* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download (19) United States N204“ 206 202
Survey
Document related concepts
Transcript
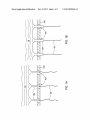
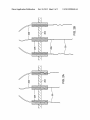
US 20130280436A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0280436 A1 Scott et al. (54) (43) Pub. Date: ELECTROSPINNING WITH REDUCED CURRENT OR USING FLUID OF REDUCED CONDUCTIVITY (51) (71) Applicant: Nanostatics Corporation, Circleville, (72) Publication Classi?cation Int Cl B05D 1/00 (2006.01) B05C 5/00 2006.01 (52) us CL OH (Us) Oct. 24, 2013 ( ) CPC .. B05D 1/007 (2013.01); B05C 5/00 (2013.01) USPC .......................... .. 427/458: 118/621' 524/577 Inventors: Ashley S. Scott, Grove City, OH (US); ’ ’ John A. Robertson, Chillicothe, OH (US); Andrew L. Washington, JR., (57) ABSTRACT A method comprises: dissolving an aromatic side chain poly Pataskala, OH (US) mer in a terpene, terpenoid, or aromatic solvent; dissolving an inorganic salt in a polar organic solvent; mixing the salt solution and the polymer solution; and using a predominantly terpene, terpenoid, or aromatic solvent phase of the mixture as electrospinning ?uid. The ?uid is electrospun from spin (21) Appl. No.: 13/787,724 ning tip(s) onto a target substrate. The inorganic salt and the (22) Filed: Mar. 6, 2013 polar organic solvent are chosen so as not to cause substantial precipitation of the polymer upon mixing With the polymer solution. The terpene, terpenoid, or aromatic solvent can (62) Related US. Application Data Division of application NO 12/728 070 ?led on Mar 19 2010 HOW Pat NO 8 5'18 319 ’ ’ ’ ' ' ’ ’ ’ comprises D-limonene, the aromatic side chain polymer can comprise polystyrene, the polar organic solvent can comprise ' ' dimethyl formamide, and the inorganic salt can comprise LiCl, AgNO3, CuCl2, or FeCl3. The method can further (60) Provisional application No. 61/161,498, ?led on Mar. comprise electrospinning the ?uid With the ?uid and spinning 19, 2009, provisional application No. 61/256,873, tips electrically isolated from a voltage source that drives the ?led on Oct. 30, 2009. electrospinning. 206 N204“ / /// ///// 202 Patent Application Publication Oct. 24, 2013 Sheet 1 0f 2 US 2013/0280436 A1 Patent Application Publication Oct. 24, 2013 Sheet 2 0f 2 US 2013/0280436 A1 Oct. 24, 2013 US 2013/0280436 A1 ELECTROSPINNING WITH REDUCED CURRENT OR USING FLUID OF REDUCED CONDUCTIVITY [0005] Conventional ?uids for electrospinning (melts, solutions, colloids, suspensions, or mixtures, including many listed in the preceding references) typically have signi?cant ?uid conductivity (e.g., ionic conductivity in a polar solvent, BENEFIT CLAIMS TO RELATED APPLICATIONS or a conducting polymer). In addition, conventional methods of electrospinning typically include a syringe pump or other driver/controller of the ?oW of ?uid to the spinning tip, and a [0001] This application is a divisional of US. non-provi sional application Ser. No. 12/728,070 entitled “Fluid formu lations for electric-?eld-driven spinning of ?bers” ?led Mar. 19, 2010 in the names of Ashley S. Scott, AndreW L. Wash ington, Jr., and John A. Robertson, Which in turn claims bene?t of (i) US. provisional App. No. 61/161,498 entitled “Electrospinning Cationic Polymers and Method” ?led Mar. 19, 2009 in the names of Ashley S. Scott, John A. Robertson, and AndreW L. Washington, Jr., and (ii) US. provisional App. No. 61/256,873 “Electrospinning With reduced current or using ?uid of reduced conductivity” ?led Oct. 30, 2009 in the names of Ashley S. Scott, John A. Robertson, and AndreW L. conductionpathbetWeen the high voltage supply and the ?uid to be spun. Such arrangements are shoWn, for example, in US. Pat. Pub. No. 2005/0224998 (hereafter, the ’998 publi cation), Which is incorporated by reference as if fully set forth herein. In FIG. 1 of the ’998 publication is shoWn an electro spinning arrangement in Which high voltage is applied directly to a spinning tip, thereby establishing a conduction path betWeen the high voltage supply and the ?uid being spun. In FIGS. 2, 5, 6A, and 6B of the ’998 publication are shoWn various electrospinning arrangements in Which an electrode is placed Within a chamber containing the ?uid to be spun, thereby establishing a conduction path betWeen the Washington, Jr. Each of said non-provisional and provisional applications is hereby incorporated by reference as if