* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Guidelines - World Health Organization
Hemolytic-uremic syndrome wikipedia , lookup
Blood sugar level wikipedia , lookup
Schmerber v. California wikipedia , lookup
Blood transfusion wikipedia , lookup
Autotransfusion wikipedia , lookup
Blood donation wikipedia , lookup
Jehovah's Witnesses and blood transfusions wikipedia , lookup
Plateletpheresis wikipedia , lookup
Hemorheology wikipedia , lookup
Men who have sex with men blood donor controversy wikipedia , lookup
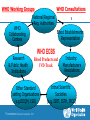
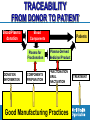
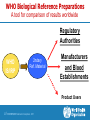
Assurance qualité des produits sanguins et autres produits biologiques Dr Ana Padilla Assurance Qualité des Médicaments Médicaments Essentiels et Produits de Santé Organization Mondiale de la Santé Web site addresses http://www.who.int/bloodproducts http://www.who.int/bloodproducts/snakeantivenoms http://www.who.int/bloodproducts/catalogue 2 | HSS/EMP/QSM: Séminaire francophone 2011 Blood Products & related Biologicals Human blood derived products Animal-derived immunoglobulins Blood components (red cells, platelets, plasma) Anti-rabies Blood Coagulation Factors Anti-venoms Polyvalent Immunoglobulins (IV, IM) Anti-tetanus toxin Specific Immunoglobulins Anti-diphteria toxin Anti-botulism toxin Anti-hepatitis B Anti-rabies Anti-tetanus Anti-rhesus (anti-D) Other biological products Anticoagulant & fibrinolysis biological therapeutic products Albumin In vitro biological diagnostic devices (IVDs): Priority: Support of international regulations 3 | HSS/EMP/QSM: Séminaire francophone 2011 Blood Products & related Biologicals Mission (Strategic Plan) A WHO normative programme: WHO is mandated by it's Member States to "…develop, establish and promote international standards for biological products." In practice, biological products cover: Vaccines, Blood and blood products; In vitro biological diagnostic devices; other biological products. An Essential Medicines Programme: To support the achievement of the health related MDGs by assisting governments and organizations to ensure equitable access to effective medicines of assured quality and their rational use by prescribers and consumers 4 | HSS/EMP/QSM: Séminaire francophone 2011 Blood Products & related Biologicals WHO standard setting functions*: Develop/establish/provide WHO Biological Reference Preparations Develop/adopt/provide evidence based WHO Guidelines on Quality Assurance and Control of specific products or procedures Support enforcement and implementation of WHO Norms and Standards:strengthen technical/regulatory capacity of NRAs & NCLs Support operational strategies to improve access to quality products (*) Consistent with the WHO mandate through the Expert Committee on Biological Standardization 5 | HSS/EMP/QSM: Séminaire francophone 2011 Blood Products and related Biologicals Target Audiencies National/Regional Regulatory Authorities National/Regional Control and National/Regional Reference Laboratories Blood Establishments and Plasma Fractionators Manufacturers of animal derived blood products Manufacturers of in vitro diagnostic tests Public Health Departments/Public Healt Officers/Ministries of Health Medical Professionals, Health Workers Procurement agencies and NGO’s 6 | HSS/EMP/QSM: Séminaire francophone 2011 WHO Working Groups WHO Consultations National/Regional Reg. Authorities WHO Blood Establishments Collaborating Representation Centres Research & Public Health Institutions WHO ECBS Blood Products and IVD Track Other Standard setting Organizations (e.g.EDQM; ISO) 7 | HSS/EMP/QSM: Séminaire francophone 2011 Industry: Manufacturers Associations Intnal Scientific Societies (e.g. ISBT, ISTH, IFCC) Produits Sanguins d'origine Humain Human Blood Derived Products Blood Plasma: a valuable human resource Medicinal products derived from human donations of blood and plasma play a critical role in health care 9 | HSS/EMP/QSM: Séminaire francophone 2011 Blood Products: Life-Saving Medicines WHA Resolution 63.12 Blood and blood components – Whole blood collected into containers, anticoagulant to prevent clotting, cold chain – Blood components, obtained from whole blood by separation (centrifuge or apheresis): • Red blood cells: Oxygen transport • Platelets: Hemostasis, preventing bleeding • Plasma: clotting factors, immunoglobulins etc. • Cryoprecipitate , FVIII source 10 | HSS/EMP/QSM: Séminaire francophone 2011 Plasma derivatives Plasma for "fractionation“, further purification of plasma proteins, e.g. • Blood Coagulation Factors, e.g. Factor VIII for treatment of hemophilia A • Specific Immunoglobulins, e.g. anti-hepatitis B, anti-rabies, anti-tetanus, anti-D • IM and IV normal IgG • Albumin, involved in the regulation of body fluids, used for resuscitation WHO List of Essential Medicines Human derived blood plasma products – Plasma for Fractionation • • • • Blood Coagulation Factors: FVIII, PCC Human Normal Immunoglobulin (IV and IM) Anti-D immunoglobulin