* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The functional anatomy of the rodent larynx in relation to audible
Survey
Document related concepts
Transcript
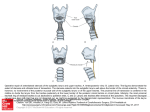
Zool. J. Linn Soc.. 56: 255-264. With 6 plates April 1975 The functional anatomy of the rodent larynx in relation to audible and ultrasonic crv production LAURENCE H.ROBERTS Department of Zoology, King’s College, University of London * Accepted for publication August I974 Rodents are well known for the production of physically quite different audible and ultrasonic cries. Both of t h m cries are known to be produced in the larynx, but the anatomy of the larynx is practically unknown, it thus being difficult to analyse how the cries are produced. The anatomy of the larynx of Mus musculus is described in detail and is found to be essentially typical of that of other mammals that can fix the thorax and make independent use of the forelimbs. Unlike the larynx of microchiropteran bats, no modifications for ultrasound production are apparent, although .the laryngeal structure is ideal for the production of audible cries. However, the audible and ultrasonic cries differ so markedly that they are unlikely to be produced by the same mechanism. The lack of laryngeal specialization therefore makes the ultrasound production mechanism largely an enigma. CONTENTS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Introduction Materials and methods Resulai Discussion Acknowledgements References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 255 257 258 260 261 262 INTRODUCTION There is very little information available in the literature on the anatomy of the rodent vocal tract and of the larynx in particular. The rodent larynx is mentioned only briefly in the definitive work of Negus (1949) on the comparative anatomy and physiology of the larynx, is not described, apart from its blood supply and nerve supply, in Greene’s (1955) volume on the anatomy of the rat and is not described by Keleman (1963). However, rodents are now known for their wide range of vocalizations in a variety of behavioural situations. Not only do myomorph rodents produce their well-known audible cries, but R m n t address: Department of Life Sciences, Polytechnic of Central London. 115 New Cawndish Street, London W1M 8JS 18 255 256 L. H. ROBERTS both adults and young also produce ultrasonic cries in many behavioural situations (Anderson, 1954; Zippelius & Schleidt, 1956; Noirot, 1965, 1966, 1968; Noirot & Pye, 1969; Sewell, 1967, 1968, 1969). Sewell (1970a) has demonstrated the communication function of ultrasonic cries between the mother and the young of Apodemus sylvaticus. and has investigated their role in mating and aggression in the adults of various species (Sales, nCe Sewell, 1972a,b). Okon (1970a,b, 1971a,b, 1972) and Smith (1972) have shown that tactile stimuli and cold stress motivate ultrasound production by young rodents, probably to reduce maternal aggression and cause retrieval by the mother respectively. Brown (1970, 1971a,b, 1973a,b) has shown a high degree of auditory sensitivity at the levels of the cochlea and inferior colliculus in several species, there being a second peak in the sensitivity curves at the frequency of a species own ultrasonic cries as well as a peak in the audible range. Despite the greatly renewed interest in rodent vocal communication, there is little information available on how the audible and ultrasonic cries are produced. This, combined with the paucity of anatomical information on the rodent vocal tract, makes it difficult t o produce a working hypothesis. The audible cries and the ultrasonic cries differ considerably in their physical structures. The audible cries consist of a fundamental, normally between 2 and 6 kHz, and a harmonic series exhibiting a formant structure. As such they are typical of other mammalian “voiced” sounds where vibrating “vocal cords” produce the fundamental and harmonic series, the fundamental is controlled by muscle action and vocal tract cavity resonances produce the formant structure. The best known example is human vowel production (Farnsworth, 1940; Dunn, 1950; Stevens & House, 1955; House & Stevens, 1958; Fant, 1960; Gopinath & Sondhi, 1970; or for brief reviews see Olson, 1967 or Holmes, 1972). The ultrasonic cries of rodents may be of high intensity and are often pure tones varying in frequency from 20-100 kHz or more from species to species. Rapid frequency and amplitude changes may occur during a cry and often instantaneous frequency and amplitude jumps occur