* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download injection in rapid tranquillisation - South West Yorkshire Partnership
Survey
Document related concepts
Transcript
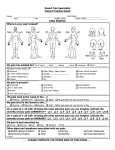
RAPID TRANQUILLISATION AND PRN PSYCHOTROPIC MEDICATION; POLICY AND GUIDANCE Document name: Rapid tranquillisation and PRN psychotropic medication; policy and guidance. Version 6 Staff group to whom it applies: Qualified medical staff, qualified nursing staff, pharmacists and pharmacy staff. All staff working on in-patient units. Distribution: Hard copy to in-patient areas Intranet Medical staff on induction Issue date: February 2016 Next review: February 2018 Developed by: Mark Payne Mohammed Fazlee Sandra Butler Contacts for advice or information: Jane Riley, Chief Pharmacist Dr Adrian Berry, Medical Director Mark Payne, Senior Clinical Pharmacist 1 CONTENTS Abbreviations ................................................................................................................. 3 Prescribing algorithm ..................................................................................................... 4 Flowchart ....................................................................................................................... 5 1 2 3 4 5 6 7 Introduction ............................................................................................................ 6 Definitions and Principles ...................................................................................... 7 Prescribing guidance ............................................................................................. 8 Formulary .............................................................................................................. 12 Carrying out Rapid Tranquillisation........................................................................ 17 Physical health monitoring ..................................................................................... 18 Managing side effects and complications .............................................................. 19 Appendix 1 Rapid Tranquillisation Episode Criteria and Review Pathway ................ 21 Appendix 2 Rapid Tranquillisation – Assessment and Progress Chart ....................... 22 Appendix 3 The Richmond Agitation – Sedation Scale............................................... 24 Appendix 4 48 Hour Intramuscular Clopixol Acuphase Monitoring Chart ................... 25 Appendix 5 Guidance on the Preparation & Administration of Aripiprazole (Abilify®) 26 Appendix 6 Haloperidol Administration ....................................................................... 27 Appendix 7 Guidance on the Preparation & Administration of Olanzapine (Zyprexa®) …………………………………………………………………………………. .... 28 Appendix 8 Advice on the Preparation & Administration of Lorazepam (Ativan®) ..... 29 Appendix 9 Policy for the use of midazolam .............................................................. 30 Appendix 10 Implementation ........................................................................................ 32 Appendix 11 Equality Impact Assessment Tool ......................................................... 33 Appendix 12 Checklist for the Review and Approval of Procedural Document ............ 34 Appendix 13 Training Needs ....................................................................................... 36 Appendix 14 Version Control Sheet ............................................................................ 38 References .................................................................................................................... 40 2 Abbreviations used in this document BNF CNS CPR D&T ECG EPSE ESR FBC IM IV LFT MDT MAV NICE NMS PO PRN QT/QTc RT SPC SWYPFT ST TFTs TIA U&Es British National Formulary Central Nervous System Cardiopulmonary resuscitation Drug and Therapeutics Sub Committee Electrocardiogram Extrapyramidal side effects Electronic Staff Record Full Blood Count Intramuscular injection Intravenous injection Liver Function Tests Multidisciplinary team Managing Aggression and Violence National Institute for Health and Care Excellence Neuroleptic malignant syndrome Oral/by mouth Medicines to be taken as and when required Interval in the cardiac cycle Rapid Tranquillisation Summary of Product Characteristics South West Yorkshire Partnership NHS Foundation Trust Speciality Trainee Thyroid Function Tests Transient Ischemic Attacks Urea and Electrolytes 3 Version 6 Feb 16 SOUTH WEST YORKSHIRE PARTNERSHIP NHS FOUNDATION TRUST Rapid Tranquillisation / PRN prescribing algorithm Consider de-escalation techniques eg: talking down, distractions, time out Have access to procyclidine injection for acute dystonic reactions and flumazenil for benzodiazepine induced respiratory depression. Have access to emergency resuscitation facilities Consider starting/increasing regular oral medication No response Consider pharmacological management Seek consultant advice re MHA status. If necessary treatment may continue under common law Try Oral Therapy Patient refusing oral / Rapid response required / No response to oral Try intramuscular injection Adult Drug (oral) Lorazepam 1-2mg or Promethazine 25-50mg Where the use of benzodiazepines is inappropriate Elderly/Physically Frail Drug (oral) Lorazepam 0.5-1mg or Promethazine 25mg Where the use of benzodiazepines is inappropriate And/or another from the list below Olanzapine 5-15mg Haloperidol 3-5mg Risperidone 2mg And/or another from the list below Olanzapine 2.5-5mg Haloperidol 1.5-2.5mg Risperidone 1-2mg Use formulation most appropriate to patient Use formulation most appropriate to patient Adult Drug (IM) Lorazepam 1-2 mg or Promethazine 25-50mg Where the use of benzodiazepines is inappropriate and/or Haloperidol 2-5mg (if known to tolerate typical antipsychotics) Elderly/Physically Frail Drug (IM) Lorazepam 500 micrograms-1mg or Promethazine 25-50mg Where the use of benzodiazepines is inappropriate and/or Haloperidol 1-2.5mg (if known to tolerate typical antipsychotics) Response Optimise regular psychotropic medication Restart or start oral medication Response Review appropriateness of continuing intramuscular therapy Monitor Patient No response within ½ hour Never mix two drugs in the same syringe. Always dilute lorazepam injection before use Review. Complexity – refer to consultant Outside normal working hours – refer to on-call consultant. The On call pharmacist is also available for advice. Consider repeating IM lorazepam (Adult Max 4 mg in 24 hours) and haloperidol 5mg injections (caution – maximum adult haloperidol dose is 12mg IM in 24 hours, avoid repeating haloperidol in the elderly above a total of 5mg IM without Consultant advice) or any of the following Aripiprazole 5.25-15mg IM (max dose 30mg in 24hrs by any route, however only IM used in Rapid Tranquillisation, do not give when other antipsychotics are prescribed) 4 Zuclopenthixol Acetate (Clopixol Acuphase) • Not recommended for RT due to delayed onset and long duration of action but may be considered as an option when: 1 Patient will be disturbed/violent over extended time period. 2. Past history of good/timely response. 3. Past history of parental administration. 