fully set high voltage supply and the ?uid. The chamber communi forth herein. ments, signi?cant current (typically greater than 1 [1A per spinning tip) ?oWs along With the spun polymer material. BACKGROUND [0002] The ?eld of the present invention relates to electro spinning of polymer nano?bers or electrospraying of small droplets. In particular, electrospinning With relatively reduced ?uid conductivity or With relatively reduced current is disclosed herein. [0003] The subject matter disclosed herein may be related to subject matter disclosed in co-oWned U.S. non-provisional cates With a plurality of spinning tips. In any of those arrange Conventional electrospinning ?uids are deposited on metal target substrates so that current carried by the spun material can ?oW out of the substrate, thereby avoiding charge buildup on the target substrate. Electrospinning onto nonconductive or insulating substrates has proved problematic due to charge buildup on the insulating substrate that eventually suppresses the electrospinning process. application Ser. No. 11/634,012 entitled “Electrospraying/ SUMMARY electrospinning array utiliZing a replacement array of indi [0006] A method comprises: dissolving an aromatic side chain polymer in a terpene, terpenoid, or aromatic solvent; vidual tip ?oW restriction” ?led Dec. 5, 2006 in the names of John A. Robertson and Ashley Steve Scott (Pub. No. US 2008/0131615 published Jun. 5, 2008) and US. provisional App. No. 61/161,498 entitled “Electrospinning cationic poly mers and method” ?led Mar. 19, 2009 in the name of Ashley dissolving an inorganic salt in a polar organic solvent; mixing the salt solution and the polymer solution; and using the predominantly terpene, terpenoid, or aromatic solvent phase S. Scott. Both of said applications are incorporated by refer of the mixture as an electrospinning ?uid. The ?uid is elec trospun from one or more spinning tips onto a target substrate. ence as if fully set forth herein. The inorganic salt and the polar organic solvent are chosen so [0004] “Electrospinning” and “electrospraying” refer to the production of, respectively, so-called “nano?bers” or “nanodroplets”, Which may be “spun” as ?bers or “sprayed” as droplets by applying high electrostatic ?elds to one or more ?uid-?lled spraying or spinning tips (i.e., noZZles or spin nerets). The high electrostatic ?eld produces a Taylor cone at each tip opening. The sprayed droplets or spun ?bers are typically collected on a target substrate. A high voltage supply provides an electrostatic potential difference (and hence the as not to cause substantial precipitation of the polymer upon mixing With the polymer solution. The terpene, terpenoid, or aromatic solvent can comprises D-limonene, the aromatic side chain polymer can comprise polystyrene, the polar organic solvent can comprise dimethyl formamide, and the inorganic salt can comprise LiCl, AgNO3, CuCl2, or FeCl3. The method can further comprise electrospinning the ?uid With the ?uid and spinning tips electrically isolated from a voltage source that drives the electrospinning. electrostatic ?eld) betWeen the spinning tip (usually at high [0007] Objects and advantages pertaining to electrospin voltage) and the target substrate (usually grounded). A num ber of revieWs of electrospinning have been published, including (i) Huang et al, “A revieW on polymer nano?bers by ning or electro spraying may become apparent upon referring to the exemplary embodiments illustrated in the draWings and disclosed in the folloWing Written description or appended electrospinning and their applications in nanocomposites,” claims. Composites Science and Technology, Vol. 63, pp. 2223-2253 (2003), (ii) Li et al, “Electrospinning of nano?bers: reinvent ing the Wheel?”, Advanced Materials, Vol. 16, pp. 1151-1170 (2004), (iii) Subbiath et al, “Electrospinning of nano?bers,” Journal ofApplied Polymer Science, Vol. 96, pp. 557-569 (2005), and (iv) Bailey, Electrostatic Spraying of Liquids BRIEF DESCRIPTION OF THE DRAWINGS [0008] FIGS. 1A and 1B illustrate schematically an exem plary electrospinning head. [0009] FIGS. 2A and 2B illustrate schematically another (John Wiley & Sons, NeW York, 1988). Details of conven exemplary electrospinning head. tional electrospinning materials and methods can be found in the preceding references and various other Works cited [0010] The embodiments shoWn in the Figures are exem