with no obvious harmonic relations. Simultaneous harmonically unrelated components may occur and some cries, especially of cricetids, are broadband “huff“-like pulses (for reviews of these sound structures see Sewell, 1970b or Roberts, 1973a). Such sound structures are very difficult to explain in terms of vibrating structures and muscle action, as are the changes in fundamental frequency of an audible cry t o that of an ultrasonic cry. Whereas vocal tract cavity resonances appear to influence the formant structures of audible cries, they do not appear to influence the ultrasonic cries (Roberts, 1975). Both types of cry have, however, been shown to be produced in the larynx by nerve sectioning experiments (Roberts, 1973a, 1975). Both are produced at the same point in the respiratory cycle, at the onset of expiration (Roberts, 1972a). The rate of air flow was found to be low during the sounds, especially during ultrasound production, and to increase at the cessation of sound. It was suggested that if rodents possess a valvular larynx typical of other mammals that can fix the thorax and make independent use of the forelimbs (Negus, 1949) sound production might be related t o a steady release of built-up pressure through the valve system followed by a more rapid release at the THE RODENT LARYNX 257 termination of the sound. Slight adjustment of the valves might then determine whether vocal cords vibrate (although as yet there is only indirect evidence for this in the literature), or whether ultrasound is produced by an as yet unspecified mechanism. As microchiropteran bats produce high intensity ultrasonic cries for echolocation a comparison of the rodent larynx with their better known larynx may be valuable. The microchiropteran larynx is relatively massive, the cartilages often being unusually ossified and the muscles, especially the cricothyroid muscle, may be highly developed (Robin, 1881; Elias, 1907; Fischer 8t Gerken, 1961; Fischer & Vomel, 1961; Wassif 8t Madkour, 1968). Associated with the larynx there may be unusual cavities in the form, for example, of cartilaginous ampoules off the trachea below the larynx (Robin, 1881; Elias, 1907; Mohres, 1953; Wassif & Madkour, 1968). However, these ultrasonic cries are produced by modified vocal cords (Griffin, 1958) and have been proposed by Pye (1967) to be typical of other mammalian “voiced” sounds comprising an harmonic series filtered by vocal tract resonances. This is despite the fact that some of the echolocation cries may at first sight be similar to certain rodent cries in consisting of a single component, although this is in fact the second harmonic (Novick, 1958; Pye, 1968; Pye & Roberts, 1970; Roberts, 1972b). The major points of Pye’s hypotheses have recently been examined and discussed in detail with only slight modifications (Roberts, 1972b, 1973b). I t would therefore appear that the ultrasounds of rodents and bats may be produced by different mechanisms. In addition Negus (1949) does not note any specializations of the rodent larynx similar to those described by other workers for bats, although his description is limited to a few features. He describes the laryngeal ventricles as being relatively shallow in the “brown rat”, the lateral epiglottic folds as being absent and the aryteno-epiglottic folds as forming the margins of the glottis. Nothing can be inferred about sound production from such a description. An investigation of the anatomy of the larynx in relation to sound production was therefore undertaken. MATERIALS AND METHODS The animals used for the histological work undertaken were three- and four-day old “En strain” albino mice, Mus musculus (from an outbred strain used by Eliane Noirot). Young mice were used as they were of a more suitable size for sectioning. Before being sacrificed the animals were all tested for normal audible and ultrasonic cry production. Heads and necks were fixed for 24 hours in formal-acetic acid and then decalcified in Pirenny’s fluid for 24 hours. The material was dehydrated in alcohol, cleared with benzene and embedded in a plasticised wax, “Paramat”. During embedding any traces of air were removed from the vocal tracts with a weak vacuum produced by a water tap suction pump. Sections were cut at 10 pm using a rotary microtome. All the sections were mounted on slides and a standard staining technique was employed using haematoxylin and eosin. In addition cartilage was stained with toluidene blue. Five complete sets of sections of the heads and necks were prepared; two of saggital sections (hence dorso-ventral