* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download chap 4 org chem
Survey
Document related concepts
Transcript
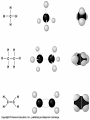
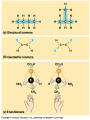
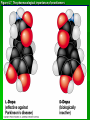
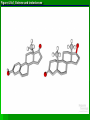
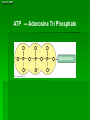
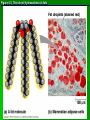
Organic Molecules AP Biology Chapter 4 Organic Chemistry Study of Carbon Compounds Organic molecules are those that have carbon. Valence electrons? 4 covalent bonds Why not Si? Figure 4.3 Valences for the major elements of organic molecules Carbon Will readily bond with itself (C) with – or = bonds (single, double or even triple) Also bonds with H – O = N, P, & S Forms long chains or backbones of organic molecules Carbon will always have four lines connecting to other atoms. Hydrocarbons Hydrocarbons are carbon compounds with carbo n and hydrogen Methane Ethane Video clip… Variations in Hydrocarbons Isomers Isomers are molecules with identical molecular formulas but different structural formulas Different types of Isomers exist: Structural Geometric Enantiomers Video clip… Identify the type of isomer for each pair. Figure 4.7 The pharmacological importance of enantiomers Figure 4.6ax Structural isomers Figure 4.8 A comparison of functional groups of female (estradiol) and male (testosterone) sex hormones Figure 4.8x1 Estrone and testosterone Figure 4.8x3 Male and female peacocks Figure 4.8x4 Male and female sage grouse Functional Groups Many organic molecules share similar properties because they have similar clusters of atoms: functional groups. Functional groups are added to a hydrocarbon chain Functional groups give the molecule a particular property, such as acidity or polarity. The seven functional groups that are most important in the chemistry of life: Hydroxyl group Carbonyl group Carboxyl group Amino group Sulfhydryl group Phosphate group Methyl group © 2011 Pearson Education, Inc. Table 4.1 Functional Groups of Organic Compounds p64-65 Functional Groups 1. Hydroxyl (-OH) Alcohols End in –ol (methanol) Found in sugars 2. Carbonyl Group (C=O) Double bond between C and O Aldehyde (at end of structure) Ketone (in center of structure) Functional groups (con’t) 3. Carboxyl Group ( -COOH) Double bonded (H-O – C = O Carboxylic acids Found in sugars, fats and amino acids Source of H+ ions 4. Amino Group (-NH2) Amines Amino acids -- add a carboxyl group Basic unit of all proteins Functional Groups (con’t) 5. Sulfhydryl Group ( -SH) Found in some proteins (amino acid –monomer) Thiols 6. Phosphate Groups ( -PO4) Found in ATP Helps in transfer of chemical energy within a cell Found in structure of another famous molecule - ? 7. Methyl Group (-CH3) Hydrophobic Insoluble in water Non- polar Found in long chains of Lipids Functional Groups: F-Group Class Name Examples Characteristics Hydroxyl Alcohols Ethanol, glycerol, sugars Polar Hydrophilic Carboxyl Carboxylic acids Acetic Acid Amino Acid Fatty acids, Sugars Polar Hydrophilic Weak acid Amino Amines Amino Acids Proteins Polar Hydrophilic Weak base Phosphate Organic phosphates DNA, ATP, Phospholipids Polar Hydrophilic Carbonyl Ketones Acetone Sugars Polar Hydrophilic Carbonyl Aldehydes Formaldehyde Sugars Polar Hydrophilic Fatty acids Oils, Waxes Non-Polar Hydrophobic Methyl Figure 4. UN04 ATP --- Adenosine Tri Phosphate Adenosine Connecting to Biochemistry In living systems, large organic molecules, called macromolecules, may consist of hundreds or thousands of atoms. Most macromolecules are polymers, molecules that consist of a single unit (monomer) repeated many times. Figure 4.0 Protein Figure 4.5 The role of hydrocarbons in fats Modeling Biological Molecules Use your book, and worksheet to build a 3-D model of the molecules listed Build the molecules in the order noted** Draw the molecule in your notebook Get a stamp for a correct model Be mindful of the “functional group” in each molecule Answer the “Formulating Generalizations” Questions in notebook