plary, and should not be construed as limiting the scope of the present disclosure or appended claims. therein, and need not be repeated here. Oct. 24, 2013 US 2013/0280436 A1 DETAILED DESCRIPTION OF EMBODIMENTS [0011] Electrospinning or electrospraying of polymer-con DMF in various proportions. The conductivity of the salt/ DMF-treated PS/DL is about 13 uS/cm in each example. taining nano?bers or small droplets, respectively, can be employed to produce a variety of useful materials. However, scaling up an electrospinning process beyond the laboratory or prototype level has proven problematic. To achieve pro duction-type quantities, multiple electrospinning tips are typically employed in an arrayed arrangement. HoWever, the conductive ?uids used and the signi?cant current (typically greater than 1 [1A per tip) carried by ?bers emerging from each tip lead to impractically large overall current and to undesirable electrostatic interactions among the electrospin ning tips and ?bers; these limit the number and density of electrospinning tips that can be successfully employed. [0012] Electrospinning ?uids are disclosed herein that exhibit substantially reduced conductivity relative to conven tional electrospinning ?uids (While maintaining suitability for electrospinning), at least partly mitigating the undesirable electrostatic interactions described above. One group of such electrospinning ?uids comprises mixtures of (i) a solution of polystyrene in D-limonene and (ii) an inorganic salt dissolved in dimethyl formamide. Polystyrene (PS) is a non-polar, non conductive polymer; D-limonene (DL) is a relatively high boiling, loW vapor pressure, non-polar solvent that occurs naturally in citrus rinds. D-limonene is attractive as a “green,” or environmentally friendly, organic solvent, and is readily available in large quantities as a byproduct of citrus process ing. Conventional electro spinning has been attempted using a solution of PS in DL, but it has been observed that the result ing ?bers are relatively large (about 700 nm) and of poor quality (Shin et al, “Nano?bers from recycle Waste expanded polystyrene using natural solvent,” Polymer Bulletin, Vol. 55 pp. 209-215 (2005)). [0013] Treatment of the PS/DL solution With a solution of inorganic salt in dimethyl formamide (DMF) markedly improves the quality of nano?bers produced by electrospin ning a PS/DL ?uid. In one example of preparation of the electrospinning ?uids, a PS/DL solution is prepared that is betWeen about 10% and about 50% PS by Weight, typically betWeen about 20% and about 40% PS by Weight, preferably betWeen about 25% and about 35% PS by Weight. A solution of about 30% PS by Weight in DL can be employed. The measured conductivity of the 30% PS/DL solution is about 0.0 uS/cm (in contrast to a conductivity of about 150 uS/cm for pure DL) and the viscosity is about 3125 cps. As noted above, the PS/DL solution does not produce nano?bers of satisfactory siZe or quality When used as the electrospinning ?uid. [0014] To the PS/DL solution is treated With a solution of inorganic salt in DMF. Examples of salts that can be employed are LiCl, CuCl2, AgNO3, and FeCl3; other suitable inorganic salts can be employed. Salt concentrations in DMF can be betWeen about 0.01% and about 10% salt by Weight, Wgt % PS in DL 30% 30% 30% 30% 30% 30% [0015] salt % salt in DMF LiCl LiCl LiCl AgNO3 LiCl CuCl2 5% Wgt 5% Wgt 5% Wgt 5% Wgt 5% Wgt 5% Wgt % salt/DMF in PS/DL 10% 20% 30% 30% 30% 30% vol vol vol vol Wgt Wgt viscosity 1640 1200 990 795 325 540 cps cps cps cps cps cps typical ?ber diameter no data no data 552.5 nm 516.7 nm 289 nm 224 nm Another exemplary salt/DMF-treated PS/DL com prises a solution of 30% by Weight of PS (mW 192k, atactic) dissolved in DL and combined With a solution of CuCl2 dissolved in DMF, With the amounts of CuCl2 and DMF chosen to yield a 3:1 mole ratio ofCuCl2 to PS and a 1 :1 mole ratio of DMF to DL. The resulting mixture does not phase separate and is used as an electrospinning ?uid in exemplary embodiments described hereinbeloW, Wherein nano?bers betWeen about 250 nm and about 300 nm are consistently produced. More generally, the amounts of salt and polar organic solvent can be chosen to result in (i) a mole ratio betWeen about 0.5:1 and about 20:1 of the salt to the polymer in the mixture and (ii) a mole ratio betWeen about 1:1 and about 1:4 of the polar organic solvent to the terpene, terpe