* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Morphology of the Parrotfish Pharyngeal Jaw Apparatus1
Survey
Document related concepts
Transcript
AMER. ZOOL., 29:319-331 (1989)
Morphology of the Parrotfish Pharyngeal Jaw Apparatus1
KENNETH WALTER GOBALET
Department of Biology, California State University,
Bakersfield, California 93311
SYNOPSIS. Analysis of the anatomy of the pharyngeal apparatus of parrotfish demonstrates extraordinary specialization of the grinding jaws. The epibranchials have lost their
gill-bearing function. The first epibranchial is the structural element of the pharyngeal
valve that is operated by the first levator externus, first branchial adductor and part one
of the transversus dorsalis muscles. Five pairs of muscles (fourth levator externus, levator
posterior lateralis and medialis, fifth branchial adductor, part two of the transversus
ventralis) are positioned to adduct the lower pharyngeal. The retractor dorsalis and fourth
obliquus dorsalis are positioned to retract the upper pharyngeal. The third levator internus
and transversus dorsalis posterior protract the upper pharyngeal. The fourth levator
externus, both parts of the levator posterior and the fifth adductor are massive and pinnate.
Deep fossae for the attachment of the fourth levator externus and levator posterior muscles
are sculpted out of the neurocranium. A ventral spike process of the prootic and expanded
hemal postzygapophyses of the first three vertebrae are skeletal features associated with
the elaborated musculature of the pharynx. Synovial joints are present between the basicranium and upper pharyngeals, between the upper pharyngeals and fourth epibranchials
and between the lower pharyngeal and cleithrum. The upper pharyngeals act as a single
unit bound by cruciate ligaments. The fourth epibranchial is a key element in the pharyngeal apparatus and serves to direct forces generated by the transversus ventralis, fifth
adductor, levator posterior lateralis, transversus dorsalis posterior and fourth obliquus
dorsalis.
about 1800 pharyngognathous species
(Stiassny and Jensen, 1987).
Based on an "adaptive breakthrough" of
the pharyngeal apparatus, Kaufman and
Liem (1982), Lauder and Liem (1983), and
Stiassny and Jensen (1987) proposed the
monophyletic suborder, the Labroidei that
include the families Pomacentridae, Cichlidae, Embiotocidae, and Labridae. The
Odacidae and Scaridae are included in the
Labridae. Their assemblage is based on the
shared characteristics of fused fifth ceratobranchials, true diarthroses between the
upper pharyngeals and the basicranium and
an undivided sphincter esophagi muscle.
This view contrasts with the traditional view
that has only three separate families, the
Labridae, Odacidae, and Scaridae in the
Labroidei (Nelson, 1984). Precise anatomical description of the majority of these
fishes is lacking. This study provides anatomical and functional information on the
pharyngeal apparatus of the traditionally
designated parrotfish family, Scaridae, that
can be used in taxonomic, ecological, and
evolutionary evaluations.
' From the Symposium on Vertebrate Functional Morphology: A Tribute to Milton Hildebrand presented at the Schultz (1969) recognized 68 species of
Annual Meeting of the American Society of Zoolo- parrotfishes. Thorough studies indicate
gists, 27-30 December 1986, at Nashville, Tennessee. that these tropical marine fish are primarINTRODUCTION
There is an aesthetic beauty in structure,
determining its function, and discovering
the interdependence of the components.
Milton Hildebrand continues to provide us
with a framework for looking for clues that
result in an appreciation of the aesthetics
of form.
This study was initiated out of a fascination for the extraordinary ability of parrotfish to pulverize their food. The information obtained is useful in the broader
context of the evolution and ecology of the
labroid fishes.
Fishes with specialized pharyngeal jaws
show considerable morphological radiation. Almost half of the 6,000 species of
freshwater fish are either cyprinids or cichlids, families with specialized pharyngeal
jaws (Liem, 1973; Sibbing, 1982; Nelson,
1984). In the marine environment the
Labroidei, as conceived by Kaufman and
Liem (1982), is a diversified suborder of
319
320
KENNETH WALTER GOBALET
minology of Nelson (1969), for other skeletal features, Rognes (1973) and Patterson
(1977). Winterbottom (1974) was generally followed for muscle terminology.
