* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Presence of Leishmania organisms in specific and non
Survey
Document related concepts
Transcript
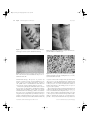
IJD_1610.fm Page 670 Friday, September 27, 2002 9:05 PM Report Oxford, UK International IJD Blackwell 0011-9059 41 Science, Journal Ltdof Dermatology 2002 Presence of Leishmania organisms in specific and non-specific skin lesions in HIV-infected individuals with visceral leishmaniasis Leishmania Bosch et al. organisms in skin lesions Ricardo J. Bosch, MD, Ana B. Rodrigo, MD, Purificación Sánchez, MD, María V. de Gálvez, MD, and Enrique Herrera, MD From the Department of Dermatology, University Hospital, School of Medicine, Malaga, Spain Correspondence Ricardo J. Bosch, MD Departamento de Dermatología Facultad de Medicina Universidad de Málaga 29010, Malaga Spain E-mail: [email protected] Abstract Background Leishmania coinfection is frequently seen in human immunodeficiency virus (HIV)-infected patients in endemic areas, and from time to time the protozoan is detected in cutaneous biopsies. Objective To establish the characteristics and possible ethiologic role of the presence of Leishmania in these lesions. Methods We studied 12 cutaneous biopsies with Leishmania organisms from nine HIVinfected patients (seven men and two women) with visceral leishmaniasis, diagnosed by bone marrow examination, seen over a period of 9 years. Results Based on clinical characteristics, evolution and response to anti-leishmanial treatment, cutaneous alterations were found to be related to the presence of the protozoan in six cases, whereas in the other six cases it was not considered responsible for the dermatological lesions (dermatofibroma, and lesions of psoriasis, Reiter’s syndrome, bacillary angiomatosis, cryptococcosis and oral aphthae). Of note was the high prevalence of specific mucocutaneous manifestations, usually accompanied by intense pruritus, great variability, and a tendency to relapse after treatment stopped. On two occasions, detection of the protozoa in skin biopsies led to the diagnosis of a previously unsuspected visceral leishmaniasis. Conclusions Cutaneous detection of Leishmania is frequent in HIV-infected individuals with visceral leishmaniasis. Sometimes Leishmania is associated with changes attributable to other dermatological processes, and its presence does not imply a causative role. A clear relationship between the systemic process and the therapeutic response is necessary to demonstrate an ethiologic role. Introduction 670 Leishmaniasis, caused by a protozoan of the genus Leishmania, includes a series of diseases which vary widely depending on the infecting species, the reservoir, the transmitting agent and the susceptibility of the host.1 Spanish dermatologists, especially in certain Mediterranean areas, quite often see children with the cutaneous form of leishmaniasis, generally caused by Leishmania tropica minor, and in which Phlebotomus papatasi acts as the vector. However, despite the fact that visceral leishmaniasis, usually caused by L. donovani or L. infantum, is also endemic in this area, cutaneous manifestations are rare, and are most often associated with non-specific eruptions during the acute phase, showing macular lesions and dischromatic, erythematous or nodular lesions of postkala-azar dermal leishmaniasis.2 International Journal of Dermatology 2002, 41, 670 –675 In a 9-year period we have detected the histopathological presence of Leishmania in various cutaneous biopsies from human immunodeficiency virus (HIV)-infected patients with visceral leishmaniasis. Although its presence does not imply that it is the cause of the dermatological process, we feel it is necessary to establish criteria to determine whether Leishmania is responsible for the disease. Current ease of travel requires specialists working outside endemic areas to be aware of these infections and their presentations.3,4 Patients and methods Nine