longitudinal sections of the larynx), one of transverse sections of 258 L. H. ROBERTS the neck (hence transverse.sections of the larynx) and two of coronal sections (hence approximately lateral longitudinal sections of the larynx). Microscopic analysis of every section was undertaken, sections being compared by scale drawings produced using a camera lucida. A micrometer slide was used for calibration. RESULTS The laryngeal skeleton and associated soft tissues were found to be well developed in all five animals. The laryngeal cartilages were quite typical with none of the modifications shown by the microchiropteran larynx (Robin, 1881 ; Elias, 1907; Wassif & Madkour, 1968). No unusual cavities were found to be associated with the larynx. The cricoid cartilage was found to be a signet ring shape, being deep dorsally, narrowing laterally and being fairly narrow ventrally lying just posterior to the thyroid cartilage in this region (Plates lA, B, 2A, 4A, B and 6A). Dorsally the cricoid extends back to overlie the first two tracheal rings whereas ventrally it does not (Plates l B , 3B, 4 B and 6A). On the anterior dorsal surface are the articulations with the paired arytenoid cartilages (see below) and ventrally a fine connective tissue membrane links the cricoid and thyroid cartilages forming the cricothyroid membrane (Plates l B , 3B and 4A). The anterior margin of the cricoid extends further towards the glottis dorsally than ventrally. The thyroid cartilage is loosely associated with the hyoid bone and more closely associated with the cricoid cartilage. The hyoid lies transversely under the base of the tongue anterior to the ventral plate of the thyroid (Plates l A , B, 2B, 3B, 4A, 5A and 6A) and extends around the lateral regions of the pharynx. Dorso-lateral extensions of the thyroid cartilage, the superior cornua, reach forward to be associated by ligaments with the dorsal extensions of the hyoid on each side (Plate 5A). The thyroid and cricoid cartilages articulate at the tip of the inferior cornu of the former, the cricothyroid joint so formed on the dorso-lateral surface of the cricoid appearing to be quite firm (Plates 2A,B). The thyroid alae, making up the body of the thyroid cartilage, are complete ventrally (Plates 2A and 3A,B and others) and, as is typical for other mammals, incomplete in the dorsal region just reaching the dorso-lateral margins of the cricoid cartilage. The thyroid alae are broad and typically horseshoe-shaped in transverse section (Plate 3A). The antero-superior region of the thyroid cartilage bears a condensation of cartilage which extends into the epiglottis as the epiglottic cartilage (Plates 1B, 3B, 4A and 6A). This raises the anterior and antero-lateral margins of the glottis (seen in saggital section in Plate 3B and in horizontal section in Plate 2B). Lateral to the margins so formed are the lateral food channels leading to the oesophagus. As Negus (1949) pointed out, the lateral margins of the glottis are the arytenoepiglottic folds. A muscle runs from the epiglottic cartilage into the tongue, contraction of which will lower the epiglottis. The epiglottis lies in an intranarial position in the sections of some animals and under the soft palate in others; it may therefore by mobile. In the lateral regions of the joint of the thyroid and epiglottic cartilages may be seen small THE RODENT LARYNX 259 condensations of cartilage extending into and supporting the margins of the glottis. These are the cartilages of Wrisberg (Plates 4A and 6A). Each of the pair of arytenoid cartilages is articulated on the dorso-lateral anterior surface of the cricoid cartilage at the closely fitting cricoarytenoid joints (Plates lA, 3A and 4B). Lateral to the joint is a short muscular process (Plate 5A) and medial to the joint an extension from the main body of the arytenoid, the vocal process, extends slightly diagonally to the margin of the glottis (Plates 4A and SB). Dorsally the arytenoids bear the cartilages of Santorini that support the posterior margin of the glottis and the entrance to the oesophagus (Plates 1 B and 6A). Between the cartilages of Santorini and the epiglottic cartilage and cartilages of Wrisberg run the upraised margins of the glottis, lateral to which are the food channels. The muscles associated with the larynx are, like the cartilages, little modified from the typical mammalian form. However, as with the laryngeal skeleton, the muscles are very well developed in the animals examined. The suspensory and associated external muscles of the larynx are largely outside the