PHARYNGEAL JAW ANATOMY
Gill arches and pharyngeal jaws
Six unpaired elements are present in the
ventral midline (Fig. 1). A tiny anteriorlyFIG. 1. Scarid branchial apparatus (modified after flared basihyal barely extends beyond the
Monod, 1951). Abbreviations: BB, basibranchial; BH, hyoid bar. The first basibranchial is very
basihyal; CB, ceratobranchial; EB, epibranchial; HB, thin and ventrally expanded. The second
hypobranchial; I, infrapharyngobranchial; LPh, lower basibranchial is a slightly dorsally convex,
pharyngeal; UH, urohyal; UPh, upper pharyngeal.
hourglass-shaped rod to which the first
hypobranchials bind. The third basiily herbivorous (Verwey, 1931; Longley branchial tapers posteriorly to a long, venand Hildebrand, 1941; Suyehiro, 1942; trally curved process. It receives the secRandall, 1967;Earle, 1972;Hobson, 1974; ond and third hypobranchials laterally. The
Smith and Paulson, 1974; Lobel and third and fourth ceratobranchials are
Ogden, 1981). Gohar and Latif (1959) and bound to a small cartilaginous fourth basiHiatt and Strasburg (1968) are the only branchial. Its anterior end lies dorsal to the
researchers who have found coral polyps caudal end of the third basibranchial. The
in the guts. The specimens for this study lower pharyngeal is caudal to the fourth
were collected in a coral-free area of the basibranchial.
Gulf of California, Mexico. Parrotfish feed
The paired elements of the gill arches
by scraping food off the substrate using are quite delicate. Of the three pairs of
robust mandibles and pulverizing it to a hypobranchials, the third has a dorsovenfine slurry in massive pharyngeal jaws. Sev- tral orientation. The ventral tip is conenty percent of the ingested material is nected to the ventral extension of the first
inorganic in nature (Randall, 1967; Go- basibranchial by a long ligament. Its narbalet, 1980).
row dorsal end sits between the third basiAnatomical studies of the pharyngeal branchials and the third ceratobranchial.
The first three ceratobranchials have
apparatus of parrotfishes have been rather
general as part of broader comparative ventrally-directed laminae from the lateral
studies (Boas, 1879; Gregory, 1933; edges. The first ceratobranchial may be
Monod, 1951; Board, 1956; Nelson, 1967a; greatly recurved in large S. perrico, resultTedman, 1980a, b\ Yamaoka, 1980; Liem ing in the angle of the arch being within
and Greenwood, 1981). This study focuses the curvature of the bone, a positional shift
exclusively on the pharyngeal apparatus undoubtedly associated with the loss of the
and associated structures of representa- gill-bearing function of the epibranchials.
The thin elongate fourth ceratobranchial
tives of Scams and Nicholsina.
is flanged anteroventrally and attaches to
MATERIALS
the ventral edge of the anterior portion of
Virtually all of the specimens used in this the neck of the fourth epibranchial. The
study were collected in the Gulf of Cali- fourth ceratobranchial bears a hemifornia, Mexico. Identifications of parrot- branch as there is no gill slit behind the
fish followed Rosenblatt and Hobson fourth arch.
(1969). For the study, 19 Scarus compressus The dorsal portion of the gills adheres
were used, 25 5. ghobban, 18 S. perrico, 10 to the skin covering the highly modified
S. rubroviolaceus, and a single Xicholsina den- dorsal pharynx. The first epibranchial is a
ticulata.
