exciting News: CXL appears to stabilize the cornea in 95 percent of patients for up to 10 years. “We hope that timely treatment of keratoconus with CXL will ultimately eliminate the need for corneal transplantation (PK) in the next 15 to 20 years,” Dr. Gangadhar said. Who is a Candidate? Patients with keratoconus and post-LASIK ectasia may be candidates. Ages 15 – 65. Young patients are the most ideal candidates. Not recommended for pregnant females or patients with corneas thinner than 400 microns. Reasonable Expectations: Insurance does not currently cover this treatment and the cost is around $2500 per eye. The primary goal of CXL is to stabilize the cornea and reduce the chance of progression and the need for a cornea transplant. “We are very excited that Dr. Gangadhar offers corneal crosslinking as a relatively non-invasive option for patients with keratoconus. His dedication to patients is evident through his continued involvement with clinical trials and training in the most current corneal procedures,” said Collin Hermreck, Director of Marketing and Public Relations, Grene Vision Group.