scope of this study but the major ones can be described quite simply. The inferior pharyngeal constrictor muscles running from the dorso-lateral surface of the thyroid and cricoid cartilages around the oesophagus can be seen in Plates 3A and 5B. The main suspensory muscle of the larynx, the thyrohyoid, can be seen in Plates lA, 2B, 3B, SA and 6B. The sternothyroid muscle can be seen in Plates 2B and 6 B and the sternohyoid muscle in Plates 4A, SA and 6A. Quite typically the muscles that move the parts of the larynx relative to each other and hence might control vocalization can be divided into three groups, the external adductors, the internal adductors and the internal abductors. The external adductor group is comprised of one pair of muscles, the cricothyroids, running from the anterior of the cricoid near the mid line to the thyroid ala and inferior cornu of the thyroid (Plates 2A,B). The muscle appears to be quite well developed in the specimens examined, but its action appears to be quite normal in approximating the anterior parts of the thyroid and cricoid by rotation around the cricothyroid joint and thus elongating the glottis and adducting the arytenoids as a result. A major role for this muscle has been proposed to be a bracing action for sound production. The bracing action certainly appears to be a feasible function for this muscle in rodents. The internal adductors, or sphincters, comprise the interarytenoid muscle, the paired lateral cricoarytenoid muscles and the paired thyroarytenoid muscles. The interarytenoid muscle is very small; movement of the arytenoids towards one another may therefore be weak. The lateral cricoarytenoid muscles (Plates lA, 4 B and SA) run from the lateral part of the cricoid to the muscular process of the arytenoids. Their action is to draw the arytenoids towards the glottis approximating the vocal processes. This action might be important, together with the bracing action of the cricothyroids, in “voiced” sound production. The remaining part of the sphincter ring, the paired thyroarytenoid muscles, are important in vocalizing animals and the rodent larynx appears to be no exception. The thyroarytenoids make up the superior and lateral part of the adductor group (Plates l A , B, 2B, 3A, 4B, SA, B and 6A). The muscles are large and run mainly from the arytenoids to the middle of the thyroid alae (Plates lA, 3A and 4B). The specimens examined show a well developed but relatively shallow ventricle on each side of the larynx dividing the fold of tissue 260 L. H. ROBERTS running from the arytenoids to the thyroid on each side into a superior and inferior thyroarytenoid fold (Plates 38, 4A and 6A). The bulk of the thyroarytenoid muscle underlies the inferior fold only (Plates lB, 2B and 6A,B). Whereas part of the muscle lies in close proximity to the inferior fold, the superior fold appears to be lacking in muscle tissue (Plate 6A) although some of the muscle may lie more deeply under this fold. This is somewhat different from the arrangement of the muscle for example in man, where there are distinct superior and inferior divisions associated with the superior and inferior thyroarytenoid folds. However, as in man, the muscle can be divided into internal and external portions (Plate SB). The internal part lies in close proximity to the inferior thyroarytenoid fold and runs from the vocal process of the arytenoid to the cricothyroid membrane and to an extension of that membrane, the conus elasticus, that runs to the margins of the glottis (Plate 6B). The close association of the internal division of the thyroarytenoid muscle and the margin of the glottis may be seen in Plate 5B. The external division of the muscle is its main bulk, running from the body of the arytenoid to the thyroid ala. The action of the thyroarytenoid muscle is to shorten and tense the inferior thyroarytenoid folds and oppose the vocal processes of the arytenoids. The internal abductor muscles are represented by the paired posterior cricoarytenoid muscles running from the muscular processes of the arytenoids to a wide area of the posterior surface of the cricoid (Plates l A , B, 2A, 3B, 4A, B and 5A,B). The action of these muscles alone will open and lengthen the glottis. The action of the sphincter group of muscles alone will shorten and close the glottis and as with other mammals (Negus, 1949) this may be important during deglutition. Again, as in other mammals when opposed by the dilator muscles the sphincters may be especially important for sound production. In most mammals with laryngeal ventricles the