"Y"-shaped element, the base of which is
For branchial elements I follow the ter- directed medially to reinforce the comb-
PARROTFISH PHARYNGEAL JAWS
like anterior portion of the pharyngeal
valve which functions to move materials
filtered by the gill rakers to the pharyngeal
mill (Board, 1956). In anterior view the
valve is notched in the midline between the
medial ends of the first epibranchials. The
pharyngeal pad located anterior to the
upper pharyngeals behind the first epibranchials and medial to the second epibranchials has a watery internal matrix
containing some muscle fibers. The second
infrapharyngobranchials are located within
the posterodorsal portion of the pad.
One of the two lateral processes of the
first epibranchial attaches to the first ceratobranchial and the other to the second
epibranchial. The posterior edge of the
second epibranchial bears a facet at its midpoint which attaches to a raised facet of
the third epibranchial. Dorsally, the rodlike portion of the ventrally-flattened second epibranchial attaches to the second
infrapharyngobranchial. The second
infrapharyngobranchial is a small boomerang-shaped bone that attaches by its dorsomedial end to the anterior end of the
upper pharyngeal within the pharyngeal
pad.
The third epibranchial is wishboneshaped with the base attached to the third
ceratobranchial. The anterior process of
the fourth epibranchial fits within the notch
between the forks. The second epibranchial attaches to the medial ramus of the
wishbone. The dorsal tip of the medial
ramus attaches to the anterior tip of the
condylar portion of the fourth epibranchial and the lateral ramus to the lateral
surface of the anterior process and its neck.
The robust fourth epibranchial is highly
modified and distinctive. It is composed of
a medially grooved condylar portion that
articulates with the upper pharyngeal. A
large lateral wing sweeps off the anterolateral portion of the condylar segment.
Contrary to what Nelson (1967a) reports,
the wing does not articulate with the cleithrum in either Scams or Nicholsina. A deep
posteriorly open notch separates the posterior portion of the condylar segment from
the posterolaterally curved wing. An anterior process hooks laterally from this
321
"neck." The dorsal portion of the medial
condylar part bears a broad posteromedially recurved process that defines the
end of the transversus dorsalis posterior
muscle. The dorsal surface of the wing is
convex and the ventral surface is deeply
concave and ridged on the perimeter.
The dorsal surfaces of the paired upper
pharyngeals bear the long condyle of the
basipharyngeal joint. The tooth row on the
ventral surface of the bone extends posteroventrally from an alveolar area where
new teeth are constantly formed to the posterior end of the tooth plate that is worn
thin. The exposed teeth are incisiform,
arranged in tandem, and interdigitate
across the midline. The wear pattern on
the occlusal surface produces alternating
ridges of enamel, dentine and bone, a pattern typical of mammalian herbivores.
Small denticles may be found between the
lateral edges of the tooth row depending
on the species.
On the lateral surface of the upper pharyngeal is the long convex peanut-shaped
condyle of the joint with the fourth epibranchial. The posterior border of the
upper pharyngeal bears a thin, squared-off
extension.
The rectangular tooth plate of the lower
pharyngeal is often worn to thin bone anteriorly from the caudal germinative alveolar
area. The incisiform teeth are apparently
replaced conveyor-like fashion in each jaw
from the germinative area as in wrasses
(Liem and Sanderson, 1986). The wear
pattern in the lower pharyngeal is opposite
to that of the upper pharyngeal so that the
most worn teeth in one pharyngeal jaw face
less worn teeth in the oppositejaw. Because
the upper pharyngeal tooth plates are narrower than the lower pharyngeal tooth
plate, the lower pharyngeal becomes concave with wear. On the lateral edge, the
teeth are smaller. Lateral to the tooth plate
are large muscular processes which are
roughly scarred except for the posterolateral facet of the pharyngocleithral joint.
A large, anteriorly-directed, keel extends
from the ventral midline of the thin anterior part of the bone. Fossae are found
lateral to the base of the rudder, and ante-
322
KENNETH WALTER GOBALET
ment with the ventral edge of the fourth
epibranchial, lateral to its anterior process.
A ligament interconnects the posteroventral portion of the first basibranchial
with the ventral portion of each third
hypobranchial.