4. Cited in an advance directive. • Never administer to those without previous antipsychotic exposure. • Consult BNF and manufacturer’s SPC regarding its use and SWYPFT guidelines and monitoring charts. RAPID TRANQUILLISATION / PRN PRESCRIBING FLOWCHART Review patient’s presentation Check for physical health monitoring; ECG, pulse, BP, U&E’s Patient requires rapid tranquillisation (RT) Patient requires pro re nata (PRN) Document treatment decision (Appendix 1) Document treatment decision Medication administered Monitor physical health / arousal for 2hrs or until ambulant (whichever is longer). Document monitoring (Appendix 2) Review and record need for RT (Appendix 1) Record intervention on Rio If requiring high doses of medicines or formulary medication ineffective contact senior medic (ST / Consultant) File paperwork and complete DATIX Debrief staff team and review medication regime in the MDT meeting 5 SOUTH WEST YORKSHIRE PARTNERSHIP NHS FOUNDATION TRUST Rapid Tranquillisation Policy 1. 1.1 Introduction Scope This policy is intended to support the delivery of appropriate, safe and effective rapid tranquillisation in the context of in-patient care within SWYPFT and identifies which clinical staff need training. This policy replaces all previous local RT related policies or procedures and represents expected (i.e. usual) practice within all districts and the regional forensic service. 1.2 Practice Variation This policy sets out the standards of care that are expected by SWYPFT when prescribing medication for the management of acute behaviour disturbance. Where clinical need indicates that practice which falls outside of the scope of this guidance is necessary, the following should be discussed with a senior psychiatrist and recorded in the patient’s clinical notes : - The exact nature of the intervention - The rationale for this intervention, including acknowledgement of usual practice, and that this is a deviation from this - A clearly defined aim of the treatment - A clearly defined timescale for review - The name of the senior psychiatrist with whom the plan has been discussed and agreed 1.3 Sources of Guidance A full list of references is given at the end of this document, but this policy takes particular note of NICE Guideline 10 and 178 (management of Violence and Psychosis respectively) and is a successor to the SWYPFT Rapid Tranquillisation Protocol version 5.3.1, September 2014 and the Guidelines for Rapid Tranquillisation for acutely disturbed behaviour for adults in Barnsley approved May 2010. 1.4 Duties 1.4.1 Healthcare organisations have an obligation to provide an effective rapid tranquillisation service to their patients and appropriate training to their staff. A suitable infrastructure is required to establish and continue support for these activities. 1.4.2 The Chief Executive is responsible for ensuring that resources and mechanisms are in place for the overall implementation, monitoring and review of this policy. Implementation of this document is outlined in appendix 10, page 35. 6 1.4.3 The Medical Director, the Director of Nursing and the Chief Pharmacist are responsible for ensuring the policy is reviewed, approved and monitored by the appropriate trust-wide group (currently the Drug & Therapeutic Sub Committee). 1.4.4 The Executive Management Team will provide policy approval and ratification. 1.4.5 The Drug & Therapeutic Sub Committee will consider the monitoring evidence put before them and request actions as appropriate. 1.4.6 The General Manager, Practice Governance Coach and Clinical Lead are responsible for ensuring that dissemination and implementation of the policy occurs within their own area of responsibility. In addition, they are responsible for providing relevant support for the training required around RT as outlined in the training needs analysis. 1.4.7 Ward Managers must ensure staff have access to training on RT (see appendix 13 training needs analysis and training matrix, Section 1 of the Medicine Code). 1.4.8 As well as training on RT there must be staff trained on the use of oxygen, pulsoximetry and defibrillators on the wards when RT is employed. 2 Definitions and Principles 2.1 Definitions Throughout this document the following definitions have been used, in line with NICE guidance: 2.1.1 p.r.n. (pro re nata): When needed. In this guideline, p.r.n. refers to the use of medication as part of a strategy to de‑escalate or prevent situations that may lead to violence or aggression; it does not refer to p.r.n. medication used on its own for rapid tranquillisation during an episode of acutely disturbed behaviour. 2.1.2 Rapid tranquillisation: Use of medication by the parenteral route (usually intramuscular or, exceptionally, intravenous) if oral medication is not possible or appropriate and urgent sedation with medication is needed. 2.1.3 A multidisciplinary team that includes a psychiatrist and a specialist pharmacist should develop and document an individualized pharmacological strategy for using routine and p.r.n. medication to calm, relax, tranquillise or sedate patients who are at risk of violence and aggression as soon as possible after admission to an inpatient psychiatric unit. 2.1.4 The administration of medication by a short-acting intramuscular injection should always be considered rapid tranquillisation and the principles of this policy adhered to, even where the patient expresses a preference for this route of administration. 2.2 Principles 7 2.2.1 Patients should be involved in their care at the earliest opportunity, those requiring p.r.n or rapid tranquillisation should not be denied this opportunity. Where it is not possible to discuss these issues with a patient prior to the administration of medication then this should be discussed with them at the earliest opportunity following the intervention. 2.2.2 Checks should be made prior to administering medication whether there is an advance decision or advance statement in place. Where there is not an advance decision or statement in place regarding the use of p.r.n or rapid tranquillisation the patient should be encouraged and supported to develop one. 2.2.3 Where a patient is subject to a restrictive intervention such as rapid tranquillisation, this should only be after less restrictive interventions have been considered and deemed ineffective or inappropriate. Even where a restrictive treatment has been required previously, the least restricitive intervention should always be considered first before repeating this intervention. 2.2.4 All treatment decisions, administrations of medication and discussions with patients about their treatment should be documented in the patients notes to inform future care. 3 Prescribing guidance 3.1 Baseline checks 3.1.1 Prior to prescribing medication for use as p.r.n or rapid tranquillisation the following should be reviewed and documented: 3.1.2 The medication prescribed for regular administration 3.1.3 Whether there are any issues with administering the regular medication e.g. refusal of doses / issues with swallowing 3.1.3 Whether any changes to the regular medication regime are indicated, and how these will be reviewed / actioned (please consider local supply arrangements for medication before making changes to the treatment regime). 3.1.4 Whether there is currently a need for p.r.n or rapid tranquillisation 3.1.5 Where a need for p.r.n or rapid tranquillisation is identified the following should be considered and documented: 3.1.5.1 3.1.5.2 3.1.5.3 The symptoms that are to be targeted by the p.r.n / rapid tranquillisation How these symptoms will be monitored How to measure if the medication administered has been effective e.g reduction in agitation / time spent out of seclusion 8 3.1.5.4 3.1.5.5 3.1.5.6 3.1.5.7 3.1.5.8 3.1.5.9 3.1.5.10 3.1.5.11 The medication that is to be prescribed, recording: a) Name of the medication b) Dose; individual and maximum c) Frequency of administration (minimum interval, and maximal frequency) d) Route of administration Any available information on historical response to medication Any available information on historical tolerability of medication The patient’s preference including any documented advance statements / advance decisions Any physical health conditions, particularly those which may affect the absorption / distribution / elimination of medication. The current physical health parameters (ECG / blood tests / cardiovascular monitoring) Any other information taken into consideration when making the decision. The timescale for review A proforma is provided (Appendix 1) to facilitate the recording of this information. 