HIV-positive patients, seven men and two women, with different degrees of immunosuppression (Table 1) showed 12 mucocutaneous lesions in which, by means of biopsy, we detected the presence of Leishmania. © 2002 The International Society of Dermatology IJD_1610.fm Page 671 Friday, September 27, 2002 9:05 PM Bosch et al. Leishmania organisms in skin lesions Report Table 1 HIV-positive patients with visceral leishmaniasis and the presence of mucocutaneous Leishmania Patient Sex Age (years) 1 F 21 24 1 Hyperpigmented facial areas 2 M 46 3 M 27 128 250 320 2 3 4 Tattoo infiltration Disseminated papular eruption Disseminated papulae 4 5 M F 31 33 220 116 5 6 Flat-topped violaceous lesions Extensive infiltrated plaques 6 M 34 32 420 46 7 8 7 M 32 72 9 Indurated papulo-nodule Papulosquamous lesions; balanitis Onychodystrophy; arthritis Papuloglobular lesions 8 M 27 191 10 9 M 28 30 300 114 11 12 CD4/mm3 Chart Tumors, papulae, and disseminated violaceous nodules Erythematous desquamative plaques Relapsing ulceration in mouth All the patients were diagnosed with visceral leishmaniasis through bone marrow examination. In seven cases the diagnosis was known before the apparition of the dermatological alterations leading to the consultation, and five of them had previously received antiprotozoan therapy, two with liposomal amphotericin B (patients 3 and 4) and three with antimonial compounds (patients 1, 6 and 8). In two cases (patients 2 and 5), the detection of Leishmania in skin biopsies led to the diagnosis of a previously unsuspected visceral leishmaniasis. Eight patients were or had previously been intravenous drug abusers, with the one remaining woman (patient 1) denying any history of drug addiction, having presumably become infected through her work as a prostitute. They were seen at our dermatology department between 1990 and 1998 and had all resided in the province of Malaga, on the Mediterranean coast in southern Spain, with none of them reporting any travel to other areas or countries. The cutaneous manifestations for which they initially presented were very varied. The common characteristic leading to their grouping in this study was the pathological finding of Leishmania organisms. The responsibility of Leishmania for the dermatological process was established based on the presence of lesions which were suggestive of well-known cutaneous manifestations of visceral leishmaniasis and which were not clinically or pathologically consistent with any other known cause. Parallelism among the cutaneous process and visceral leishmaniasis was also considered. Moreover, in all cases we verify the response of the cutaneous lesions to antileishmanial therapy, whereas other treatments had already failed. © 2002 The International Society of Dermatology Histopathology (Leishmania presence) Clinical features Increased epidermal melanine Lymphohistiocytic infiltrate Deep well-defined granulomas Slight lymphohistiocytic infiltrate Psoriasiform dermatitis Lymphohistiocytic infiltrate Superficial granulomas Massive histiocytic infiltrate Dermal band respected Dermatofibroma Psoriasiform dermatitis Lymphohistiocytic infiltrate Cryptococcosis gelatinous pattern Cryptococcus spore coexistence Vascular proliferation with Warthin-Starry + bacilli Typical psoriasis Non-specific ulcer Results The presence of Leishmania in the dermis was considered responsible for the processes leading to the biopsy of the cutaneous alterations of patients 1–4 and the extensive infiltrated plaques of patient 5. The facial itching hyperpigmentation in patient 1 disappeared with antiprotozoan treatment, whereas it persisted with systemic antihistamine derivatives and topical steroids. The evolutionary correlation with visceral leishmaniasis was especially evident in patient 2, in whom the appearance of infiltration over a tattoo (Fig. 1) was simultaneous with hematological involvement. This same patient later developed an itching papulo-erythematous eruption (Fig. 2), in