The joint between the posterolateral
portion of the muscular process of the lower
pharyngeal and the flat facet on the cleithrum is synovial. The cleithrum is not
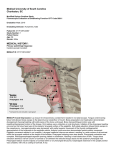
grooved for this joint as reported by Gregory (1933) or Al-Hussaini (1945). A reticFIG. 2. Ventral view of the neurocranium of Scarus ulum of loose connective tissue fibers conrubroviolaceus. Abbreviations: AStF, anterior subtem- nects the anteroventral portion of the
poral fossa; BPhGr, basipharyngeal groove; DStF, deep muscular process with the edge of the
subtemporal fossa; HmSoc, sockets of hyomandibula; medial flange of the cleithrum ventral to
LEth, lateral ethmoid; LOcF, lateral occipital fossa;
PtoPopPr, preopercular process of pterotic; VoF, the medial notch, the posterior pharyngeal
ligament of wrasses (Yamaoka, 1978).
ventral fossa of vomer.
Muscles of the pharynx
rior to the thick alveolar part of the bone.
The keel is thick along its dorsal edge and
may bear a hole in the base.
Significant joints and ligaments of the
pharyngeal apparatus
The medial faces of the upper pharyngeals are connected across the midline ventral to the posterior end of the cranial condyles by cruciate ligaments. The fibers fan
out from a narrow attachment at the dorsal
part of one element to a wide ventral
attachment on the opposite element. These
ligaments are also cruciate in Nicholsina.
The independent motion of upper pharyngeals suggested by Al-Hussaini (1945) is
thus impossible.
The long synovial basipharyngeal joint
between the upper pharyngeals and the
grooved parasphenoid (Fig. 2) extends from
the occipital condyle to the level of the
post-orbital process. The joint is shorter in
Nicholsina than in Scarus. This is the key
joint of the Labroidei of Lauder and Liem
(1983).
A long oval synovial joint is found
between the upper pharyngeals and the
fourth epibranchials. The synovial joint
between the upper pharyngeal and third
epibranchial of wrasses (Yamaoka, 1978) is
lacking. The squared-off dorsal part of the
fourth ceratobranchial is bound by a liga-
The posteroventral part of the neurocranium is highly sculptured with ridges
and concavities for the branchial musculature (Fig. 2). A prominent spike (absent
in Nicholsina) projects ventrally from the
lateral commissure of the prootic. This
spike serves as the tendinous origins of the
levatores externi 1, 2, and 3 (L Ext). L Ext
1 (Figs. 3, 4, 5, 6A) has a fleshy insertion
along the dorsolateral surface of the middle of the shaft of the first epibranchial; L
Ext 2 (Figs. 3, 5, 6B) by a thick tendon
beside the posterior facet of the second
epibranchial; L Ext 3 (Figs. 3, 5, 6C) on
the tip of the lateral ramus of the third
epibranchial. L Ext 2 and 3 are spindleshaped.
The massive L Ext 4 (Figs. 3, 5, 7) originates from the prootic anterior and medial
to the spike and from the anterior and deep
subtemporal fossae of the neurocranium
(Fig. 2). The anterior subtemporal fossa is
located between the sockets of the joint
with the hyomandibula. Posterior and
medial to this fossa is the deep subtemporal
fossa which reaches the skull roof. The L
Ext 4 is highly pinnate, with five layers of
fibers attaching to myocommata. The flat
tendon of insertion passes caudal to the
neck of the fourth epibranchial and joins
the major interior myocommata of the
levator posterior medialis muscle to insert
PARROTFISH PHARYNGEAL JAWS
Vo-Enpt
323
Lig
AAP ( c u t )
TrDA
1»3
PhCE
TrV2
FIG. 3. Lateral view of the branchial muscles of Scarus. The keel of the lower pharyngeal is medial to the
transversi ventrales. Abbreviations: AAP, adductor arcus palatini; Ad, branchial adductor; AO, adductor
operculi; ClCPh, cleithral condyle of lower pharyngeal; LExt, levator externus; Lint, levator internus; LPostL,
levator posterior lateralis; OblDors, obliquus dorsalis; OblV, obliquus ventralis; ProPect, protractor pectoralis;
PhCE, pharyngocleithralis externus; RComm, pharyngohyoideus; TrDA, transversus dorsalis anterior; TrV,
transversus ventralis, VoEnptLig, vomer-endopterygoid ligament.