inferior thyroarytenoid folds, tensed by the thyroarytenoid muscles, act as the “vocal cords’’ for “voiced” sound production (Negus, 1949). The rodent larynx would appear to be no exception to this. Although the inferior folds are not very sharp in the specimens examined the functional arrangement appears to be ideally suited for a typical voice production mechanism. In the rodents the function of the superior folds, considering the lack of intrinsic muscle tissue, is more difficult to interpret. Where there is a well developed musculature, as in man, the superior folds are thought to act as valves to prevent air leaving the lungs, thus aiding fixation of the thorax. It is possible in the rodent larynx that more remote musculature is capable of bringing about this action. The relatively blunt inferior folds may function as inlet or outlet valves as required. DISCUSSION The rodent larynx, at least that of Mus musculus. clearly has none of the modifications for ultrasound production possessed by the larynx of microchiropteran bats. There is none of the massive development of laryngeal cartilages and associated musculature nor the unusual structure of the inferior and superior thyroarytenoid folds or the development of cavities off the vocal tract. However, the ultrasonic cries of bats are specialized voiced sounds THE RODENT LARYNX 26 1 produced by the modified “vocal cords” (Griffin, 1958; Novick & Griffin, 1961; Pye, 1967, 1968; Roberts, 1972b, 1973b), whereas rodents produce quite typical mammalian audible cries as well as their ultrasonic cries from the larynx (Roberts, 1975). The rodent larynx is clearly well developed at an early age, but care must be taken in assuming that this development is solely for sound production as the larynx is involved in the control of respiratory airflow and prevention of inundation by food material as well as sound production. However, it is quite clear from the description of laryngeal anatomy of mouse pups given here that the larynx is ideally suited for the production of audible cries by a normal mammalian voice mechanism (as discussed in the introduction). I t is also clear, however, that the physical structures of the audible and ultrasonic cries differ so markedly that it is most unlikely that they are produced by the same mechanism for the reasons discussed in the introduction t o this paper. There are, though, no apparent modifications to the rodent larynx for ultrasound production. Therefore little information on the ultrasound production mechanism can be gained from the study of the anatomy of the larynx, except perhaps that the mechanism must be an unusual one. Although there are strong indications of two distinct sound production mechanisms in the rodent larynx, two factors suggest that the mechanisms are closely related in some ways. Firstly, the respiratory patterns are very similar during audible and ultrasonic cry production (Roberts, 1972a), indicating that the valve systems of the larynx might be acting in similar ways in each case. Secondly, the two cry types may on occasions be closely linked physically as one can break almost instantaneously into the other, but they do not occur simulataneously (Roberts, 1972a, 1973a). This might indicate that only a fine adjustment of the valve system is needed t o switch from a typical voiced mechanism t o an as yet unspecified ultrasound production mechanism or vice versa. Evidence from the structure of the rodent ultrasonic cries and experimental evidence is now indicating that ultrasound production is based on a specialized whistle mechanism (to be published elsewhere), and that this is in no way incompatible with the results reported here and with the discussions above. ACKNOWLEDGEMENTS I would like to thank Mr John Wells of King’s College, University of London for his advice on histological techniques, and Professor J. D. Pye for his advice and encouragement throughout the work reported here. I would also like to thank Professor D. R. Arthur for providing the facilities for carrying out the histological preparation in King’s College, Department of Zoology and Professor N. Singer, Head of the Department of Life Sciences of The Polytechnic of Central London for providing facilities for detailed analysis of the material and photographic facilities. The initial part of this work was carried out during the tenure of a Science Research Council studentship. 