which leishmanias were also detected whereas no leishmanias were found in a biopsy of nearby unaltered skin taken at the same time. This same situation, with pruritus and top-flattened papules, also occurred in patient 3. The presence of a greater degree of infiltration was more obvious in patient 4, with the presentation of disseminated papules, and even more obvious in patient 5, who showed large infiltrated plaques (Fig. 3). The last two patients also had intense pruritus. In contrast, in the indurated nodule of patient 5 and the cutaneous disorders of patients 6– 9, the presence of Leishmania was considered a chance biopsy finding, and Leishmania was not therefore considered to be the cause of the cutaneous process for which the patients had originally consulted. This conclusion was reached after the clinical, analytical, and pathological demonstration of another disorder responsible for the manifestations, after the detection of leishmanias in areas of non-affected skin, or after verifying independence to International Journal of Dermatology 2002, 41, 670– 675 671 IJD_1610.fm Page 672 Friday, September 27, 2002 9:05 PM 672 Report Leishmania organisms in skin lesions Figure 1 Papulonodular lesions over a tattoo which infiltrated and disappeared under systemic antileishmanial therapy Bosch et al. Figure 3 Intensely itchy erythematous eruption with an intense parasitic infiltration of the histiocytes Figure 2 Pruriginous flat-topped papular eruption observed in Figure 4 Presence of large amounts of leishmanias in the biopsy, three of the six cutaneous lesions attributed to the presence of leishmanias in the skin which is usual in these patients, enabling them to be seen easily with a hematoxilin-eosin stain antileishmanial therapy. The presence of protozoa was detected in cutaneous lesions associated with Reiter’s syndrome, cryptococcosis, bacillary angiomatosis, psoriasis or oral aphthae and in a dermatofibroma which had been removed to rule out the possibility of Kaposi’s sarcoma. Of note in the pathological examination was the existence in most sections of a large number of leishmanias, both intracellularly and extracellularly, clearly visible even with the usual hematoxilin-eosin stain (Fig. 4). In those cases attributable to the presence of leishmanias, the histological features ranged from scarce lymphohistiocytic infiltrate, via the presentation of dermal granulomas, to the existence of a massive infiltration of histiocytes laden with leishmanias which only respected a narrow band of collagen in the superficial dermis (Fig. 5). In six cases the lymphohistiocytic infiltrate with leishmanias coexisted with histopathological changes typical of other processes, on occasions as characteristic as those of bacillary angiomatosis or the striking presence of spores of Cryptococcus neoformans. After dermatological study and clinical and hematological evaluation of the patient, therapy was initiated on nine occasions, seven with antimonial compounds (cases 1, 2, 3, 5, 6, 8 and 11), and two with liposomal amphotericin B (cases 4 and 9). The response to these treatments was important for establishing or discarding the causal role of Leishmania. Two patients (6 and 8) treated with antimonial compounds died. International Journal of Dermatology 2002, 41, 670 –675 © 2002 The International Society of Dermatology IJD_1610.fm Page 673 Friday, September 27, 2002 9:05 PM Bosch et al. Figure 5 Subepidermal band respected by the parasite-laden histiocytes, similar to that seen in lepromatous leprosy. Some role of cardiac side-effects of these drugs in conjunction with other alterations was suspected, but the actual cause could not be determined as permission for autopsy was not granted. Discussion Over recent years, the natural immune response has been altered in many patients by the action of HIV. Although the repercussions for cutaneous and mucocutaneous leishmaniasis have not been great, several reports have appeared of unusual forms of