on the longitudinal ridge of the muscular
process of the lower pharyngeal (Fig. 5). A
common aponeurosis from these two muscles also attaches to the neck of the fourth
epibranchial. The equivalent muscle in the
cichlid Haplochromis elegans is formed from
the fusion of L Ext 4 and obliquus posterior
(Aerts, 1982). This is also a compound
muscle in wrasses (Liem and Sanderson,
1986) and embiotocids (Liem, 1986).
The massive levator posterior is distinctively divided into medial and lateral portions (Figs. 3, 5). The levator posterior
medialis originates from the lateral occipital fossa, the dominant feature of the posterior aspect of the neurocranium (Fig. 2).
This depression is as deep as the deep subtemporal fossa. The muscle has at least
three layers separated by myocommata.
There is no apparent separation that would
indicate a compound ontogenetic origin of
the muscle as in the cichlid Astatotilapia elegans (Claeys and Aerts, 1984), embiotocids
(Liem, 1986) or wrasses (Liem and San-
derson, 1986). This muscle may be a caudal
expansion of the L Ext 4. If so the levator
posterior lateralis would be homologous to
the levator posterior of generalists like the
serranids. The tough tendon of insertion
of the levator posterior medialis is directed
ventrally and joins that of the L Ext 4 to
insert on the longitudinal ridge of the muscular process of the lower pharyngeal (Fig.
5). Its lateral epimycium is continuous with
that of the posterior L Ext 4 and attaches
to the neck of the fourth epibranchial. The
levator posterior lateralis is parallel fibered
laterally and has two internal medial
myocommata. It inserts on the dorsal surface of the wing of the fourth epibranchial
(Figs. 3, 5, 7) from its origin in the small
fossa on the posterior face of the pterotic,
along the ridge separating the deep subtemporal fossa from the ventral occipital
fossa. The origin extends dorsally through
the intercalar onto the lateral edge of the
epiotic.
The second levator internus (Figs. 3, 4,
324
KENNETH WALTER GOBALET
Ob*.Dor*.4
TrOA 4 {rifltlt Silly)
FIG. 4. Pharyngeal valve of Scarus viewed from the
anterior. Abbreviations: Ad, branchial adductor; CB,
ceratobranchial; EB, epibranchial; I, infrapharyngobranchial; LExt, levator externus; Lint, levator internus; TrDA, transversus dorsalis anterior; UPh, upper
pharyngeal.
5, 6B) has a fleshy origin from the anterior
edge of the prootic anterior to the base of
the ventral spike. This muscle passes posterior to the second infrapharyngobranchial to insert independent of any bony
element in the epithelium of the pharyngeal pad. This is probably a unique scarid
feature since it inserts in the pad in Nicholsina, a primitive scarid, and not in wrasses
(Quignard, 1962). The third levator internus (Fig. 5) is a parallel-fibered strap that
originates from and medial to the prootic
spike and inserts on the lateral surface of
the alveolar portion of the upper pharyngeal.
The rectus dorsalis muscle (Fig. 6A)
interconnects the lateral end of the more
dorsomedial of the two lateral processes of
the first epibranchial with the anterior surface of the dorsal portion of the second
epibranchial. It is difficult to distinguish
from the second adductor and may attach
to the second infrapharyngobranchial.
Nelson (19676) found this muscle (obliquus
inferioris) only as a secondary formation
in teleosts.