Anti-tetanus immunoglobulin Blood-derived medicinal products for the treatment of haemophilia and immune diseases are included in the WHO Model List of Essential Medicines 11 | HSS/EMP/QSM: Séminaire francophone 2011 TRACEABILITY FROM DONOR TO PATIENT Blood/Plasma donation DONATION INFORMATION Blood Components Patients Plasma for Fractionation Plasma-Derived Medicinal Product COMPONENTS PREPARATION FRACTIONATION VIRAL INACTIVATION Good Manufacturing Practices 12 | HSS/EMP/QSM: Séminaire francophone 2011 TREATMENT Good Manufacturing Practices (GMP)*: an essential tool for improvement of safety GMP implementation in Blood/Plasma Establishments: a key element to Quality and safety of plasma for fractionation Plasma contract fractionation programs Supporting access to blood plasma products *Proposed WHO Guidelines: Final document for submission at ECBS 2010 13 | HSS/EMP/QSM: Séminaire francophone 2011 Plasma Contract Fractionation Programs (Need for GMP implementation) GMP- common principles Quality Assurance Program PLASMA SUPPLIER GMP Licensing Nat.Reg. Authority Licensing GMP Nat.Reg. Authority FRACTIONATOR across countries 14 | HSS/EMP/QSM: Séminaire francophone 2011 The ‘Achilles’ project*: A WHO initiative to assure safety and availability of blood products in developing countries * WHA Resolution 63.12 on "Availability, quality and safety of blood products" (adopted May 2010) Overall Goals (WHA Resolution 63.12) The “Achilles” project To raise quality standards in blood establishments (BE) To reduce risk of transmission of infectious diseases Effective regulatory systems for blood products worldwide To make safe blood products available to patients 17 | HSS/EMP/QSM: Séminaire francophone 2011 The “Achilles” project What do we have? Materials and mechanisms on which training and technical capacity can be provided to BE and regulatory authorities: WHO Guidelines: Production, control and regulation of plasma for fractionation; Viral Inactivation and Removal procedures; GMP for BE Biological reference materials: quality control of blood products and of blood safety related in vitro biological diagnostic devices (IVDs) Good Manufacturing Practices for Blood Establishments Assessment tool for blood regulatory systems Coordination of international expertise: ECBS, BRN, WHOCC….. Expertise from other quality assurance programs in WHO 18 | HSS/EMP/QSM: Séminaire francophone 2011 WHO Guidelines and Recommendations http://www.who.int/bloodproducts/en/ WHO Guidelines on good manufacturing practices for blood establishments WHO Recommendations for the production, control and regulation of human plasma for fractionation WHO Guidelines on viral inactivation and removal procedures intended to assure the viral safety of human blood plasma products 19 | HSS/EMP/QSM: Séminaire francophone 2011 Préparations de Référence Internationale (Étalons Internationals) Definition of Biologicals (WHO) Biological sources Crude, semi purified extracts or purified fractions of microbial, animal or human tissues Produced by biological processes Traditional/ Recombinant DNA/ Other biological technologies Biological assay - Complex molecular structure - Cannot be characterized by physicochemical criteria alone 21 | HSS/EMP/QSM: Séminaire francophone 2011 WHO Biological Reference Standards* Global measurement standards Tool for comparison of biological measurement results worldwide Facilitate transfer of laboratory science into worldwide clinical practice Underpin apropriate clinical dosage Facilitate convergence of international regulations (e.g. blood products; blood safety related IVDs) *Established by the Expert Committee on Biological Standardization 22 | HSS/EMP/QSM: Séminaire francophone 2011 WHO Biological Reference Preparations A tool for comparison of results worldwide Regulatory Authorities WHO IS/IRP 2ndary Ref. Material Manufacturers and Blood Establishments Product Users 23 | HSS/EMP/QSM: Séminaire francophone 2011 WHO Biological Reference Preparations Blood Products and related Biologicals Number of preparations 120 100 60% of total IS or Ref Panels established between 1999-2009 80 60 40 20 0 In vitro Diagnostic Tests Therapeutic products Blood Safety and General Hematology Coagul.Factors/ Thrombolytic Agents Immunological Reagents Total 55 13 10 78 0 25 11 36 WHO Catalogue of Biological Reference Preparations: www.who.int/bloodproducts 24 | HSS/EMP/QSM: Séminaire francophone 2011 Documents 25 | HSS/EMP/QSM: Séminaire francophone 2011 WHO Biological Reference Standards* Development & Establishment 1. Selection of candidate materials 7. Characterization of final product 2. Characterization of candidate materials 8. Stability studies (incl. statistical analysis) 3. Dilution of materials (dilution matrix) 9. WHO international collaborative study (incl. statistical analyses) 4. Inactivation (if needed) 5. Freeze-drying 6. Feasibility studies 10. WHOCC & Working Groups 11. Report to ECBS and decision 12. Storage and distribution *Recommendations for the preparation, characterization and establishment of international and other biological reference standards (revised 2004); Annex 2, WHO TRS, No 932, 2005. 