262 L. H. ROBERTS REFERENCES ANDERSON, J. W.. 1954. The production of ultrasonic sounds by laboratory rats and other mammals. Science. N. Y.. I 19: 808. BROWN, A. M., 1970. Bimodal cochlear response curves in rodents. Nature Lond., 228: 576-7. BROWN, A. M., 1971a. High frequency responsiveness in rodents at the level of the inferior colliculus. Nature Lond.. 232: 223-4. BROWN, A. M.. 1971b. Auditory responses to high frequencies in small mammals. Ph.D. thesis, Univ. of London. BROWN, A. M.. 19731. High frequency peaks in the cochlear microphonic response of rodents. J. comp. P h y ~ i ~ l83: . , 377-92. BROWN, A. M.. 1973b. High levels of responsiveness from the inferior colliculus of rodents at ultrasonic frequencies. J. comp. Physiol.. 83: 393-406. D O " . H. K.. 1950. The calculation of vowel resonances and an electrical vocal tract. J. acoust. Soc. A m , 22: 740-53. ELIAS. H., 1907. Zur anatomic des Kehlkopfes der Mikrochiropteren. Morph. Jb.. 37: 70-118. FANT. C., 1960. Acoustic theory of speech production. s'Gravenhage: Mouton & CO.. The Hague. FARNSWORTH. D. W.. 1940. High speed motion pictures of the human vocal cords. Bell Labs. Rec.. 18: 203-8. FISCHER. H. & GERKEN. H.. 1961. Le larynx d e la chauve-souris (Myotis myoris) et le larynx humain. Annls. 010-lar., 78: 577-85. FISCHER, H. & VOMEL. H. J., 1961. De Ultraschallapparat des Larynx von Myoris myotis. Morphol. Jb.. 102: 200-26. COPINATH. B. & SONDHl, M. M., 1970. Determination of the shape of the human vocal tract from acoustical measurements. Bell Syst. tech. J.. 49: 1195-214. GREENE, E. C., 1955. The anatomy of the rat. l h u . Am. phil. Soc. (N.S.),27. GRIFFIN, D. R., 1958. Listening in the dark. New Haven: Yale University Press. HOLMES. J. N.. 1972. Speech synthesis. London: Mills & Boon. HOUSE, A. S. & STEVENS, K. H.. 1958. Estimation of formant band widths from measurements of transient response of the vocal tract. J. Speech Hear. Res.. I: 309-1 5. KELEMAN, C., 1963. Comparative anatomy and performance of the vocal organ in vertebrates. In R. C. Busnel. (Ed.), Acoustic behaviour of animals: 489-521. Amsterdam: Elsevier. MOHRES, F. P.. 1953. h e r die Ultraschallorientieurung der Hufeisennasen (Chiroptera-Rhinolophidae). Z vergl. Physiol.. 34: 547-88. NEGUS. V. E., 1949. The comparative anatomy and physiology of the larynx. Hafner. NOIROT, E.. 1965. Ultrasons et comportements maternels chez les petits rongeurs. Annls Soc. r. rool. Belg.. 95: 47-56. NOIROT. E., 1966. Ultrasounds in young rodents. 1. Changes with age in albino mice. Anim. Behov., 14: 459-62. NOIROT, E., 1968. Ultrasounds in young rodents. II. Changes with age in albino rats. Anim. Behav.. 16: 129-34. NOIROT, E. & PYE, J. D.. 1969. Sound analysis of ultrasonic distress calls of mouse pups as a function of their w.Anim Behav.. 17: 340-9. NOVICK, A., 1958. Orientation in palaeotropical bats. I. Microchiroptera. J. exp. Zool., 138: 81-154. NOVICK, A. & GRIFFIN, D. R.. 1961. Laryngeal mechanisms in bats for the production of orientation sounds. J. exp. Zool., 148: 125-46. OKON, E. E., 19700. The effect of environmental temperature o n the production of ultrasounds by isolated non-handled albino m o w pups. J. Zool.. Lond.. 162: 71-83. OKON, E. E.. 1970b. The ultrasonic responses of albino mouse pups to tactile stimuli. J. Zool.. Lond.. 162: 485-92. OKON, E. E.. 1971a. The temperature relations of vocalizations in infant golden hamsters and Wistar rats. J. Zool.. Lond, 164: 227-37. OKON, E. E.. 1971b. Motiwtion for the production of ultrasounds in infant rodents. Ph.D. thesis, Univ. of Lond. OKON, E. E., 1972. Factors affecting ultrasound production in infant rodents. J. Zool., Lond.. 168: 139-41. OLSON, H. F.. 1967. Music. physics and engineering, 2nd ed. New York: Dover Publications. PYE. J. D., 1967. Synthesizing the waveforms of bat's pulses. In R. G. Busnel, (Ed.), Animal sonar systems I: 43-65. Jouy-cn-Josas, France: Laboratoire dc Physiologic Acoustique. PYE. J. D., 1968. Animal sonar in air. Ultrasonics. 6: 32-8. PYE, J. D. & ROBERTS, L. H., 1970. Ear movements in a hipposiderid bat. Narure Lond.. 225: 285-6. ROBERTS, L. H., 19721. Correlation of respiration and ultrasound production in rodents and bats. J. Zool.. Lond., 168: 439-49. ROBERTS, L. H., 1972b. Variable resonance in constant frequency bats. J. Zool.. Lond.. 166: 337-48. THE RODENT LARYNX 263 ROBERTS, L. H., 19730. Comparative studies of sound production in small mammals with specicll reference to ultrasound. Ph.D. thesis, Univ. of Lond. ROBERTS, L. H., 1973b. Cavity resonances in the production of orientation cries. Period. biol.. 75: 27- 32. Proceedings of the 3rd International Bat Research Conference, Plitvice, Yugoslavia, 1972. ROBERTS, L. H., 1975. Evidence for the larynx as the source of both the ultrasonic and audible cries of rodents. J. 2001..Lond.. 175: 243-57. ROBIN, M. H. A., 1881. Recherches anatomiques sur les mammifkres de I’ordre Chiroptkres. Annls Sci. nut., 12(6): 180. SALES, G. D., 1972a. Ultrasound and mating behaviour in rodents with some observations on other bchavioural situations. J. 2001..Lond., 168: 149-64. SALES, G. D., 1972b. Ultrasound and aggressive behaviour in rats and other small mammals. Anim. Behav.. 