3.2 Prescribing medication for use as P.R.N 3.2.1 When prescribing p.r.n. medication as part of a strategy to de‑escalate or prevent situations that may lead to violence and aggression: 3.2.1.1 Do not prescribe p.r.n. medication routinely or automatically on admission 3.2.1.2 Tailor p.r.n. medication to individual need and include discussion with the patient if possible 3.2.1.3 Ensure there is clear documentation of the rationale and circumstances in which p.r.n. medication may be used and that these are included in the care plan and on the prescription. 3.2.1.4 Do not exceed the maximum daily dose stated in the British National Formulary (BNF) for each drug, or exceed 100% of the BNF maximum for a single drug when combined with the person's standard dose and their dose for rapid tranquillisation unless this is planned to achieve an agreed therapeutic goal, documented, and carried out under the direction of a senior doctor (ST / Consultant) 3.2.1.5 Ensure that the interval between p.r.n. doses is specified. 3.2.1.6 Prescriptions for p.r.n medication should state: a) Name of medication b) Dose or dose range c) Route of administration d) Frequency of administration / minimum interval between doses e) Maximum dose in 24 hours f) Prescribers signature g) The indication for use h) Start date for the prescription 9 3.2.1.7 Where a medication is prescribed by more than one route, this should be indicated on the prescription. Prescriptions allowing the administration of a medication by more than one route e.g PO/IM are only acceptable where there is the facility to record the route of administration on the drug chart and the doses are bioequivalent. 3.2.2 The multidisciplinary team should review the pharmacological strategy and the use of medication at least once a week and more frequently if events are escalating and restrictive interventions are being planned or used. The review should be recorded in the MDT review and include: a) b) c) d) e) f) g) 3.3 clarification of target symptoms the likely timescale for response to medication the total daily dose of medication, prescribed and administered, including p.r.n. medication the number of and reason for any missed doses therapeutic response the emergence of unwanted effects. Consideration of the development of dependence or tolerance to the medication Prescribing medication for use as rapid tranquillisation 3.3.1 When prescribing medication for use as rapid tranquillisation: 3.3.1.1 3.3.1.2 3.3.1.3 3.3.1.4 Do not prescribe rapid tranquillisation for ongoing use Tailor each episode of rapid tranquillisation to the patients needs, giving consideration to: a) Previous response to medication b) Previous tolerability of medication c) Patients preference d) Concurrent prescribed medication e) Comorbid health conditions Prescriptions should have a set duration of treatment , initial prescriptions should be for no more than 24 hours treatment, subsequent prescriptions should be based on a comprehensive review of the efficacy and tolerability of the initial prescription. Referral for review by the MDT should be at the earliest opportunity. Where an ongoing need is identified for the use of rapid tranquillisation the MDT should review the need and make the same documentation as they would for prn medication (see 3.2) 3.3.2 If rapid tranquillisation is being used as an ongoing part of a patients care plan, a senior doctor (ST / Consultant) should review all medication at least once a day. This review should be documented in the patients notes. 3.3.3 When IM medication is used the incident and associated monitoring should be reported via the local incident reporting system. 10 3.4 Use of medication in combination with physical intervention 3.4.1 Patients may occasionally require physical restraint to prevent violence to themselves or others. Swift, safe and effective drug treatment may then be needed to effect rapid tranquillisation and to allow subsequent evaluation and appropriate management. Medication should be used in the context of a combination of approaches to the management of the agitated patient and at all stages continue talking and using non-drug approaches and use the least restrictive option available. 3.4.2 Medication for RT, particularly in the context of physical intervention, should be used with caution owing to the following risks:a) b) c) d) e) f) g) loss of consciousness instead of tranquillisation sedation with loss of alertness loss of airway cardiovascular and respiratory collapse interaction with medicines already prescribed or illicit substances taken possible damage to patient-staff relationship underlying coincidental physical disorder 3.4.3 It should noted that the risks due to medication are increased when combined with physical restraint due to increased physical activity and restriction of motion. 11 4 Formulary Drug Lorazepam Forms available 1mg tablets 1mg/1ml intramuscular injection Dosing Adult: 1-2mg, up to 4mg/24hrs. Elderly / Frail: use half adult dose. 12-18yrs: as per adult dose. At least 1hr between doses. Adult: 1-2mg, up to 4mg/24hrs. Elderly / Frail: use half adult dose. 12-18yrs: as per adult dose. Additional information Tablets can be dispersed in water where there are issues with swallowing (off-label use). Absorption is no more rapid than oral: use in those unable / unwilling to take oral medication only. Injection needs to be diluted 1:1 with water for injection prior to administration. At least 1hr between doses. Promethazine Haloperidol 25mg tablets 25mg/5ml liquid 25mg/ml intramuscular injection 500microgram capsules 1.5mg tablets 5mg tablets 5mg/5ml liquid 5mg/ml intramuscular injection Adult: 25-50mg, up to 100mg/24hrs. Elderly / Frail: as per adult dose. At least 2hrs between doses. Oral Dosing Adult: 3-5mg, up to 20mg/24hrs. Elderly / Frail: use half adult dose. At least 2hrs between doses. Intramuscular Dosing Adult: 2-5mg, up to 12mg/24hrs. Elderly / Frail: use half adult dose. At least 1hr between doses. Olanzapine 2.5mg tablets 5mg tablets / orodispersible tablets In cases of non-response to standard doses, consider dosing by weight: 25–30 micrograms/kg (usual range 1.5–2.5 mg), repeated every 6 hours if necessary. Consider using lower starting doses in the elderly / frail until tolerability is established, due to risk of sedation / postural hypotension / anticholinergic side effects. NB: dose / maximum varies according to route of administration due to differences in bioavailability. 5mg PO = 3mg IM Consider prescribing bioequivalent doses PO and IM, and giving stated maximum as a number of doses per 24hrs: e.g. Haloperidol 5mg PO or 3mg IM, no more than 4 doses to be given in a 24hr period. Adult: 5-10mg, up to 20mg in 24hrs. Elderly / Frail: use half adult dose. 12 10mg tablets / orodispersible tablets 15mg tablets Risperidone 500microgram tablets / orodispersible tablets 1mg tablets / orodispersible tablets 2mg tablets / orodispersible tablets 1mg/ml liquid Aripiprazole 7.5mg/ml intramuscular injection Zuclopenthixol acetate 50mg/ml intramuscular injection Midazolam 1mg/ml intramuscular injection 5mg/ml intramuscular injection At least 4hrs between doses. Adult: 2mg, up to 2mg/24hrs Licensed for use in dementia for up to 6 weeks Elderly / Frail: 1-2mg, up to treatment. 