The fourth obliquus dorsalis (Fig. 7) connects the lateral surface of the alveolar portion of the upper pharyngeal with the dorsal surface of the condylar portion of the
fourth epibranchial anterior to the
recurved process and anteromedial to the
dorsal crest of the neck. Its orientation is
nearly anterior-posterior.
FIG. 5. Dorsal view of the pharynx of Scarus. Abbreviations: EB, epibranchial; 1, infrapharyngobranchial;
L.Ext., levator externus; L.Int., levator internus;
L.Post.L., levator posterior lateralis; L.Post.M., levator posterior medialis; Obi.Dors., obliquus dorsalis;
R.Dors., retractor dorsalis; Sph.O., sphincter esophagi; Tr.D.A., transversus dorsalis anterior; Tr.D.P.,
transversus dorsalis posterior; UphCo, basicranial
condyle of upper pharyngeal.
The retractor dorsalis (Fig. 5) has long
parallel fibers which originate from the
ventral surfaces of the expanded hemal
postzygapophyses of the first three vertebrae. The muscle inserts by a tendon to
the medial and lateral aspects of the thin
posterior portion of the upper pharyngeal.
The two rectrator dorsales meet below the
dorsal aorta before dividing again before
their insertions.
Specialization of the dorsal pharynx (into
a villous pharyngeal valve supported by the
first epibranchial and the fleshy pharyngeal pad between the first epibranchial and
the upper pharyngeal) has led to specialization of the transversi dorsales anterior
muscles. Four independent muscles may
occur, though variation exists among specimens of the same species. All four muscles
are apparently present in Sparisoma cretense
(Board, 1956) and a single specimen of
Nicholsina.
The transversi dorsales anterior part 1
(Figs. 3, 4, 5, 6A) are small muscles that
attach to the lateral surface of the condylar
portion of the fourth epibranchials ventral
to the recurved dorsal process. Their fibers
project anteriorly ventral to the neck of
the fourth epibranchial and the third and
second epibranchials, to attach to the shaft
of the first epibranchial. Board (1956) calls
this a retractor muscle of the pharyngeal
PARROTFISH PHARYNGEAL JAWS
325
FIG. 7. Lateral view of the fourth and fifth arches
of Scarus. The transversi ventrales attach to the keel
of the lower pharyngeal. Abbreviations: Ad, branchial adductor; CB, ceratobranchial; CICPh, cleithral
condyle of lower pharyngeal; EB, epibranchial; LExt,
levator externus; LPh, lower pharyngeal; LPost, levator posterior; OblD, obliquus dorsalis; PhOE, pharyngocleithralis externus; PhClI, pharyngocleithralis
internus; Rcomm, pharyngohyoideus; RectV, rectus
ventralis; TrV, transversus ventralis.
interconnects the fossa of the posterodorsal portion of the condylar region of the
fourth epibranchial posteromedial to the
recurved process with the posterior portion of the lateral surface of the upper pharyngeal. In Nicholsina the posterior ends of
the transversi dorsales posterior also fail to
cross the midline. This may be a unique
feature of scarids.
valve. Transversus dorsalis anterior part 2
Five branchial adductors are present.
(Fig. 4, 5) is a small bundle of fibers that The first three interconnect the lateral surinterconnects the dorsomedial portion of faces of their respective ceratobranchial
the first epibranchials across the midline. and epibranchials (Fig. 6). As in wrasses
It may be an extension of the transversi they are broadly attached to the epibrandorsales anterior part 1. Muscle fibers of chials (Liem and Sanderson, 1986). The
the transversi dorsales anterior part 3 (Figs. first adductor is separated by a gap from
4, 5, 6B) have fleshy attachments to the the epibranchial-ceratobranchial joint
anterior faces of the second infrapharyn- (Figs. 4, 6A). Contraction of the first and
gobranchials and cross and angle poste- second adductors would probably depress
riorly to fan out within the tissue ventral the pharyngeal valve and pad. The fourth
to the alveolar portion of the upper pha- adductor is the tiny probably vestigial musryngeals. A diffuse sheet of muscle fibers, cle that fills the angle between the fourth
the transversus dorsalis anterior part 4 (Fig. ceratobranchial and the ventral surface of
4), originates with the transversus dorsalis the neck of the fourth epibranchial (Fig.