20: 88-100. SEWELL. G. D., 1967. Ultrasound in adult rodents. Narure Lond.. 215: 512. SEWELL, G. D., 1968. Ultrasound in rodents. Nature Lond.. 21 7: 682-3. SEWELL, C. D.. 1969. UItrasound in small mammals. Ph.D. thesis, Univ. of Lond. SEWELL. G. D., 1970a. Ultrasonic communication in rodents. Nature Lond., 227: 410. SEWELL, C. D.,1970b. Ultrasonic signals from rodents. Ultrasonics. 8: 26-30. SMITH. J. C., 1972. Sound production by infant Peromyscus maniculatus (Rodentia-Myomorpha). J. 2001.. Lond., 168: 369-79. STEVENS, K. H. & HOUSE, A. S., 1955. Development of a qualitative description of vowel articulation. J. acoust Soc. Am., 27: 161-8. WASSlF, K. & MADKOUR, C. M., 1968. The anatomy of the hyoid bone, larynx and upper parts of the trachea in some Egyptian bats. Bull. zool. Soc. Egypt, 22: 15-26. ZIPPELIUS, H. M. & SCHLEIDT, W. M., 1956. UltraschaII-Laute bei jungen Musen. Naturwissenschaften, 43: 502. ABBREVIATIONS USED IN PLATES ant. d A An Ab B C e cb D E F c H I J anterior dorsal arytenoid cartilage muscular process of arytenoid cartilage vocal process of arytenoid cartilage cricoid cartilage thyroid cartilage inferior cotnu of thyroid cartilage superior comu of thyroid cartilage hyoid bone cricoarytenoid joint cricothyroid joint tracheal cartilage epiglottic cartilage cartilage of Santorini cartilage of Wrisberg K L M N 0 P Q R S T U V W X Y z inferior constrictor muscle thyrohyoid muscle sternohyoid muscle sternothyroid muscle cricothyroid muscle lateral cricoarytenoid muscle thyroarytenoid muscle posterior cricoarytenoid muscle cricothyroid membrane conus elasticus ventricle superior thyroarytenoid fold inferior t hyroarytenoid fold buccal cavity palate -phagus EXPLANATION OF PLATES PLATE 1 A. Saggital section of the larynx of an albino mouse pup lateral to the mid-line through the side of the cricoid cartilage and lateral part of one arytenoid cartilage. B. Saggital section of the Iarynxpf an albino m o m pup, closer to the mid-line than that of A, through the bulk on one arytenoid cartilage. PLATE 2 A. Transwrse section of the larynx of an albino mouse pup through the cricoid cartilage, showing the joint of the cricoid with the inferior comu of the thyroid cartilage. B. Longitudinal horizontal section of the larynx of an albiao m o m pup (the section is not M exact horizontal plane, the right half Wig slightly more ventral). 264 L. H. ROBERTS PLATE 3 A. Transverse section of the larynx of an albino mouse pup at the level of the thyroid cartilage (above the level of the section shown in Plate 2A). B. Soggital section of the larynx of an albino mouse pup slightly lateral to the mid-line (posteriorly the section passes through the medial part of one arytenoid cartilage rather than between the arytenoids). PLATE 4 A. Saggital section of the larynx of an albino mouse pup taken a little laterally to that in Rate 3 8 . B. Saggital section of the larynx of an albino mouse pup lateral to the mid-line through the lateral part of the cricoid ring, showing the thyroarytenoid muscle underlying the thyroarytenoid folds and the cricoarytenoid joint. PLATE 5 A. Saggital section of the larynx of an albino mouse pup through the lateral part of the arytenoid cartilage. the muscular process (i.e. more lateral to the section in Plate 4B). B. Transverse section of the larynx of an albino mouse pup at the level of the vocal processes of the arytenoid cartilages. PLATE 6 A. Saggital section of the larynx of an albino mouse pup through the bulk of one arytenoid cartilage and the innermost margin of the cricoid ring (i.e. slightly lateral to the section shown in Plate 4A). B. Longitudinal horizontal section o f the larynx of an albino mouse pup through the bulk of the thyroid cartilage (i.e. at a lower level than the section shown in Plate 28). 2001. J . Linn. SOC., 56 (197s) L. H. ROBERTS Plate 1 (Facingp. 264) 2001.J. Linn. SOC., 56 (197.5) L. H.ROBERTS Plate 2 2001.7. Linn. Soc.. 56 (1975) L. H. ROBERTS Plntc 3 2001.J . Linn. SOC..56 (1975) Plate 4 Zod. J. Linn. SOC., 56 (1975) L. I I . ROBERTS Plate 6 %d.J. Litin. Soc., 56 ( 1975) I 1,. 14. ROBERTS Imm I.