these diseases in HIV-positive patients.5–13 In contrast, in countries around the Mediterranean basin, including Spain, where the infection is endemic, there is a high prevalence of visceral (“kala-azar”) leishmaniasis among HIVpositive patients,14–18 with an estimated 1–3% of these patients being infected.19 In fact, although leishmaniasis is not at present included among the definitive criteria for acquired immunodeficiency syndrome (AIDS), its inclusion has been proposed.20–23 This high prevalence is mainly a result of reactivation of a latent infection by immunosuppression, with antileishmania serology tests and CD4+ T-cell counts being proposed for its prophylactic and therapeutic control.24,25 The influence of immunosuppression is also supported by the frequent treatment failures in these patients and by the reduction of these observations over recent years, since the use of antiretroviral therapy has prevented situations of severe immunosuppression. The predominance of intravenous drug abusers among our patients is the result of intravenous drug abuse being the most common risk factor in our area, although the possibility has also been suggested that these patients act as reserves for transmission by contaminated needles.26,27 © 2002 The International Society of Dermatology Leishmania organisms in skin lesions Report Paralleling the increase in cases of visceral leishmnaisis in HIV-positive patients are reports of different cutaneous manifestations in which leishmanias have been detected, though the etiologic and pathogenic significance of this finding with regard to cutaneous manifestations may be different, as we suggested in our initial report some years ago.28 Occasionally, the presence of protozoa in dermal histiocytes is attributable solely to the existence of a general impregnation of the macrophagocytic system, which is especially intense in immunosuppressed patients. The detection therefore of cutaneous amastigotes in a patient with visceral leishmaniasis does not necessarily imply that they are the cause of the dermatological process under study. Leishmanias have been noted in healthy skin29 as well as in lesions from widely different processes including Kaposi’s sarcoma,30–33 herpes simplex and zoster,34,35 and a dermatofibroma.36 From our cases, it is possible to include within this group the clinically and histopathologically typical psoriasis and the erythematous desquamative eruption seen in a typical case of Reiter’s syndrome. Moreover, their coexistence with the infectious agents responsible for bacillary angiomatosis and cryptococcosis, which has not previously been reported, was clear. Finally, they were also detected in a dermatofibroma and in an ulcerated lesion of the oral mucosa, which from its evolution and features was an oral aphtha. In two cases this incidental observation led to the diagnosis of an unsuspected visceral leishmaniasis, which in the absence of clinical and hematological data should be confirmed by bone marrow study, including cultures in adequate media. Although not mentioned in comparative studies,37 cutaneous involvement during the course of visceral leishmaniasis seems to be more frequent in HIV-positive patients than in immunocompetent persons. Manifestations reported comprise a wide range of changes, such as hyperpigmented lesions, which gave rise to the term “kala-azar” (black fever) in one of the languages of India, and diverse papular and nodular eruptions, more or less extensive and florid, which indicate the involvement or persistence of cutaneous leishmanias.38–42 Other manifestations with varying clinical aspects have also been reported, including erythroderma, dermatomyositis and psoriasis.43–45 Our experience confirms this great clinical and pathological variability. The presence of infiltration over a tattoo has been reported previously36 and in our case it is gaudy, the infiltrative relapses coinciding with systemic worsening. Of note in three of the patients (cases 