2mg/24hrs Dementia: 250-500micrograms, up Consider using in patients already prescribed to 2mg/24hrs risperidone, due to inflexibility of dosing. At least 6hrs between doses. Adult: 5.25mg / 7.5mg / 9.75mg / Not recommended in combination with other 15mg , up to 30mg/24hrs or 3 antipsychotics. Suggest to use in those injections. patients treated with regular aripiprazole. Elderly / Frail: as per adult dosing. At least 2hrs between doses. Adults: 50-150mg, maximum 400mg Consider when: / 4 injections in 14 days. - Repeated IM administration has been Elderly / Frail: 50-100mg, maximum necessary 400mg / 4 injections in 14 days. - Patient expresses a preference for this At least 24hrs must be left after the first dose before administering subsequent doses. At least 48hrs must be left after subsequent doses before a further dose is administered. Adult: 2.5-7.5mg, maximum 15mg/24hrs. Elderly / Frail: 1-2mg, maximum 7.5mg/24hrs. At least 1hr between doses. Not for use in: - Pregnancy - Antipsychotic naiive patients - Patients who have experienced intolerable EPSE with typical antipsychotic medication ONLY APPROVED FOR USE IN CASES OF LORAZEPAM SHORTAGE. NB: this is a controlled drug, and so consideration needs to be given to storage / administration restrictions. 13 4.1 Choice of treatment 4.1.1 The following should be considered when choosing which treatment is appropriate for use, and documented in the patients notes: a) The patients preferences or advance statements and decisions b) Pre-existing physical health conditions c) Whether there is a chance the patient may be pregnant, and whether this has been tested d) Previous response to medication, including adverse effects e) Potential for interaction with other medications f) The total daily dose of medication prescribed and administered 4.1.2 The Trust recommends the following approach be considered: 4.1.2.1 Ensure appropriate p.r.n medication is available 4.1.2.2 Where rapid tranquillisation is required: a) Use lorazepam alone where there is no clear psychotic component to the presentation b) Antipsychotic medication should be avoided where there is prologation of the QT / QTc interval (see: Reference document for monitoring the physical health of service users taking psychotropic medication ) c) Do not prescribe more than one antipsychotic medication and one sedative medication at a time. d) Where clinically necessary a sedative and antipsychotic medication can be administered at the same time, however co-administration of these medications should not be considered “standard treatment”. 4.1.2.3 Combined antipsychotic dosages (regular / PRN / rapid tranquillisation) should not exceed 100% BNF maximum for a single drug unless this is planned to achieve an agreed therapeutic goal, documented, and carried out under the direction of a senior doctor (ST / Consultant) 4.1.2.4 Zuclopenthixol acetate is not considered to be part of rapid tranquillisation, it should form part of a patient’s ongoing management plan, and as such should only be prescribed by the regular MDT, except where there is a clear indication that this is part of the MDT care plan, or it is stated in an advance directive. 4.1.3 Zuclopenthixol acetate injection is not recommended for RT due to its significantly delayed onset and relatively long duration of action. However, it may be considered as an option in the management of RT when:a) Initial calming has been achieved and it is likely that repeated doses of IM antipsychotics will be necessary. b) It is clearly expected that the patient will be disturbed/violent over an extended period of time and will refuse oral medication. c) A patient has a past history of good and timely response to it. d) An advance directive indicates that it is the treatment of choice. 4.1.4 Zuclopenthixol acetate SHOULD NOT BE USED on individuals who: a) Are antipsychotic (neuroleptic) naïve i.e. patients without any previous exposure to antipsychotic medication b) Are sensitive to extrapyramidal side effects c) Have cardiac disease d) Have hepatic or renal impairment 14 e) Are pregnant 4.1.5 The efficacy and tolerability of each individual dose should be assessed before a decision is made regarding the prescribing or administration of further doses. 15 4.2 Decision support tool This tool provides a summary of the information available on the use of these medications in patients with a documented co-morbidity, it is intended as a guide, and should not be used as a replacement for clinical judgement. Drug Cardiac disease Respiritory Hepatic Renal Pregnancy Epilepsy depression impairment impairment * Effect on QT** No effect Additional info Zuclopenthixol acetate No effect Contra-indicated in patients with depressed CNS function Midazolam No effect Lorazepam Contra-indicated in sleep apnoea Low Contra-indicated in effect patients with depressed CNS function Moderate Contra-indicated in severe effect / uncontrolled cardiac disease Low effect Moderate effect No effect Promethazine Haloperidol Olanzapine Risperidone Aripiprazole Key Reccomended Seek further guidance*** Avoid *the use of any medication during pregnancy carries some risk, even the “recommended” treatments are not risk free. See trust policy on prescribing psychotropic medication in pregnancy **when used at therapeutic doses ***further guidance should be sought from a senior doctor (ST / Consultant) or a pharmacist For up-to-date guidance on interactions or dose information please see the electronic BNF (http://www.evidence.nhs.uk/formulary/bnf/current) 16 5 5.1 5.2 Carrying Out Rapid Tranquillisation The advice of the senior doctor (ST / Consultant) should be available at all times and should be used accordingly. The patient should be able to respond to communication throughout. 5.3 The aim is to achieve a state of calm sufficient to minimise the risk posed to the patient or to others. 5.4 Keep the drug regimen simple and document the treatment plan. 5.5 The initial doses of medicine should be small and within the recommended BNF limits unless the patient is categorically known from previous episodes to require larger doses. 5.6 are Sufficient time should be allowed for the therapeutic response before doses repeated. 5.7 Review of the efficacy and tolerability of the medication administered should take place regularly, at intervals appropriate to the patients presentation. 5.8 After using intramuscular medication, and when the risks of harm have been contained, conduct an immediate post‑incident review in line with the policy on Management of Aggression and Violence, including a nurse, doctor and where appropriate the patient, to identify and address physical harm to patients or staff, ongoing risks and the emotional impact on patients and staff, including witnesses. 5.9 There should be regular review of the doses of intramuscular medication administered in order to identify any patterns of use which may inform future care planning. 5.10 When a patient is transferred between units, a full medical history, including the patient’s response to medications, any adverse effects, and an advance directive should accompany them. Where possible, the patient’s account of their experience of RT should also be included. 5.11 On discharge, all such information should be filed in the persons healthcare record . 17 6 Physical health monitoring 6.1 Following the administration of p.r.n medication there is no additional physical health monitoring required. 