noid, or aromatic solvent in the mixture. [0016] The electrospinning of the ?uids described above exhibit electrospinning characteristics that differ substan tially from those of conventional electrospinning ?uids in several Ways. Nano?bers can be spun from the salt/DMF treated PS/DL onto insulating substrates (e.g., Mylar®, Typar®, paper, and so forth) as Well as conducting substrates. The ?oW rate during spinning of the salt/DMF-treated PS/DL electrospinning ?uid is substantially larger than that of con ventional electrospinning ?uids (20-500 uL/min/nozzle While producing nano?bers of less than 500 nm diameter, versus 1-2 uL/min/nozzle for conventional ?uids). The cur rent carried by the spun nano?bers is substantially reduced for the salt-DMF-treated PS/DL (less than about 0.3 [LA/nozzle versus greater than about 1 [LA/nozzle for conven tional ?uids). The nano?bers produced by electro spinning the salt/DMF-treated PS/DL typically spread over a smaller area When spun than nano?bers spun from conventional electro spinning ?uids (e. g., a spot about 0.5 inch in diameter versus about 2 inches in diameter When spun from a noZZle about 7 inches from the target substrate). Instead of requiring a syringe pump or similar mechanism to drive ?uid ?oW (as is the case When using conventional electrospinning ?uids), ?uid head pressure behind the noZZle can be suf?cient to sustain electrospinning of the salt/DMF-treated PS/DL ?uid (in one example only about 1 inch of ?uid pressure Was suf?cient). In contrast to nano?bers produced by conven typically betWeen about 0.02% and about 5% salt by Weight, preferably betWeen about 0.05% and about 1% salt by Weight. tional electrospinning ?uids, Which can vary Widely (for The PS/DL solution and the salt/DMF solution are mixed in a diameter based on operating conditions such as voltage or example, from less than 200 nm to greater than 1 pm) in their selected proportion. At higher salt/DMF proportions, the ?oW rate, electrospinning the salt/DMF-treated PS/DL ?uid resulting mixture phase-separates over a period of several typically produces nano?bers in about a 250-300 nm range over a Wider range of operating conditions. In one example, electrospinning With a ?uid head pressure of about 1 psi and an applied voltage of 80 kV results in a ?oW rate of about 59 uL/min/nozzle and ?bers of about 278 nm average diameter. In another example, the same pressure and ?oW rate With an hours to several days (into separate ?uid layers, sometimes also With a solid precipitate). The salt/DMF-treated PS/DL (the phase-separated PS/DL layer, if phase separation occurs) is used as the electrospinning ?uid. The table beloW summa riZes some observed results using PS/DL treated With salt/ Oct. 24, 2013 US 2013/0280436 A1 applied voltage of 40 kV yields ?bers of about 282 nm aver age diameter. In yet another example, applying a ?uid head pressure of about 10 psi and applying about 80 kV results in a ?oW rate of about 135 uL/min/nozzle and ?bers of about 235 deposits substantially more surface charge onto an insulating target substrate (loud, visible spark When discharged versus nm average diameter. no audible or visible discharge) compared to isolated-noZZle [0017] electrospinning With salt/DMF-treated PS/DL. [0020] An exemplary electrospinning head 200 is shoWn in FIGS. 2A and 2B. The head 200 comprises (i) a metal plate Alternative solvents can be employed for dissolving the polystyrene (or other polymer); examples of candidate solvents include but are not limited to: limonene derivatives (e.g., carveol or carvone); other terpene-based solvents or terpenoid derivatives (e.g., ot-pinene, [3-pinene, 2-pinanol, camphene, ot-myrcene, cis-ot-ocimene, linalool, nerol, geraniol, citronellol,Y-terpinene, ot-phellandrene, p-cymene, terpinolene (1 ,4(8)-menthadiene), isolimonene (2,8-mentha diene), lp-limonene (1 (7),8-menthadiene), or 1(7),4(8)-men a substantial, readily visible and audible corona discharge near the noZZle (versus only a audible corona discharge), and 202, (ii) an array of 1 10 metal tubes 204 (stainless steel in this example) about 1 inch long arranged in a 3 inch by 3 inch square grid pattern on 1A inch centers With a noZZle end thadiene); aromatic solvents (e.g., benZene or toluene); tet extending about 1/2 inch from one surface of the plate, and (iii) a bundle of 110 corresponding capillary tubes 206 (Te?on® of other suitable