3, 4 and 5) was the papular eruption, mainly involving the extremities, with certain clinical features resembling lichen planus. An important clinical detail, also present in another two of the six cases, attributable to the presence of leishmanias was the intense pruritus accompanying the lesions. This symptom may well be of use when considering the possibility of visceral leishmaniasis as an explanation for these specific cutaneous manifestations. At no time did we see any lesions in the oral mucosa, which could International Journal of Dermatology 2002, 41, 670– 675 673 IJD_1610.fm Page 674 Friday, September 27, 2002 9:05 PM 674 Report Leishmania organisms in skin lesions have been considered attributable to the direct activity of Leishmania, although this has been reported on occasion.27,46–48 The histopathological findings in cutaneous lesions are variable and often atypical,42,49 as in other organs.50 Of interest in our series, together with the non-specific images and variable granulomatous appearance, was the case with an intense histiocytic infiltrate laden with leishmanias respecting a narrow band in the superficial dermis, similar to that seen in lepromatous leprosy. Its coincidence with severe immunosuppression confirms it as an anergic form and suggests the existence in leishmaniasis of a similar situation to leprosy, with clinical and pathological differences depending on the immune response. The large amount of leishmanias present in most cases enabled them to be seen easily with routine stains, and emphasizes the importance of the histopathological study of these lesions. In fact, it led to the diagnosis of clinically non-specific lesions, and on two occasions resulted in the diagnosis of a previously unsuspected visceral leishmaniasis. The demonstration of leishmanias is essential but, as we have seen, does not necessarily indicate that they have a causal role in the lesions under study. It is necessary to discard other processes and, more especially, to demonstrate a good evolutionary correlation with the systemic symptoms and with the response to antiprotozoan therapy. Seven of the nine patients in which leishmanias were detected had lymphocyte counts ≤ 250 CD4/mm3, many of these corresponding to the period prior to the introduction of multiple antiretroviral therapy, when situations of severe immunosuppression were common. At that time the HIV viral load was not determined, but later studies showed the existence of a relationship between this and the quantification of leishmanias in bone marrow, as well as their influence in the response to antileishmanial therapy.51 The tendency of the infection to persist is demonstrated by the fact that eight of the cases in which leishmanias were detected arose in patients who had previously received antimonial or liposomal amphotericin B treatment. The prognosis in these patients is uncertain, so they should undergo close follow-up and be given secondary prophylaxis52 at least until there is a clear improvement in their immune status.53 Acknowledgments The authors thank Ian Johnstone for help with the English language version of the paper. References 1 Herwaldt BL. Leishmaniasis. Lancet 1999; 354: 1191–1199. 2 Ramesh V, Mukherjee A. Post-kala-azar dermal leishmaniasis. Int J Dermatol 1995; 34: 85 – 91. International Journal of Dermatology 2002, 41, 670 –675 Bosch et al. 3 Albrecht H, Sobottka I, Emminger C, et al. Visceral leishmaniasis emerging as an important opportunistic infection in HIV-infected persons living in areas nonenedemic for leishmania donovani. Arch Pathol Laboratory Med 1996; 120: 189–198. 4 Rosen T. Leishmaniasis and the JEADV. J Eur Acad Dermatol Venereol 1998; 10: 212–213. 5 Echevarria J, Campos P, Chang J, et al. Mucocutaneous leishmaniasis and AIDS. Case report. Trans R Soc Trop Med Hyg 1993; 87: 186. 6 Belaich S, Bouscarat F, Picard C. Leishmaniasis and AIDS. In: Burgdorf WHC, Katz SI, eds. Dermatology Progress and Perspectives. New York: The Partenon Publishing Group 1993, pp. 407–409. 