26 | HSS/EMP/QSM: Séminaire francophone 2011 In vitro diagnostic devices (IVDs)* Medical devices used in vitro for the examination of human specimens IVDs for infectious markers Viruses, bacteria, parasites, unconventional agents IVDs for Blood/plasma screening (blood safety) Confirmation of infection Diagnosis and monitoring Tests methods Serological assays (e. g. ELISA) Nucleic acid amplification techniques (NAT) *Priority: pathogens with impact on blood safety and international regulations 27 | HSS/EMP/QSM: Séminaire francophone 2011 ECBS: HIV (IVD Technologies) http://www.who.int/bloodproducts/en/ WHO International Standard or Reference Panel Test Current Serology HIV-1 p24 antigen, 1st IS (IU) NAT Test developers, Anti-HIV, Ref Panel (no unitage) manufacturers, (HIV-1 subtypes: A, B, C, CRF_01, O; HIV-2) regulators, blood establishments, HIV-1 RNA 2nd IS (IU) fractionators, HIV-1 RNA Genotype 1st Ref Panel (no unitage) reference laboratories, diagnostic (A,B,C,D, AE, F, G, AG-GH, groups N & O) laboratories HIV-2 RNA 1st IS (IU) 1st 28 | HSS/EMP/QSM: Séminaire francophone 2011 Users ECBS: Hepatitis Viruses (IVD Technologies) http://www.who.int/bloodproducts/en/ Test Serology NAT WHO International Standard or Reference Panel Current Users Hepatitis B surface antigen, 2nd IS (IU) - adw2 Anti-Hepatitis B virus core antibodies (IU) HBsAg genotype reference panel Hepatitis A virus RNA 1st IS (IU) Hepatitis B virus DNA 2nd IS (IU) – genotype A2 Hepatitis B virus DNA Genotype 1st Reference Panel Genotypes A, B, C, D, E, F, G (no unitage) Hepatitis C virus RNA 2nd IS (IU) Test developers, manufacturers, regulators, blood establishments, fractionators, reference laboratories, diagnostic laboratories Current IS both for HBsAg and HBV DNA are genotype A2: 1% of HBV infections worldwide 29 | HSS/EMP/QSM: Séminaire francophone 2011 How do we work? – ECBS Blood Products and related Biologicals Track – WHO Blood Regulators Network: scientific/regulatory – WHO Collaborating Centres: Priority setting in the development of IBRPs – WHO disease control programmes (infectious diseases): Overview of global epidemiological data – WHO Working groups for specific topics: research & public health institutions; manufacturers associations; – Coordination with other standard setting organizations and professional organizations: ISBT, ISTH, IFCC, EDQM, EC 30 | HSS/EMP/QSM: Séminaire francophone 2011 Immunoglobulines d'origine animal Produit thérapeutique d'origine animal WHO Essential Medicines List Animal derived blood products – Snake anti-venom immunoglobulins 32 | HSS/EMP/QSM: Séminaire francophone 2011 SNAKE ANTIVENOM IMMUNOGLOBULINS VERY POOR REGULATORY CONTROL: Technology in the public domain A - Collection of venoms B – Horse Immunization Protocols C – Starting material of animal derived sera D – Fractionation & Purification process WHO Guidelines and Recommendations ANIMAL DERIVED BLOOD PRODUCTS WHO Guidelines on production, control and regulation of snake antivenom immunoglobulins WHO Database: clinically important venomous snakes species and its worldwide geographical distribution together with antivenoms for treatment of snakebite envenomings WHO website hosting both the Guidelines and database (maps, pictures, products, manufacturers) 34 | HSS/EMP/QSM: Séminaire francophone 2011 WHO Database: Medically Important Snakes Distribution maps, pictures & antivenoms Red or orange question marks (?) (Indicates expected presence not yet confirmed due to lack of exploration Allocation to CATEGORY 1 shown in red (Indicates common, widespread species that causes numerous snake bites with high morbidity, disability or mortality) Allocation to CATEGORY 2 shown in orange (Indicates highly venomous and capable of causing morbidity, disability or mortality, but exact country data lacking, or less frequently implicated in these countries) www.who.int/bloodproducts/snakeantivenoms 35 | HSS/EMP/QSM: Séminaire francophone 2011 WHO Database: Medically Important Snakes Distribution maps, pictures & antivenoms www.who.int/bloodproducts/snakeantivenoms WHO Guidelines 36 | HSS/EMP/QSM: Séminaire francophone 2011 WHO web site: Target Audiences Central information source for data on the current availability of antivenoms for specific species. Aimed at a wide audience, that includes: – – – – – National Regulatory Agencies Ministries of Health Antivenom Manufacturers Medical Professionals, Health Workers Procurement Personnel in Industry and NGO’s Objective is to use the web site to distribute accurate data that can be used to plan improvements to existing supply and distribution. 37 | HSS/EMP/QSM: Séminaire francophone 2011 Web site addresses http://www.who.int/bloodproducts http://www.who.int/bloodproducts/snakeantivenoms http://www.who.int/bloodproducts/catalogue E-mail addresses: [email protected]; [email protected] 38 | HSS/EMP/QSM: Séminaire francophone 2011