6.2 Following the administration of rapid tranquillisation the following parameters should be monitored: a) Blood pressure b) Pulse c) Temperature d) Respirations e) Alertness (Using the RASS, Appendix 3) f) Hydration g) Pulse oximetry (where the patient is unconscious) 6.3 Monitoring should be carried out at 15 minute intervals for the first hour, and 30 minute intervals for the second hour. Where the patient is not ambulatory at 2 hours monitoring should continue at least every 30 minutes until the patient is ambulatory. 6.4 An ECG should be carried out where the patient has received a dose of antipsychtoics which takes them above a combined 100% BNF maximum. 6.5 Where it is not possible to monitor the required parameters the reasons for this should be clearly documented and reviewed with the prescriber or the clinical team prior to the administration of further doses. 6.6 Where it is not possible to record all of the required parameters this should not prevent the team from recording those which are possible. 6.7 Monitoring forms should be submitted as part of an incident report via the local incident reporting system, and filed in the patients notes. 18 7 Management of side effects and complications 7.1 Recognition and management of side effects which require medical attention Complications Symptoms/Signs Management Acute Procyclidine 5-10mg IM: for severe dystonias Severe painful reactions. including muscular stiffness Procyclidine 5-10mg PO: for moderate oculogyric reactions. crisis Hypotension Lie patient flat and raise legs Monitor (orthostatic or Fall in blood closely. <50mmHg pressure Seek medical advice. diastolic) Increasing temperature, Neuroleptic Withhold antipsychotics. fluctuating blood Malignant Monitor closely. pressure, Syndrome Liaise with general medical team. muscular rigidity, (NMS) Creatinine Kinase levels confusion, altered consciousness Arrhythmias Respiratory Depression Perform an ECG. Slow (<50/minute) Withhold antipsychotics. or irregular pulse Monitor closely and liaise with specialist medical team immediately. Reducing respiratory rate, reducing consciousness Give oxygen. Raise legs. If necessary ventilate mechanically. If respiratory rate drops below 10/minute or Oxygen Saturation <90% ring emergency services as per cardiopulmonary resuscitation procedures. 7.2 Respiratory depression is a well characterized side effect of benzodiazepines, the risks of this are increased by: a) Underlying respiratory disease b) Existing compromised respiratory function c) Co-administration with other medications known to suppress respiratory function e.g opiates. d) Administration via the parenteral route e) Administration of higher doses 7.3 Where a patient has received benzodiazepines and experiences respiratory depression flumazenil should be administered. At the time of administration the emergency services should be contacted. 19 7.4 Guidance on the use of Flumazenil 7.4.1 Flumazenil is a benzodiazepine antagonist, it will only reverse the effects of benzodiazepine administration. If the respiratory depression is thought to be due to other causes then do not administer flumazenil. 7.4.2 Flumazenil is a non-specific benzodiazepine antagonist, it will reverse the effects of all benzodiazepine treatment. Where there is a prescription for regular benzodiazepine treatment the impact of the reversal of this therapeutic effect should be considered. 7.4.2 Flumazenil is required to be given by IV injection so this is only possible by trained medical personnel. 7.4.3 How to give flumazenil: i. 200 micrograms IV over 15 seconds. ii. If consciousness is not resumed within 60 seconds give 100 micrograms over 10 seconds. iii. Repeat at 60 second intervals. Maximum dose 1mg/24 hours. 7.4.4 Continue to monitor after respiratory rate returns to normal. 7.4.5 Flumazenil has a short duration of action so further doses may be required. Patients may become agitated or anxious on awakening. 20 Appendix 1 RAPID TRANQUILLISATION EPISODE CRITERIA AND REVIEW PATHWAY, SWYPFT NAME WARD RiO NUMBER MHA STATUS DOB ETHNICITY (OR AFFIX LABEL HERE) Criteria of Need for RT episode: The patient currently is suffering from: (ring all symptoms) Severe Aggression Severe Agitation Severe Disinhibition Other (please specify)…………………………………………………………… Symptomatic improvement to be by……………………………………………….. There is an immediate risk to self or others (tick box) Non-pharmacological interventions not de-escalated behaviour measured There is an immediate need for Rapid Tranquillisation The following criteria have been considered ( - considered, - unavailable) ECG TFT FBC Previous response Patient preference U&E LFT Previous tolerability Physical health conditions Medication regime prescribed by: Print Sign Medication regime initiated by: Print Sign Date Time Reviews of need for RT (enter next due review and time after each review) Planned Actual review Medication review Print review date / date / time Effective? Tolerated? time Sign 21 Appendix 2 RAPID TRANQUILLISATION – ASSESSMENT AND PROGRESS CHART Patient name RiO Number Date Time Before IM medication is administered, the following has been considered Physical examination Recent U & E Recent Drug Screen Recent ECG History of EPSE Past response to RT This review led to these investigations or actions (specify any deemed necessary) Time Actual date and time Temp BP Pulse bpm Resprn. Per min pO2 RASS score Comments Signature 0 mins 15 mins 30 mins 45 mins 60 mins 90 mins 120 mins 22 RAPID TRANQUILLISATION – ASSESSMENT AND PROGRESS CHART: GUIDANCE Temperature Blood pressure Pulse (Beats / min) Respirations (per min) 1. pO2 RASS If temperature increasing monitor closely for Neuroleptic Malignant Syndrome: 1. Fluctuating blood pressure 2. Muscular rigidity 3. Confusion 4. Altered consciousness If any of these feature present seek urgent medical advice. If Diastolic <50 mmHg: 1. Lie patient flat and raise legs 2. Monitor closely. 3. Seek medical advice. If pulse slow (<50 bpm) or irregular: 1. Perform an ECG. 2. Withhold antipsychotics. 3. Monitor closely 4. Seek medical advice If respirations slowing: 1. Give oxygen. 2. Raise legs. 3. If necessary ventilate mechanically. 4. Seek medical advice If respiratory rate drops below 10/minute or Oxygen Saturation <90% ring emergency services as per cardiopulmonary resuscitation procedures. If benzodiazepines have been administered consider administration of Flumazenil. If < 0 1. Increase frequency of monitoring 2. If decreasing rapidly seek medical advice 23 Appendix 3 The Richmond Agitation – Sedation Scale (SWYPFT adaptations) Score Term Description +4 Combative: +3 Very agitated Overtly combative or violent; immediate danger to staff Frequently irritable/aggressive. damaging property. +2 Agitated Marked restlessness such as pacing, some irritability. +1 Restless: vigorous Anxious or apprehensive but movements not aggressive or 0 Alert and calm -1 Drowsy: Not fully alert, but has sustained (more than 10 seconds) awakening, with eye contact to voice -2 Light sedation: Briefly (less than 10 seconds) awakens with eye contact to voice -3 Moderate sedation: Any movement (but no eye contact) to voice -4 Deep sedation: No response to voice, but any movement to physical stimulation -5 Unarousable: No response to voice or physical stimulation Procedure 1. Observe patient. Is patient alert and calm? (score 0) Does patient have behaviour that is consistent with restlessness or agitation? 2. If patient is not alert, in a loud speaking voice state patient’s name and direct patient to open eyes and look at speaker. Repeat once if necessary. Can prompt patient to continue looking at speaker. 