7 Miralles ES, Nunez M, Hilara Y, et al. Mucocutaneous leishmaniasis and HIV. Dermatology 1994; 189: 275–277. 8 Inhof M, Schöfer H, Milbradtr R, Liutz TH. Mucocutaneous leishmaniasis in a European HIV-infected patient. Eur J Dermatol 1995; 5: 594–596. 9 Herrera E, Sánchez Bosch RJ. Disseminated cutaneous leishmaniasis in an HIV-infected patient. Int J STD AIDS 1995; 6: 125–126. 10 Durand I, Beylot-Barry M, Weill FX, et al. Disseminated cutaneous leishmaniasis revealing human immunodeficiency virus infection. Ann Dermatol Venereol 1998; 125: 268– 270. 11 Rosatelli JB, Souza CS, Soares FA, et al. Generalized cutaneous leishmaniasis in acquired immunodeficiency syndrome. J Eur Acad Dermatol Venereol 1998; 10: 229– 232. 12 de Souza I, Daibes R, de Guimaraens Farias M, et al. American cutaneous leishmaniasis due to Leishmania (Viannia) guyanensis as an initial clinical presentation of human immunodeficiency virus infection. J Eur Acad Dermatol Venereol 1998; 10: 214–217. 13 Mattos M, Caiza A, Fernandes O, et al. American cutaneous leishmaniasis associated with HIV infection: report of four cases. J Eur Acad Dermatol Venereol 1998; 10: 218–225. 14 Alvar J, Gutierrez Solar B, Molina R, et al. Prevalence of Leishmaniasis infection among AIDS patients. Lancet 1992; 339: 1427. 15 Dedet JP, Lambert M, Pratlong F. Leishmaniasis and human immunodeficiency virus infections. Presse Med 1995; 24: 1036–1040. 16 Rosenthal E, Marty P, Pazot-Martin J, et al. Visceral leishmaniasis and HIV-1 co-infection in southern France. Tras R Soc Trop Med Hyg 1995; 89: 159–162. 17 Agostoni C, Doprigoni N, Malfitano A, et al. Mediterranean leishmaniasis in HIV-infected patients: epidemiological, clinical, and diagnostic features of 22 cases. Infection 1998; 26: 93–99. 18 Pineda JA, Gallardo JA, Macias J, et al. Prevalence of and factors associated with visceral leishmaniasis in human immunodeficiency virus type 1-infected patients in southern Spain. J Clin Microbiol 1998; 36: 2419 – 2422. 19 World Health Organisation. AIDS, leishmanias dangers of clash highlighted. TDR News 1991; 36: 1. © 2002 The International Society of Dermatology IJD_1610.fm Page 675 Friday, September 27, 2002 9:05 PM Bosch et al. 20 Montalban C, Martinez Fernández R, Calleja JL, et al. Visceral leishmaniasis (kala-azar) as an opportunistic infection in patients infected with the human immunodeficiency virus in Spain. Rev Infect Dis 1989; 4: 655–660. 21 Rousaud F. Visceral leishmaniasis in patients infected by HIV. An AIDS diagnostic criteria? Rev Clin Esp 1993; 192: 462. 22 Albrecht H. Redefining AIDS: towards a modification of the current AIDS care definition. Clin Infect Dis 1997; 24: 64– 74. 23 Yebra M, Segovia J, Montalban C, Vargas JA. Is kala-azar a diagnostic criterion for acquired immunodeficiency syndrome? Med Clin (Barc) 1989; 93: 16. 24 Kubar J, Marty P, Lelievre A, et al. Visceral leishmaniasis in HIV-positive patients: primary infection, reactivation and latent infection. Impact of the CD4+ T-lymphocyte counts. AIDS 1998; 12: 2147–2153. 25 Gradoni L, Scalone A, Gramiccia M. HIV-Leishmania coinfections in Italy: serological data as an indication of the sequence of acquisition of the two infections. Trans R Soc Trop Med Hyg 1993; 87: 94–96. 26 Alvar J, Jimenez M. Could infected drug-users be potential Leishmania infantum reservoirs? AIDS 1994; 8: 854. 27 Amela C, Lopez-Gay D, Alberdi JC, Castilla J. Injecting drug use as risk factor for visceral leishmaniasis in AIDS patients. Eur J Epidemiol 1996; 12: 91–92. 28 Herrera E, Bosch RJ, Fernandez F, et al. The presence and significance of leishmania in mucocutaneous biopsies from HIV+ patients with visceral leishmaniasis. Eur J Dermatol 1996; 6: 501–504. 29 Belda A, Diaz F, Martinez B, et al. Visceral leishmaniasis and AIDS. Report of 2 cases with cutaneous dissemination. Ann Med Interna 1994; 11: 398–400. 30 Romeu J, Milla F, Batlle M, et al. Visceral leishmaniasis involving lung and cutaneous Kaposi’s sarcoma lesion. AIDS 1991; 5: 1272. 