dielectric insulating material), each about 8 inches long and about 450 pm in inner diameter. The capillary rahydrofuran or other ethers; or other similar solvents or tubes 206 are connected to a ?uid reservoir (not shoWn) at one mixtures thereof. Alternative non-polar, non-conductive polymers can be employed; examples of candidate polymers end and are each inserted into the back end (opposite the noZZle end) of a corresponding one of the metal tubes 204. The ?uid reservoir can be controlled to apply a desired level of head pressure on the ?uid in the reservoir. A high voltage include but are not limited to: styrene butadienes, other aro matic side chain polymers, polymethylmethacrylate (PMMA) or other acrylate polymers, polyvinylchloride (PVC), or copolymers or derivatives thereof. Alternative salts can be employed; examples include but are not limited to LiCl, AgNO3, CuCl2, or FeCl3. In addition to treating the polymer solution so that it spins, silver salts can also impart desirable antimicrobial properties onto the deposited electro spun nano?bers. Alternative solvents can be employed for dissolving the salt to treat the non-polar polymer solution that preferably do not reduce the solubility of the polymer in its supply (not shoWn) applies voltage to the metal plate 202 (and hence all the metal tubes 204) to cause electrospinning of the ?uid in the reservoir through the capillary tubes 206. [0021] The capillary tubes 206 can be recessed about 1/2 inch (or other suitable distance) into the metal tubes 204 (as in FIG. 2A) so that the electrospinning head 200 acts as a con ventional spinning head, With a conduction path betWeen the electrospinning solution and the voltage supply (through Were formed using a conventional electrospinning arrange ment, in Which a conduction path is established betWeen the metal plate 202 and metal tubes 204, Which are in contact With the spun ?bers 20 after they leave the recessed ends of the capillary tubes 206).Alternatively, the capillary tubes 206 can be extended out from the noZZle ends of the metal tubes 204 by about 1/2 inch (or other suitable distance; as in FIG. 2B) to act as an electrically isolated noZZle, eliminating any conduc electrospinning ?uid and the high voltage supply (either through ground or through the supply’s voltage output). voltage supply. solvent; examples include but are not limited to: DMF, N-me thyl-2-pyrrolidone (NMP), or tetrahydrofuran (THF). [0018] The electrospun nano?bers listed in the table above tion path betWeen the electrospinning solution and the high HoWever, the salt/DMF-treated PS/DL electrospinning ?uids [0022] can also undergo electrospinning Without any electrical con parison of electrospinning behavior and spun material betWeen the differing operating conditions. The capillary duction path to the electrospinning ?uid (i.e., if the electro spinning ?uid and the noZZles are electrically isolated, Which means both from the voltage supply and from ground). The high voltage applied to drive the electrospinning process is The arrangements of FIGS. 2A and 2B enables com tubes 206 act as ?uid ?oW restrictors according to the Hagen Poiseuille equation and function in a manner analogous to that of the ?oW restrictors disclosed in US 2008/0131615 applied to a plate, screen, or mesh 102 that is electrically (incorporated above). For a given ?uid composition and ?oW insulated from the electrospinning ?uid 10 and provided With passages or perforations 103 for the electrospinning noZZles 104 (alternatively, With a single opening that accommodates all of the noZZles 104). The noZZles 104 are also electrically rate, the voltage can be adjusted to minimiZe the amount of insulated from the plate, grid, or mesh 102 and are arranged to convey the electrospinning ?uid 10 through the passages 103, Whether ?ush With (FIG. 1A) or extending through (FIG. 1B) ?uid that drips or ?oWs (not as ?bers) from the spinning head, presumably by optimiZing the balance of electrostatic and hydrodynamic forces acting on the ?uid jet. Conversely, for a given ?uid composition and voltage, the ?oW rate can be chosen to minimiZe the amount of ?uid that drips or ?oWs (not as ?bers) from the spinning head. That optimiZed ?oW rate is the plate, grid, or mesh 102. The applied electrostatic ?eld drives the electrospinning process, but With substantially less current ?oW per noZZle and substantially less charge depos ited