24 Appendix 4 48 hour Intramuscular Zuclopenthixol Acetate Monitoring Chart Date of injection……………………………..Time of injection…………………………..Dose……………….. Date Time Temp Pulse Resp BP EPSE’s Comment/Action Within 2 hours 1 4 hourly onwards 2 3 4 5 6 7 8 25 Appendix 5 Guidance on the Preparation and Administration of Aripiprazole (Abilify®) Intramuscular (IM) Injection Indicated for the rapid control of agitation and disturbed behaviours in patients with schizophrenia or in patients with manic episodes in Bipolar I Disorder, when oral therapy is not appropriate. For intramuscular use only. Do not administer intravenously or subcutaneously. The aripiprazole injection strength is 7.5mg/ml The aripiprazole vial contains 9.75mg in 1.3ml The recommended initial dose for aripiprazole solution for injection is 9.75 mg (1.3 ml), administered as a single intramuscular injection. The effective dose range of aripiprazole solution for injection is 5.25-15 mg as a single injection. Patients with hepatic impairment should be managed cautiously. A lower dose of 5.25 mg (0.7 ml) may be given, on the basis of individual clinical status, which should also include consideration other medication already administered either for maintenance or acute treatment. A second injection may be administered 2 hours after the first injection, on the basis of individual clinical status. No more than three injections should be given in any 24-hour period. The maximum dose in 24 hours of aripiprazole is 30 mg (including all formulations of aripiprazole). The following shows the volume required for doses of aripiprazole injection: Dose of aripirazole required 5.25mg 9.75mg 15mg Volume of aripiprazole inj. (7.5mg/ml) 0.7ml 1.3ml (complete vial) 2ml (2 vials needed) 26 Appendix 6 HALOPERIDOL ADMINISTRATION – ORAL and INTRAMUSCULAR EQUIVALENT DOSES Intramuscular doses have a greater bioavailability than oral doses, therefore the maximum recommended daily dose for each route of administration is different. Use separate lines on the treatment sheet for each route of administration . Maximum oral dose in 24 hours is 20mg Maximum IM dose in 24 hours is 12mg Any doses above these should be monitored according to High Dose Antipsychotic guidance If a patient has received both haloperidol IM and oral in the last 24 hours. Use the conversion chart below to calculate how much the patient has received in total: APPROXIMATE EQUIVALENT DOSES (mg) Oral Haloperidol 0.5 1 1.5 2.5 4.2 5 7.5 8.3 10 12.5 16.7 20 IM Haloperidol 0.3 0.6 0.9 1.5 2.5 3 4.5 5 6 7.5 10 12 For example: Patient has been given 1 x 5mg haloperidol IM, followed 30 minutes later by 5mg orally, then 30 minutes later by another 5mg orally. Convert to all oral doses, i.e. 8.3mg + 5mg + 5mg = 18.3mg oral equivalent OR Convert to all IM doses, i.e. 5mg + 3mg + 3mg = 11mg IM equivalent Therefore the patient may receive a further 10mg oral equivalent or 5mg IM equivalent haloperidol within the 24 hour period. References - Psychotropic Drug Directory 2012 eMC – SPC Haloperidol 5.5.04 Medicines Bulletin Number 3 Writing Prescriptions: Providing a Clear Record of Drug Therapy 27 Appendix 7 Guidance on the Preparation and Administration of Olanzapine (Zyprexa®) Intramuscular (IM) Injection Indicated for the rapid control of agitation and disturbed behaviours in patients with schizophrenia or manic episode, when oral therapy is not appropriate. For intramuscular use only. Do not administer intravenously or subcutaneously. Administration is a maximum of three consecutive days. The maximum dose in 24 hours is 20mg (including all formulations of olanzapine). The recommended initial dose is 10mg. A lower dose (5mg or 7.5mg) may be given, on the basis of individual clinical status (e.g. renal and/or hepatic impairment). A second injection, 5-10mg, may be administered 2 hours after the first injection. Not more than three injections should be given in any 24-hour period. Patients receiving olanzapine intramuscularly should be closely monitored for the first 4 hours following the injection for hypotension, bradyarrhythmia and hypoventilation. Intramuscular olanzapine and intramuscular lorazepam must not be administered within 1 hour of each other. If olanzapine IM is prescribed as part of rapid tranquillisation ALWAYS follow the Rapid Tranquillisation Policy, including patient monitoring parameters. The following shows the volume required for doses of olanzapine injection: Dose of olanzapine required 2.5mg 5mg 7.5mg 10mg Volume of olanzapine inj. 5mg in ml 0.5ml 1.0ml 1.5ml 2.0ml Reconstitution 1. Withdraw 2.1ml of water for injection into a sterile syringe. Inject into a vial of olanzapine. 2. Rotate the vial until the contents have completely dissolved, giving a yellow coloured solution. Inspected visually for particulate matter prior to administration. 3. The vial contains 11 mg olanzapine as a solution of 5mg/ml. 4. Discard the syringe and any unused solution in accordance with appropriate clinical procedures. 5. Use the solution immediately within 1 hour of reconstitution. 28 Appendix 8 Advice on the Preparation and Administration of Lorazepam (Ativan®) Intramuscular (IM) Injection IM lorazepam must only be administered diluted 1:1 with sodium chloride 0.9% or water for injection. IM Lorazepam must not be mixed with any diluents other than sodium chloride 0.9% or water for injection. Lorazepam injection is only manufactured as one strength: 4mg in 1ml. The following shows the volume required for doses of lorazepam injection 4mg in 1ml: Dose required 0.5mg 1mg 2mg 3mg 4mg Vol. of lorazepam = = = = = 0.125ml 0.25ml 0.50ml 0.75ml 1.00ml Vol. of diluent + + + + + 0.125ml 0.25ml 0.50ml 0.75ml 1.00ml Example: For a prescription of lorazepam 2mg IM Draw up 0.5ml of lorazepam 4mg in 1ml and 0.5ml of sodium chloride 0.9% or 0.5ml water for injection Always remember to mix lorazepam 1:1 with diluent If lorazepam IM is prescribed as part of rapid tranquillisation ALWAYS follow the Rapid Tranquillisation Policy, including patient monitoring parameters. Lorazepam injection MUST be stored in the FRIDGE 29 Appendix 9 Policy for the use of midazolam injection in rapid tranquillisation Introduction This policy outlines the use of intramuscular midazolam for rapid tranquillisation, or the treatment of agitation or aggression. This is only to be considered for action on receipt of a memorandum from either the Chief Pharmacist, the Deputy Chief Pharmacist or the Principal Pharmacist for Wakefield and Forensic. declaring an official shortage of lorazepam injection and authorising the use of intramuscular midazolam Midazolam is a benzodiazepine available as an intramuscular injection. It is not licensed for agitation, but has been investigated in a randomised trial. All formulations of midazolam were changed to controlled drug (schedule 3) status in 2008. Within the trust this means that it should be stored in the controlled drugs cupboard (midazolam does not need to be stored in a refrigerator) and all receipt and administration of midazolam must be recorded in the ward controlled drugs register. Prescribing and administering midazolam Where an intramuscular benzodiazepine is required, in the event of an official shortage of lorazepam, midazolam should be prescribed instead. The recommended dose in adults is 2.5mg (2.5ml), or 5mg (5ml) if judged to be clinically appropriate. A further dose may be given 30 minutes later if required. This may be titrated up to 5mg or 7.5mg according to response. The maximum cumulative dose should not exceed 15mg in 24 hours. In elderly patients the initial dose should be 1 to 2mg (1 to 2ml). In working age adults where