* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download a 14-year-old boy with onychomycosis
Survey
Document related concepts
Transcript
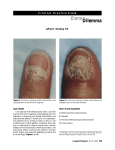
CASE STUDY A 14-YEAR-OLD BOY WITH ONYCHOMYCOSIS — Sheila Fallon Friedlander, MD BACKGROUND A 14-year-old boy has severe nail fungus affecting several toes and a tender red streak beginning on the left distal toe and proceeding up the posterior aspect of his leg (Figure 1). He is otherwise healthy. His mother reports a family history of “foot fungus” in his older brother and father. The patient has had nail fungus for several years but only recently noticed the tender streak in his leg. He has been treated previously with microsized griseofulvin 1 g/d for 8 months (patient weight is 60 kg). There was no improvement in the patient’s toenails with this medication, thus he discontinued using it 6 months before this office visit. FOLLOW-UP The patient’s laboratory tests were repeated at 4 weeks, and the test results were within the standard ranges. After 6 months, the patient’s tinea pedis had resolved and his nails showed significant improvement (Figure 4). DISCUSSION Griseofulvin is the only US Food and Drug Administration (FDA)-approved therapy for ony- Figure 1. Presentation PHYSICAL EXAMINATION The red streak of induration extends from the patient’s toe proximally to the lateral aspect of his lower leg. He shows signs of erythema, maceration, and tinea of his interdigital web spaces (Figures 2A and 2B), in addition to discoloration and subungual debris of multiple toenails. DIAGNOSIS An aspirate of the leading edge of the red streak showed Gram-positive cocci and a culture grew Group A streptococci. A potassium hydroxide preparation of subungual debris of the affected nails showed septate hyphae, and cultures of the patient’s toenail clippings grew Trichophyton rubrum on dermatophyte test medium and Mycosel agar (Figures 3A and 3B). TREATMENT The patient responded quickly to systemic antibiotic therapy and topical antifungal therapy to the interdigital web spaces. The patient’s baseline complete blood cell count and liver function tests were obtained. He was prescribed terbinafine 250 mg daily for 3 months. Advanced Studies in Medicine n Figure 2. Tinea of Interdigital Web Spaces A B S629 CASE STUDY Figure 3. Cultures of Toenail Clippings Growing Trichophyton Rubrum A B A, Dermatophyte test medium and B, Mycosel agar. Figure 4. Follow-up at 6 Months chomycosis in children, and it previously had been ineffective in this patient. Other systemic therapy options for onychomycosis in children include terbinafine, itraconazole, and fluconazole. Griseofulvin is available in microsized and ultramicrosized formulations. Microsized griseofulvin is administered as 125 mg/5 cc or 250-mg tablets. The ultramicrosized formulation is also available in 330mg tablets. Maximum doses are 1 g daily. The most common adverse effects with griseofulvin are headache, gastrointestinal disturbances, and (rarely) hepatotoxicity and leukopenia. Griseofulvin may exacerbate lupus or porphyria. Prolonged therapy is often required and the cure rates are suboptimal.1 Terbinafine is fungicidal and acts by inhibiting squalene epoxidase and ergosterol synthesis for the organism’s membrane. It is available in 250-mg tablets. The most common adverse effects include rash, gas- S630 trointestinal disturbance, headache, taste disturbance, and, rarely, hepatotoxicity, neutropenia, and a flare of subacute cutaneous lupus erythematosus. Monitoring methods for patients who are on therapy are controversial, but many experts would recommend a baseline complete blood cell count and liver function tests in pediatric patients, with a repeat of these tests after 2 to 4 weeks of therapy or if symptoms develop. Terbinafine should be administered for 6 weeks when treating fingernails, and 12 weeks when treating toenails. The dosing schedule by patient weight is shown in Table 1.2,3 Terbinafine provides the highest cure rates in adult onychomycosis.2 However, it is not currently approved by the FDA for use in children, but it can be used offlabel. Good cure rates with minimal adverse effects have been observed in children ages 1 to 4 years.4-6 In fact, complete cure was seen in 32 of 41 cases (78%) after 12 weeks of treatment. Reported adverse effects in children include urticaria, reversible neutropenia, and rare taste disturbance. Terbinafine can be administered to children with or without food.3 Although terbinafine is a reasonable first-line therapy, families should be counseled regarding off-label use and the rare adverse events associated with the drug (eg, liver toxicity and neutropenia). Itraconazole is also fungistatic and a good option for onychomycosis treatment. The drug acts by inhibiting fungal cell wall ergosterol synthesis. It is available in 2 formulations that are convenient for children: 100-mg capsules that should be taken on a full stomach and can be opened and mixed with fatty foods (eg, peanut butter) for ease of delivery, and a 10mg/mL solution that should be taken on an empty stom- Table 1. Terbinafine Dosing in Children for Onychomycosis Child’s Weight, kg Dose, mg <20 62.5 20–40 125 >40 250 Adapted with permission from Gupta and Skinner. Pediatr Dermatol. 