* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Structural Characterization of Photoluminescent Porous Silicon with
Symmetry in quantum mechanics wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Quantum machine learning wikipedia , lookup
Hidden variable theory wikipedia , lookup
Canonical quantization wikipedia , lookup
Hydrogen atom wikipedia , lookup
Atomic theory wikipedia , lookup
Quantum state wikipedia , lookup
History of quantum field theory wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
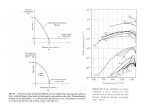
158 Brazilian Journal of Physics, vol. 27, no. 4, december, 1997 Structural Characterization of Photoluminescent Porous Silicon with FTIR Spectroscopy Walter Jaimes Salcedo, Francisco J. Ramirez Fernandez, Elisabete Galeazzo Laboratorio de Microeletr^onica LME - DEE - EPUSP Caixa postal 8174, CEP 01065-970, S~ao Paulo, SP Fax (011) 818-5718 e-mail [email protected] Received February 2, 1997 This paper presents an analytical model which is proposed to determine morphological parameters of porous silicon (PS) using results obtained with the Fourier Transform Infrared (FTIR) characterization techniques. Using the proposed model, it was found that porous silicon layers have porous size of 6.6 nm, specic surface of 600 m2/cm3 and the residual crystal size 3.7 nm. The PS and oxidized PS were characterized with Photoluminescence (PL) and FTIR techniques. These techniques give some evidences of the possible quantum connement eect in PS layer. However it is necessary to have caution at this point. Introduction Signicant attention has recently been directed towards porous silicon due to its visible photoluminescence (PL). The strong visible light emission in porous silicon is quite surprising, in view of the fact that bulk crystalline silicon has an indirect gap at 1.1 eV at room temperature, which results in a very inecient radiative recombination producing light in the infrared[1]. Porous silicon can exhibit a large variety of morphologies and particles sizes. In nanostructures, the weak emission problem could be overcome by the removal of local or global periodicity, with energies that scale inversely with the size of the connement region[2]. In the case of silicon, particles sizes with dimensions below 5 nm would enhance the oscillator strength, as well as produce a sizable blue shift of the optical gap from 1.1eV into the range of 1.5 to 1.9 eV due to quantum connement[3]. Experimental results Porous silicon layers (PS) were formed by electrochemical anodization on P - type silicon wafer (100) with resistivity of 20 cm, in 40% (w/o) HF solution at current density of 20 mA/cm2 for 5, 10 and 15 min. The back face of wafers were doped with boron followed by Al metalization to improve the uniformity of current ow during anodization and to obtain homogeneous porous layers. The porosity and thickness of the layers were determined gravimetrically by weight loss and etching in KOH solution [4]. After anodization, some layers were anodically oxidized in aqueous solution of KNO3 at a constant current density of 1 mA/cm2. Oxidation proceeds from the interface between the porous layer and silicon substrate towards the surface.[5] Experimental measurements The infrared spectra of PS were measured in absorvance mode with BIO - RAD FTIR Spectrometer and they were referenced against a single silicon crystal sample. Fig. 1 shows spectral curves of PS and oxidized PS. W. J. Salcedo et. al. 159 In the infrared spectra of PS, the surface bonding are showed in accordance with the interpretation of table 16;8. A noticeable blueshitf of the whole luminescence spectra was observed. The PL peaks emission of PS and oxidized PS were at = 725 nm and = 650nm respectively. Discussion The frequency of bulk bonding vibrations of Si-Hn in the silicon relative to surface bonding vibrations, downshift. This fact can be calculated from a model where the downshift is caused by a depolarizing produced by a vibrating dipole within a spherical cavity of radius R[9]. 2 ! = 2s ;+11 Re 3! s The Si-Hn vibrationals bonding were considered to analyze structural characteristic of PS because they have absorption peaks in region with few density of absorption peaks. The absorption intensity which belong the Si-Hn bonding presents linear dependency with anodization time. I= Z (!)d! = a + bt (1) Where, is the absorvance. The room temperature PL spectra of PS and oxidized PS are showed in Fig. 2. The PL emission was excited by an Ar+ laser line of 450nm. The emitted light was detected by a photomultiplier tube. (2) With: e : apparent charge associated with the dipole moment per unit of displacement, es: dielectric constant of Si, !w: center frequency for an absorption band. Applying equation, (2) with s = 12, e = 0:4e and R the covalent radius of Si to ! = 2100 cm;1, it is obtained ! = -80 cm;1. Absorption peaks relevant to the Si-Hn bonds of PS layers evidence dislocations of 4 cm;1 when compared with results of Si-Hn surface bonds reported[9,10]. Then, the Si-Hn bonds of PS samples are surface bonds. In order to develop a model to obtain the structural parameters of PS layer, it is dened the geometrical parameters for PS layer. The graphic representation of these parameters are shown in the Fig. 3. Another consideration is that all PS surface is covered with Si-Hn bonds. The parameters are dened as follow: d is the average pores size, q is the average size of the residual 160 Brazilian Journal of Physics, vol. 27, no. 4, december, 1997 silicon crystal between pores. The average size of residual silicon crystallite between pores is obtained using the following relationship: p q = 2 3 3 1 + md d ; d Then, the density of PS layer is given by: (3) ! 