renal, hepatic, respiratory or cardiac impairment is judged to be significant enough to compromise the patient’s well-being, this starting dose can also be employed. This may be repeated after 30 minutes. The maximum cumulative dose should not exceed 7.5mg in 24 hours. Midazolam is available at a strength of 1mg /1ml in either a 2ml or 5ml injection. It does not require dilution before administration. Administration must be recorded in the controlled drug register. Wards must ensure they have access to flumazenil injection before administration. After administration patients should be monitored according to the requirements of the rapid tranquillisation policy, for four hours post dose. 30 Explanatory Notes Repeated episodes of supply failure of lorazepam injection mean that an alternative intramuscular (IM) benzodiazepine may be needed. Diazepam is inappropriate for use as an intramuscular injection, despite this route being listed in the British National Formulary (BNF) and manufacturer’s literature. Absorption following an IM injection is inconsistent, leading to unpredictable therapeutic effect. Midazolam is a benzodiazepine available as an intramuscular injection. It is indicated for sedation before or during diagnostic or therapeutic procedures; premedication before anaesthesia; induction of anaesthesia; and sedation in intensive care units. It has also been investigated in rapid tranquillisation in randomized trials (TREC, 2003) Doses used in these studies range from 2.5mg to 15mg, however higher doses led to a large proportion of patients being asleep within 20 minutes. The risk of respiratory depression with midazolam is thought to be greater than with lorazepam, so a more cautious approach to initial doses is reasonable. Absorption from intramuscular sites is rapid and complete, with peak plasma concentrations reached within thirty minutes. Onset of sedation and subsequent arousal has been reported to be quicker than lorazepam 1. Elimination is also rapid, but repeated administration can lead to accumulation, and increased risk of adverse effects such as respiratory depression. Monitoring of the patient should continue for at least four hours after administration, using the monitoring chart in the rapid tranquillisation policy. Following IM administration patients may experience anterograde amnesia, with the patient not able to remember events which occurred when the product was at maximum effect. Midazolam products were reclassified as controlled drugs (schedule 3) on 1st January 2008. This means that it is subject to controlled drug procedures, and receipt and administration must be recorded in the controlled drug register. The National Patient Safety Agency produced a Rapid Response Report concerning risks of overdose caused by confusion between the different strengths of midazolam injection (NPSA, 2008). The only strength which should be used in the trust for rapid tranquilisation is the 1mg per ml preparation. 31 Appendix 10 Implementation Dissemination This policy will be implemented and disseminated throughout the organisation immediately following approval and will be published on the Trust Intranet site. Local dissemination of updates will be coordinated by the pharmacy team in conjuction with the appropriate TRIO (Clinical Lead / Practice Governance Coach / General Manager) Staff will be alerted to changes to the policy through the Trust management briefing process. Associated Documentation: 1. The antipsychotic prescribing guidelines: Aripiprazole communication Haloperidol communication 2. Cardiopulmonary resuscitation policy. 3. Management of violence and aggression. Review The Drug and Therapeutics Sub Committee will review this policy 2 years after the implementation, although it may be fully or part reviewed on any occasion prior to this formal review in response to legislative changes or significant procedural changes in nursing, medical or pharmacy practice. The D&T will however continuously monitor its implementation and practice through an agreed programme of clinical audit as well as reviewing annual reports, incident reports and minutes. Compliance will be checked via the following audit parameters 1. Rapid Tranquillisation episode criteria and review pathway. a. Record of completion b. Completion of required data 2. Rapid Tranquillisation – assessment and progress chart. a. Record of completion b. Evidence of reporting via local reporting system c. Completion of required data 3. Training a. Percentage of staff trained This will be carried out by the clinical governance sub-group of the D&T with each locality/area being subject to audit at least every two years. 32 Appendix 11 - Equality Impact Assessment Tool To be completed and attached to any policy document when submitted to the Executive Management Team for consideration and approval. Yes/No 1. Comments Does the policy/guidance affect one group less or more favourably than another on the basis of: Race Ethnic origins travellers) No (including gypsies and No Nationality No Gender No Culture No Religion or belief No Sexual orientation including lesbian, gay and bisexual people No Age Yes Older people may require lower doses of medication Disability - learning disabilities, physical disability, sensory impairment and mental health problems Yes People with physical disability may require different doses of medicines 2. Is there any evidence that some groups are affected differently? Yes Older people and people with a physical disability 3. If you have identified potential discrimination, are any exceptions valid, legal and/or justifiable? Yes 4. Is the impact of the policy/guidance likely to be negative? NO 5. If so can the impact be avoided? N/A 6. What alternatives are there to achieving the policy/guidance without the impact? N/A 7. Can we reduce the impact by taking different action? N/A If you have identified a potential discriminatory impact of this policy, please refer it to the Director of Corporate Development or Head of Involvement and Inclusion together with any suggestions as to the action required to avoid/reduce this impact. For advice in respect of answering the above questions, please contact the Director of Corporate Development or Head of Involvement and Inclusion. 33 Appendix 12 - Checklist for the Review and Approval of Procedural Document To be completed and attached to any policy document when submitted to EMT for consideration and approval. Title of document being reviewed: 1. 2. 4. 5. Comments Title Is the title clear and unambiguous? Yes Is it clear whether the document is a guideline, policy, protocol or standard? Yes Rationale Are reasons for development of the document stated? 3. Yes/No/ Unsure Yes Development Process Is the method described in brief? Yes Are people involved in the development identified? Yes Do you feel a reasonable attempt has been made to ensure relevant expertise has been used? Yes Is there evidence of consultation with stakeholders and users? Yes Content Is the objective of the document clear? Yes Is the target population clear and unambiguous? Yes Are the intended outcomes described? Yes Are the statements clear and unambiguous? Yes Evidence Base Is the type of evidence to support the document identified explicitly? Yes Are key references cited? Yes Are the references cited in full? Yes Are supporting documents referenced? 6. Approval Does the document identify which committee/group will approve it? Yes If appropriate have the joint Human Resources/staff side committee (or equivalent) approved the document? 7. Dissemination and Implementation Is there an outline/plan to identify how this will be done? Yes 34 Title of document being reviewed: Does the plan include the necessary training/support to ensure compliance? 