31 Taillan B, Marty P, Scneider S, et al. Visceral leishmaniasis involving a cutaneous Kaposi’s sarcoma lesion and free areas of the skin. Eur J Med 1992; 4: 255. 32 Gallego MA, Aguilar A, Plaza S, et al. Kaposi’s sarcoma with an intense parasitization by Leishmania. Cutis 1996; 57: 103–105. 33 Abajo P, Bueno GF, Fraga J, et al. Leishmaniasis and Kaposi’s sarcoma in an HIV-infected patient. Am J Dermatopathol 1997; 19: 101–103. 34 España A, Hermida JM, Mantilla P, Buzón L. Cutaneous and systemic leishmaniasis with herpes virus infection in an AIDS patient [Letter]. Rev Clin Esp 1990; 187: 437. 35 Barrio J, Lecona M, Cosin J, et al. Leishmania infection occurring in herpes zoster lesions in an HIV-positive patient. Br J Dermatol 1996; 134: 164–166. 36 Colebunders R, Depraetere K, Verstraeten Th, et al. Unusual cutaneous lesions in two patients with visceral leishmaniasis and HIV infection. J Am Acad Dermatol 1999; 41: 847–850. 37 Reus S, Sanchez R, Portilla J, et al. Visceral leishmaniasis: a comparative study of patients with and without human © 2002 The International Society of Dermatology Leishmania organisms in skin lesions Report 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 immunodeficiency virus infection. Enferm Infecc Microbiol Clin 1999; 17: 515–520. Cnudde F, Raccurt C, Boulard F, et al. Diffuse cutaneous leishmaniasis with visceral dissemination in an AIDS patient in Ghadeloupe, West Indies. AIDS 1994; 8: 559–560. Scaglia M, Malfitano A, Douville H, et al. Dermonodular and visceral leishmanoiasis due to Leishmania infantum with a new isoenzyme pattern report of a case involving a patient with AIDS. Clin Infect Dis 1996; 22: 376–377. Rios Buceta L, Buezo GF, Penas PF, et al. Post kala-azar dermal Leishmaniasis in an HIV-patient. Int J Dermatol 1996; 35: 303–304. Ara M, Maillo C, Peon G, et al. Visceral leishmaniasis with cutaneous lesions in a patient infected with human immunodeficiency virus. Br J Dermatol 1998; 139: 114–117. Villareal M, Yebra M, García M, Salas C. Nodular cutaneous lesions in a patient with human immunodeficiency virus infection. Rev Clin Esp 1998; 198: 469–470. Belda A, Pascual JM, Prats A, García J. Erythroderma as presentation form of visceral leishmaniasis in a patient with human immunodeficiency virus infection. Rev Clin Esp 1992; 191: 454. Dauden E, Penas PF, Rios L, et al. Leishmaniasis presenting as a dermatomyositis-like eruption in AIDS. J Am Acad Dermatol 1996; 35: 316–319. Rubio FA, Robayna G, Herranz P, et al. Leishmaniasis presenting as a psoriasiform eruption in AIDS. Br J Dermatol 1997; 136: 792–794. Michiels JF, Monteil RA, Hofman P, et al. Oral leishmaniasis and Kaposi’s sarcoma in an AIDS patient. J Oral Pathol Med 1994; 23: 45–46. Banuls J, Boix V, Portilla J, Silvestre JF. Leishmaniasis as a cause of oral disease in HIV infection. AIDS 1995; 9: 96–98. Vazquez-Pineiro T, Fernandez JM, Gonzalo JC, et al. Visceral leishmaniasis: a lingual presentation in a patient with HIV infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998; 86: 179–182. Perrin C, Taillan B, Hofman P, et al. Atypical cutaneous histological features of visceral leishmaniasis in acquired immunodeficiency syndrome. Am J Dermatopathol 1995; 17: 145–150. Hofman V, Marty P, Perrin C, et al. The histological spectrum of visceral leishmaniasis caused by Leishmania infantum MON-1 in acquired immune deficiency syndrome. Hum Pathol 2000; 31: 75–84. Berhe N, Wolday D, Hailu A, et al. HIV viral load and response to antileishmanial chemotherapy in co-infected patients. AIDS 1999; 13: 1921–1925. Murray HW. Kala-azar as an AIDS-related opportunistic infection. AIDS Patient Care STDS 1999; 13: 459–465. Soriano V, Dona C, Rodriguez-Rosado R, et al. Discontinuation of secondary prophylaxis for opportunistic infections in HIV-infected patients receiving highly antiretroviral therapy. AIDS 2000; 14: 283–286. International Journal of Dermatology 2002, 41, 670– 675 675