2004;21:74-79.2 Vol. 5 (6D) n June 2005 CASE STUDY ach. Adverse effects with itraconazole can be problematic: Diarrhea is associated with the solution formulation. Also, the cyclodextrin in the solution has been associated with pancreatic adenomas in rats; it is unclear what relevance this has in humans. Hepatitis and neutropenia are possible with itraconazole, but they are rare occurrences; headache and gastrointestinal disturbance are more commonly seen adverse events. Pulse therapy with itraconazole is recommended for onychomycosis: 1 week on, 3 weeks off. For toenail infections, 3 pulses are recommended; for fingernail infections, 2 pulses are often sufficient. The dosing schedule is shown in Table 2.2 Most reported cases of itraconazole use in children used pulse dosing to decrease cost and minimize adverse effects.2,7 The limited reports of itraconazole use in children show high cure rates: a 94% cure rate in 17 prepubertal children treated with pulse therapy for 3 to 5 months and a complete cure in 49 of 63 children (78%) treated with itraconazole capsules.2,8 Fluconazole is another option and has been FDA-approved for pediatric candidal yeast infections, but it is not approved for use in dermatophyte infections. Severe onychomycosis in children, especially the “total dystrophic” type of the disease, requires systemic therapy and, perhaps, more than the traditional 3month course of treatment. Adjunctive topical therapy, such as chemical avulsion of infected nails with a 40% urea paste, may prove beneficial. Nails, such as those nails shown in Figure 5, probably need chemical or mechanical avulsion in addition to systemic therapy. In this patient, chemical avulsion of the nails with a 40% urea paste and retreatment with oral antifungal agents probably would increase the likelihood of cure. There are several reasons why treating toenail fungal infections are important: These infections can cause pain and difficulty in walking. Also, patients with tinea pedis and onychomycosis are at an increased risk for developing bacterial cellulitis of the contiguous leg, as was seen in the patient in this case study. Disruption of the skin barrier, particularly in interdigital web spaces, may allow for the ingress and invasion of bacterial species that can lead to cellulitis. In fact, onychomycosis is second only to a history of bacterial cellulitis in increasing the risk (Table 3).9 Nonetheless, families of pediatric patients may not wish to treat fungal toenail infections in their children, which is a reasonable option if the disease is not associated with any symptoms. Advanced Studies in Medicine n Table 2. Itraconazole Dosing in Children for Onychomycosis Child’s Weight, kg Dosing 10–20 50 mg 3 times weekly 20–30 100 mg/d 30–40 100 mg/d, alternate with 200 mg/d 40–50 200 mg/d >50 200 mg twice daily Pulse dose = 5 mg/kg/day. Adapted with permission from Gupta and Skinner. Pediatr Dermatol. 2004;21:74-79.2 Table 3. Disruption of Skin Barrier Increases the Risk of Cellulitis Nonmycological risk factors OR CI, % History of bacterial cellulitis 29 11.7–72 Disruption of cutaneous barrier 18.2 9.2–36.2 Ulcer on target leg 9 3.7–21.8 Chronic leg edema 8.5 4.3–16.9 History of deep vein thrombosis 5.6 2.5–12.6 Varicose eczema 4.6 1.4–14.7 Abolition of peripheral pulse 2.9 1.3–6.1 Hyperpigmentation 2.9 1.8–4.5 Overweight 2.85 2.0–4.0 History of venous insufficiency 2.8 1.9–4.1 History of varicose veins 2.2 1.5–3.3 History of venous leg surgery 1.8 1.05–3.2 Results from a case-control study (243 cases, 467 controls) of patients with acute bacterial cellulitis of the leg showed that disruption of the skin barrier, particularly in interdigital web spaces, may allow for the ingress and invasion of bacterial species that can lead to cellulitis. CI = confidence interval; OR = odds ratio. Adapted with permission from Roujeau et al. Dermatology. 2004;209:301-307.9 S631 CASE STUDY Figure 5. Good Candidates for Nail Avulsion Nails such as these probably need chemical or mechanical avulsion, in addition to systemic therapy. As with my case study earlier in this monograph, this patient had a strong family history of onychomycosis, which the mother generally referred to as “foot fungus.” With onychomycosis, there often is a family history of disease. Huang and Paller found that 59% of pediatric patients had family members who were affected, and Friedlander found that 100% of 33 affected children had first-degree relatives with the disease (Unpublished data, ongoing trial).8 CLINICIAN INTERVIEW Dr Friedlander is a pediatric dermatologist with subspecialty training in infectious diseases and is a clinical professor of pediatrics and medicine at the University of California, San Diego School of Medicine, and the affiliated Children’s Hospital and Health Center. She treats children with congenital and acquired skin conditions and is particularly interested in cutaneous infections and pediatric vascular lesions. A senior clinical editor for Advanced Studies in Medicine (ASiM) interviewed Dr Friedlander about this case study involving a 14-year-old boy with onychomycosis, asking her to provide more insight into the practical clinical aspects of diagnosing and treating onychomycosis in primary care and the special