8. 9. 10. 11. Yes/No/ Unsure Comments Yes Document Control Does the document identify where it will be held? Yes Have archiving arrangements for superseded documents been addressed? Yes Historical documentation to be kept on file electronically for 2yrs. Process to Monitor Compliance and Effectiveness Are there measurable standards or KPIs to support the monitoring of compliance with and effectiveness of the document? Yes Is there a plan to review or audit compliance with the document? Yes Review Date Is the review date identified? Yes Is the frequency of review identified? If so is it acceptable? Yes Overall Responsibility for the Document Is it clear who will be responsible for implementation and review of the document? Jane Riley, Chief Pharmacis t 35 Appendix 13 TRAINING NEEDS 1. Medical staffing 1.1. All medical staff working within the Trust will be directed to the rapid tranquillisation guidance on the intranet at induction. 1.2. All medical staff are required to be trained on the use of rapid tranquillisation. Junior medical staff will be required to undertake training annually. Middle grade medical staff will be required to attend an update every 2 years. Consultant Psychiatrists will be required to attend an update every 2 years. 2. Nursing Staff 2.1. There will be a rapid tranquillisation policy on each in-patient area and a laminated copy of the algorithm in the clinic room. 2.2. Rapid tranquillisation will form part of the induction programme for the nursing staff, including locum and agency staff. 2.3. Band 5+ nursing staff who work in the areas defined below. 2.3.1. They will be required to attend training on rapid tranquillisation annually 2.3.2. Although rapid tranquillisation is only suitable to be carried out on inpatient areas it is important for staff working in crisis teams to be aware of the processes. 2.3.3. It is the responsibility of the BDUs and the ward manager to ensure staff are trained in the use of rapid tranquillisation. 3. Pharmacists 3.1. Rapid tranquillisation will form part of the induction programme for the pharmacists including locum and agency staff. 3.2. All pharmacists who work in mental health are required to be trained on the use of medicines in rapid tranquillisation. They will be required to attend training annually. 4. Records of training will be held on the Trusts Education and Training Management system. 4.1. All medical staff, ward based qualified nursing staff and pharmacists should be familiar with the use and dangers of RT, the medications used in RT as well as those used to reverse the effects (e.g. flumazenil in respiratory depression). 36 4.2. At least one member of staff, per ward, per shift should be be trained to CPR, Basic Life Support and Advisory De-fibrillation. They should be trained to use the oxygen available on the ward and in puloximetry. Areas identified as requiring mandatory training in Rapid Tranquillisation Calderdale Beechdale Elmdale Ashdale Kirklees 8 Fox View Ward 19 Ward 18 Enfield Down Wakefield Chantry Horizon Centre Priory Trinity 1 Trinity 2 Sycamores Poplars Saville Park View House Castle Lodge Forensic Services Low Secure The Bretton Centre: Sandal, Thornhill, Ryburn and Almondbury Newhaven Medium Secure Appleton Bronte Chippendale Gaskell Hepworth Priestley Waterton Barnsley Melton Suite (PICU) Clark Ward (Female acute) Beamshaw (Male Acute) Willow (older adult) Intensive Home Based Treatment staff to be trained in all localities, this training is considered essential for this group. 37 Appendix 14 - Version Control Sheet This sheet should provide a history of previous versions of the policy and changes made Version Date Author Statu s Comment / changes 1 2002 Lynn Haygarth, Professor Curran, David Hargreaves, Professor Wattis, Nisreen Booya 2 August 2005 Updated by Lynn Haygarth and David Hargreaves Updated to include olanzapine IM injection 3 Septembe r 2008 Used principles in Bradford District Care Trust policy with consent. Updated by Lynn Haygarth Updated to include aripiprazole IM injection and requirement for ECG when prescribing haloperidol Format brought in line with trust requirements. 4 June 2010 Updated in line with new guidance Updated by Lynn Haygarth The introduction of a flow chart. Increase use of the forms for assessment and progress and the consideration of the process as a rapid tranquillisation pathway. Appendices have been introduced for the administration of the IM injectables. 5 November 2011 Updated to include practice in Barnsley. Updated by Mark Payne 5.1 December 2012 Updated to include Lorazepam IM by Paul Hardy Change in the therapeutic positioning of midazolam IM Addition of risperidone oral as treatment option Amended interval for post-intervention monitoring Reformatting of flow chart Lorazepam IM injection has recently become available again. To enable either Lorazepam IM or Midazolam IM to be used within the Trust. 5.2 January 2013 Updated to stand down midazolam memo by Lynn Haygarth Following D&T it was decided that lorazepam to be IM benzodiazepine of first choice and that midazolam 1mg/1ml injection to be removed from wards 5.3 Septembe r 2013 Updated The Richmond Agitation – Sedation Scale Paul Hardy Updated the Richmond Agitiation Sedation Scale for ease of use. 38 5.3.1 Septembe r 2014 Mark Payne Updated doses for haloperidol in line with changes to BNF / SPC 6 February 2016 Mark Payne Mohammed Fazlee Sandra Butler Updated in line with NICE NG 10 Reformatted document Updated monitoring documents / pathway 39 References Bazire, S. (2014). Psychotropic drug directory 2014. Dorsington: Lloyd-Reinhold Communications. British National Formulary. (Accessed online, http://www.evidence.nhs.uk, Jan 2016) Electronic Medicines Compendium. (Accessed online, http://www.medicines.org.uk, Jan 2016) Huf, G et al. (2003). Rapid tranquillisation for agitated patients in emergency psychiatric rooms: a randomised trial of midazolam versus haloperidol plus promethazine. BMJ, 327(7417), pp.708-711. Jayakody, K et al. (2012). Zuclopenthixol acetate for acute schizophrenia and similar serious mental illnesses. Cochrane Database Syst Rev, 18(4). NICE (2013). CG155: Psychosis and schizophrenia in children and young people: recognition and management. Published online (www.NICE.org.uk) NICE (2014). CG178: Psychosis and schizophrenia in adults: prevention and management. Published online (www.NICE.org.uk) NICE (2015), NG10 Violence and aggression: short-term management in mental health, health and community settings. Published online Powney, MJ, Adams, CE, Jones, H. (2012). Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation). Cochrane Database Syst Rev, 11. SWYPFT Antipsychotics in Clinical Practice: Guidelines for safe and effective use in adults with schizophrenia and includes information on the use in early onset psychosis in adolescence, (Medical Director), Version 4.2, Mar 2013. SWYPFT Cardiopulmonary Resuscitation Policy, (Director of Nursing), Version 5, Sept 2015. SWYPFT Management of Aggression and Violence: Personal Safety and Violence Reduction policy, procedures and guidance, (Director of nursing, clinical governance and safety), Version 4, Sept 2015. SWYPFT Rapid Tranquillisation Policy, (Chief Pharmacist), Version 5.3.1, Sept 2015 SWYPFT Reference document for monitoring the physical health of service users taking psychotropic medication, (Medical Director) Version 3, Mar 2012. Taylor, D., Paton, C. and Kapur, S. (2015). The Maudsley prescribing guidelines in psychiatry (12 edition). Chichester: Wiley-Blackwell. 40