1 PSL = 1 ; p ; 2 3 1 + md 2 Si (4) The porosity of PS is dened as the quantity of silicon removed during anodization compared with the silicon concentration before anodization evaluated in the same volume. The porosity is obtained by the relation. P = Si ; PSL Si (5) Where P is the porosity, Si is the density of silicon. Then, the porosity as function of geometrical parameters is written as: 2 1 P = p 1+ m (6) 2 3 d The other important parameter is the specic surface. The following equation results of the geometric model utilized: 1 P 3 Aesp = X (m) + 8:10 d(nm) (m2=cm3) (7) p A complete description of the PS layer structure is given by Aesp , P, d parameters. The equation (7) shows a relation between these parameters. Using this geometrical parameters, the surface concentration of the Si-Hn bonds in the PS layer is determined. This concentration is obtained from the following equation: NSi;Hn = 6:75 1013 + 6:10 1014Aesp Xp (8) Where, NSi;Hn is the Si ; Hn bonds concentration on PS surface (cm;2), Aesp . The Specic surface (m2/cm3), and Xp is the PS layer thickness (mm). The silicon dilution rate is approached for 1 mm/min [11,12]. Then, it means that Xp = t (m). Using the proposed model, the Si ; Hn bonds concentration is linearly dependant of anodization time. This result agrees with experimental results obtained in FTIR techniques. The Si ; Hn bonds concentration can be obtained by evaluating the infrared absorption band by the mean of the following equation [10,13] Z NSi;Hn K!0;1 (!)d! (9) Where K is the absorption force. It is considered the mean value[13] of K = 9:0 1019. The geometric parameters of PS are obtained using the proposed model relating with experimental results of infrared spectra (eq. 9) and porosity of 50% which was determined by gravimetric techniques. It was obtained the following values: d = (6:65 0:15) nm, Aesp = (601:8 13:6) m2/cm3 and q = (3:69 0:08) nm. These values showed be independent of anodization time. Quantum connement for charge carries due to the very small dimensions of the wires of silicon in PS is hoped. The quantum connement leads to an increase in the eective band gap energy. The eective mass model leads to following expression: 2 2 1 1 h (10) E = q2 m + m e n Where, me is the eective mass of electron and mn is the eective mass of holes. For our PS it was obtained the following value: E = (0:64 0:03) eV. The PS layer, without oxidation, has the PL peak at = 725 nm, with a eective band gap of 1.711ev. The increase of band gap was E = 0:650 eV. In the quantum connement frame work the correspondently residual crystallite size value is q = 3.65 nm. The oxidized PS presents a PL emission peak at = 650 nm with a eective band gap energy of EBG = 1:908 eV. The increase of band gap is of E = 0:79 eV. In the quantum connement frame work the correspondently residual crystallite size value is q = 3.31 nm. The comparison of `qs' values, that they were estimated for PS and PSoxidized from the PL spectra, give the following ratio: q = 13:4% q The result obtained, using infrared spectroscopy on preceding two samples, shows variation of Si ; Hn bonds concentration corresponding to: W. J. Salcedo et. al. IPSL ; IPSLoxid = I = (14:1 2:6)% IPSL IPSL IPS AespPS and IPSoxid I = Aesp = (14:1 2=6)% I Aesp It can be observed that the q=q and I=I ratios are similar. These results give some evidences that there is a possible quantum connement eect in the PS layer. However the literature report a possible surface eect [14-18] of the PL phenomena in the PS layer. At this point it is necessary to have caution. Conclusions The model proposed is an eective auxiliary tool to evaluate the parameters related to the porous structure. The PL spectra are consistent with evaluation obtained using this model. Our experimental observation pointed out the quantum connement as possible cause of the PL emission in the PS. Even though many group have reported the existence of quantum connement eect, the origin and mechanics of the luminescence in the PS layer remain controversial. Acknowledgements We acknowledge to CNPq and Grant-PADCT 620118/94-6 for the nancial support. 161 References [1] W. Michaelis and M. H. Pilkuhn, Phys. Stat. Sol. 36, 311, (1969). [2] V. Lehmann, U. Gosele, Appl. Phys. Lett. V. 58, 8, 856 (1991). [3] D. J. Lockwood, Solid State Commun. V 92, 1-2, 101 (1994). [4] E. Galeazzo, et al. 11o CBECIMAT, A guas de S~ao Pedro, SP, 11 a 14 de Dezembro de 1994. [5] Holimaoui A., et al., Appl. Phys. Lett., No 59, V. 3, 15 July 1991. [6] L.M. Peter; Phys Rev. Lett. V. 62, No 3, pp. 308, 16 Jan 1989. [7] P. Gupta, et al; Phys. Rev. B, V. 37, No 14, pp. 8234, 15 May 1988-I. [8] P. Gupta, et al; Surf. Sci. V. 245, 360 (1991). [9] M. Cardona; Phys. Stat. Sol. (b), V. 118, 463 (1983). [10] R. C. Anderson, et al; J. Electrochem. Soc., V. 140, No 5, 1393, May 1993. [11] V. Lehmann, H. Foll; J. Electrochem. Soc. V. 137, 653 (1990). [12] X. G. Zhang; J. Electrochem. Soc. V. 138, No 12, 3750 (1991). [13] H. J. Stein, S. M. Myers, D. M. Follstaedt; J. Appl. Phys. V. 73, No 6, 2755, 15 Mar. 1993. [14] H. D. Fuchs, M. Stuzmann, M.S. Brandt, M. Rosenbauer, cJ. Weber, A. Breitsschwerdt, P. Deak, and M. Cardona, Phys. Rev. B 48(11), 8172 (1993). [15] P. Deak, M. Rosenbauer, M. Stuzmann, J. Weber, M. S. Brandt, Phys. Rev. B, 69 (17), 2531 (1992). [16] M. Stuzmann, Phys. Stat. Sol. (b)192, 273 (1995). [17] F. Koch, V. Petrova,-Koch and T. Muschik, J. Lumin. 57, 271 (1993). [18] M. Stuzmann, M.S. Brandt, M. Rosenbauer, H. D. Fuchs, S. Finkbeiner, H. D. Fuchs and P. Deak, J. Lumin. 57, 321 (1993).