onto the target substrate, relative to the conventional arrangement With a conductive path. In the isolated-noZZle typically larger When the ?uid is spun from electrically iso arrangement, the noZZles 104 can be formed from an suitable electrospun onto an insulating Mylar® target substrate, onto insulating material (e.g., Te?on®, polyethylene, ceramic, glass, and so on). [0019] Conventional ?uids have also been observed to undergo electrospinning Without a conduction path betWeen the ?uid and the high voltage supply. It has been observed qualitatively that such isolated-noZZle spinning With conven tional ?uids requires up to four times the applied voltage to initiate electrospinning (e. g., 60 kV versus 15 kV), produces lated capillary tubes 206 than When it is spun from the metal tubes 204 due to the differing balance of those forces. [0023] Using head 200, a salt/DMF-treated PS/DL ?uid (the non-phase-separating composition described above) Was a non-insulating scrim substrate, onto an aluminum foil target substrate (in each of those three cases With the substrate resting on a conductive ground plate, screen, or mesh), and onto an electrically isolated aluminum foil target substrate. In all cases a head pressure of about 0.5 psi Was applied to the ?uid in the reservoir and the ?oW rate Was about 5 .7 uL/min/ noZZle. The electrospinning ?uid Was in contact With the high voltage supply through plate 202 and metal tubes 204. As Oct. 24, 2013 US 2013/0280436 A1 shown in the table below, When electrospinning a salt/DMF treated PS/DL ?uid, varying the voltage or the nature of the target substrate has remarkably little effect on the average siZe of the resulting nano?bers. Similar ?ber siZes (about 250-300 nm) Were obtained When spinning the salt/DMF -treated PS/DL (non-phase separated composition) from electrically isolated noZZles. substrate voltage avg ?ber diameter scrim on ground plate scrim on ground plate scrim on ground plate scrim on ground plate Mylar ® on ground plate Mylar ® on ground plate Mylar ® on ground plate Mylar ® on ground plate aluminum foil (grounded) aluminum foil (isolated) 40 kV 60 kV 74 kV 90 kV 75 kV 76 kV 80 kV 90 kV 71 kV 71 kV 264.8 nm 239.8 nm 237.0 nm 254.1 nm 288.1 nm 252.4 nm 254.6 nm 247.0 nm 251.7 nm 254.3 nm [0024] It is intended that equivalents of the disclosed exem plary embodiments and methods shall fall Within the scope of the present disclosure or appended claims. It is intended that the disclosed exemplary embodiments and methods, and equivalents thereof, may be modi?ed While remaining Within the scope of the present disclosure or appended claims. [0025] For purposes of the present disclosure and appended claims, the conjunction “or” is to be construed inclusively (e.g., “a dog or a cat” Would be interpreted as “a dog, or a cat, or both”; e. g., “a dog, a cat, or a mouse” Would be interpreted as “a dog, or a cat, or a mouse, or any tWo, or all three”), unless: (i) it is explicitly stated otherWise, e.g., by use of “either . . . or”, “only one of . . . ”, or similar language; or (ii) tWo or more of the listed alternatives are mutually exclusive Within the particular context, in Which case “or” Would encompass only those combinations involving non-mutually Wherein the inorganic salt and the polar organic solvent do not cause substantial precipitation of the polymer upon mixing With the polymer solution. 2. The method of claim 1 Wherein the polymer solution is betWeen about 20% and about 40% by Weight of the polymer, the salt solution is betWeen about 0.02% and about 5% by Weight of the salt, and the mixture is betWeen about 10% and about 30% by volume of the salt solution. 3. The method of claim 1 Wherein the polymer solution is betWeen about 20% and about 40% by Weight of the polymer, the amount of salt results in a mole ratio betWeen about 0.5:1 and about 20:1 of the salt to the polymer in the mixture, and the amount of polar organic solvent results in a mole ratio betWeen about 1:1 and about 1:4 of the polar organic solvent to the terpene, terpenoid, or aromatic solvent in the mixture. 4. The method of claim 1 Wherein the terpene, terpenoid, or aromatic solvent comprises D-limonene, the aromatic side chain polymer comprises polystyrene, the polar organic sol vent comprises dimethyl formamide, and the inorganic salt comprises LiCl, AgNO3, CuCl2, or FeCl3. 5. A composition of matter comprising the electrospinning ?uid of claim 1. 