considerations in pediatric patients. ASiM: How often does cost become an issue when you are talking about treatment? How do you respond to patients who don’t have the money? Dr Friedlander: Most insurance carriers or healthcare plans will cover griseofulvin, and some will cover terbinafine. I have not had much success in getting S632 ciclopirox covered for my pediatric patients. Griseofulvin is the only drug that is FDA approved for children, which was not effective in this case study. If a patient has not responded to griseofulvin, then I can request, and usually receive [coverage for], terbinafine. Fluconazole also could be prescribed, but I think terbinafine is more effective. Cost is an issue because 9 months of griseofulvin is usually more expensive than 3 months of terbinafine. Some are concerned that the newer drugs are more expensive, but all treatments can be expensive. It would be interesting to see the cost data on ciclopirox nail lacquer versus oral therapies. I remind patients who are concerned about treatment costs that onychomycosis is a health issue, in addition to a cosmetic issue. If the patient is symptomatic, I try to submit coverage requests to their insurance company. There was a time when the HMOs (Health Maintenance Organizations) were attempting to deny coverage for toenail infection treatment. Practitioners were able to show that there were complications from toenail fungus, particularly in patients with compromised immune systems and in patients with diabetes, who can develop severe cellulitis. In addition, for any patient there is the issue of pain from deformed nails. Thus, now it is often possible to get medications covered by insurance. Does everyone who has toenail fungus need to be treated? Probably not; however, if the disease progresses, patients can experience discomfort, difficulty wearing shoes, and embarrassment from the discolored thickened nails. Also, they are at risk for developing a secondary infection. ASiM: When should the primary care physician refer children to a dermatologist or a podiatrist? Dr Friedlander: If the child’s disease is problematic to the family, the primary care physicians and podiatrists can obtain a culture. If the practitioner obtains a culture from the patient and is satisfied with the diagnosis, they should be able to initiate therapy. Primary care physicians certainly can prescribe topical ciclopirox nail lacquer, and they can prescribe other agents if they think it is appropriate. Although clinicians may not wholly approve of off-label usage, I think it is reasonable for them to use the off-label agents, if documentation is done. Then, if a patient is unresponsive to the treatment, the primary care physician can refer the patient to a specialist. It all depends on the “comfort level” of the practitioner. Vol. 5 (6D) n June 2005 CASE STUDY General practitioners may have less experience with treating pediatric onychomycosis because the disease is less common in children. Therefore, if clinicians are doubtful about the diagnosis, the patient should be referred to a specialist. ASiM: We know aggressive treatment is important in patients who are HIV (human immunodeficiency virus) positive and in patients with diabetes. Why should children with onychomycosis be treated, especially when there are no secondary infections, as was true in this case? Dr Friedlander: You can look at this problem in another way. This disease is a major concern for many families. They may want to have treatment started early before extensive nail involvement occurs. Obviously, discomfort is sometimes a problem for the patient. If the nails are dystrophic, the children’s shoes will not fit properly, and they may be embarrassed to participate in outdoor sports and swimming or to wear sandals. The clinician should acknowledge the patient’s degree of discomfort, the symptoms involved, and the family’s concerns. Advanced Studies in Medicine n REFERENCES 1. Gupta, AK, Sibbald RG, Lynde CW, et al. Onychomycosis in children: prevalence and treatment strategies. J Am Acad Dermatol. 1997:36:395-402. 2. Gupta AK, Skinner AR. Onychomycosis in children: a brief overview with treatment strategies. Pediatr Dermatol. 2004;21:74-79. 3. Jones TC. Overview of the use of terbinafine (Lamisil) in children. Br J Dermatol. 1995;132:683-689. 4. Aly R, Hafeez ZH, Rodwell C, et al. Rapid response of Trichophyton tonsurans-induced onychomycosis after treatment with terbinafine [published correction appears in Int J Dermatol. 2002;41:826]. Int J Dermatol. 2002;41:357-359. 5. Ungpakorn R, Reangchainam S, Kullavanijaya P. Onychomycosis in a 2-year-old child successfully treated with oral terbinafine. J Am Acad Dermatol. 1998;39:654-655. 6. Aguilar C, Mueller KK. Reversible agranulocytosis associated with oral terbinafine in a pediatric patient. J Am Acad Dermatol. 2001;45:632-634. 7. Friedlander SF, Suarez S. Pediatric antifungal therapy. Dermatol Clin. 1998;16:527-537. 8. Huang PH, Paller AS. Itraconazole pulse therapy for dermatophyte onychomycosis in children. Arch Pediatr Adolesc Med. 2000;154:614-618. 9. Roujeau JC, Sigurgeirsson B, Korting HC, et al. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. Dermatology. 2004;209:301-307. S633 NOTES S634 Vol. 5 (6D) n June 2005