6. A composition of matter comprising a plurality nano? bers produced by the process of claim 1. 7. The method of claim 1 further comprising alloWing the predominantly terpene, terpenoid, or aromatic solvent phase of the mixture to separate and then removing it from the mixture. 8. The method of claim 1 Wherein the spinning tips and the electrospinning ?uid are electrically isolated from a voltage source that drives the electrospinning. 9. The method of claim 1 Wherein the target substrate is electrically insulating. 10. The method of claim 1 Wherein the target substrate is electrically isolated. 11. The method of claim 1 Wherein an average diameter of exclusive alternatives. For purposes of the present disclosure electrospun ?bers produced by the electrospinning of the or appended claims, the Words “comprising,” “including,” ?uid is substantially independent of a voltage applied to drive the electro spinning or Whether the ?uid and the spinning tips “having,” and variants thereof shall be construed as open ended terminology, With the same meaning as if the phrase “at least” Were appended after each instance thereof. [0026] In the appended claims, if the provisions of 35 USC §1 12 1] 6 are desired to be invoked in an apparatus claim, then the Word “means” Will appear in that apparatus claim. If those provisions are desired to be invoked in a method claim, the Words “a step for” Will appear in that method claim. Con versely, if the Words “means” or “a step for” do not appear in a claim, then the provisions of 35 USC §112 1] 6 are not intended to be invoked for that claim. 1. A method comprising: dissolving an aromatic side chain polymer in a terpene, terpenoid, or aromatic solvent to form a polymer solu tion; dissolving an amount of inorganic salt in an amount of polar organic solvent to form a salt solution; mixing the salt solution and the polymer solution to form a mixture; and using a resulting predominantly terpene, terpenoid, or aro matic solvent phase of the mixture as an electrospinning are electrically isolated. 12. An apparatus comprising: a ?rst substantially planar conductive grid, mesh, or perfo rated plate; one or more spinning tips arranged to eject ?uid unim peded through the ?rst grid, mesh, or plate; a ?uid reservoir connected to deliver ?uid to be ejected by the spinning tips; a second substantially planar conductive grid, mesh, or plate arranged substantially parallel to and spaced apart from the ?rst grid, mesh, or plate so that ?uid ejected from the spinning tips is directed toWard the second grid, mesh, or plate; and a voltage supply connected to provide an electrical poten tial difference betWeen the ?rst grid, mesh, or plate and the second grid, mesh, or plate, Wherein the apparatus can be arranged in a ?rst con?gura tion With the ?uid reservoir and the spinning tips elec trically isolated. 13. The apparatus of claim 12 Wherein the apparatus can be arranged in a second con?guration With a conduction path ?uid by electro spinning the ?uid from one or more spin betWeen (i) the voltage supply and (ii) the ?uid reservoir or ning tips onto a target substrate, the spinning tips. Oct. 24, 2013 US 2013/0280436 A1 14. The apparatus of claim 13 wherein: each spinning tip comprises (i) a corresponding metal tube electrically connected to the ?rst grid, mesh, orplate and extending toWard the second grid mesh, or plate, and (ii) a corresponding electrically insulating capillary tube positioned Within the corresponding metal tube and con nected to the ?uid reservoir; the capillary tubes are longitudinally moveable Within the corresponding metal tubes; in the ?rst con?guration the insulating capillary tubes are recessed Within the corresponding metal tubes; and in the second con?guration the insulating capillary tubes extend from the corresponding metal tubes toWard the second grid, mesh, or plate. 15. A composition of matter comprising the electrospin ning ?uid of claim 2. 16. A composition of matter comprising the electrospin ning ?uid of claim 3. 17. A composition of matter comprising the electrospin ning ?uid of claim 4. 18. A composition of matter comprising a plurality nano? bers produced by the process of claim 2. 19. A composition of matter comprising a plurality nano? bers produced by the process of claim 3. 20. A composition of matter comprising a plurality nano? bers produced by the process of claim 4. * * * * *