anterior part 1 on the fourth epibranchial 7). The fourth adductor in Nicholsina has
and crosses to the midline within the pha- two distinct fiber bundles.
ryngeal pad. I agree with Stiassny and JenThe massive, pinnate fifth adductor has
sen (1987) that there is ambiguity in relat- a fleshy origin from the ventral surface of
ing these muscles with those of other the neck and lateral wing of the fourth
laborids.
epibranchial (Figs. 3, 7). Fibers insert on
The transversus dorsalis posterior (Fig. the common tendon of the fourth levator
5) has lost its connection to the midline and externus and levator posterior medialis and
FIG. 6. Lateral view of the first (A), second (B), and
third (C) branchial arches. Abbreviations: Ad, branchial adductor; CB, ceratobranchial; EB, epibranchial; HB, hypobranchial; I, infrapharyngobranchial;
LExt, levator externus; Lig, ligament; Lint, levator
internus; OblV, obliquus ventralis; RectD, rectus dorsalis; RectV, rectus ventralis; STH, sternohyoideus;
TrDA, transversus dorsalis anterior.
326
KENNETH WALTER GOBALET
on the lateral and anteromedial surface of
the muscular process of the lower pharyngeal. Four internal myocommata add to
the complexity of the muscle. No distinct
obliquus posterior was identified though a
study of ontogeny might demonstrate its
fusion with another muscle.
The first obliquus ventralis is split into
two antagonistically positioned parts, each
of which interconnects the first hypobranchial and first ceratobranchial. The larger
portion interconnects processes on the lateral surface of the bones (Figs, 3, 6 A). The
smaller part is medially positioned. The
second obliquus ventralis also has two distinct medial and laterally (Fig. 6B) positioned antagonistic sections. Nicholsina has
the same arrangement of the obliqui ventrales as Scarus. The third obliquus ventralis is undivided.
The fourth rectus ventralis (Fig. 6C)
interconnects the ventral surface of the
posterior portion of the medial flange of
the fourth ceratobranchial with the ventral
end of the third hypobranchial. The
arrangement of the obliqui ventrales and
fourth rectus ventralis does not follow the
pattern described by Stiassny and Jensen
(1987) with an attachment to a semicircular ligament. The arrangement in parrotfishes appears to be a specialization. The
pharyngohyoideus (Fig. 7) has a long narrow tendon that attaches to the anterior
surface of the muscular process of the lower
pharyngeal. The anteroventral attachment is to the dorsal surface of the urohyal.
The transversus ventralis part 1 (Fig. 7)
is parallel-fibered and interconnects the
ventral edge of the medial flange of the
fourth ceratobranchial with the ventral
edge of the keel of the lower pharyngeal.
The fibers have a dorsoventral orientation
and are lateral to the transversus ventralis
part 2.
Transversus ventralis part 2 originates
from the lateral surface of the anterior
portion of the keel of the lower pharyngeal
and coalesces upon the ventral surface of
the fourth ceratobranchial just below the
ceratobranchial-epibranchial joint. Calling
this a transversus ventralis muscle is consistent with Anker (1978) and Quignard
(1962).
The sphincter esophagi interconnects the
lateral edges of the toothed portions of the
lower and upper pharyngeals. The connection is baggy and provides a small storage space. The sphincter esophagi connects the fourth ceratobranchials across the
midline and additional fibers are found
within the walls of the distinctive pharyngeal pocket, an outpocketing between the
fourth ceratobranchial and the lower pharyngeal. Its opening to the pharynx is a
slit. It contains materials yet to be pulverized by the pharyngeal jaws. Small muscle
fascicles have separated from the sphincter
esophagi to serve the pharyngeal pocket.
Two are consistently present. One originates from the dorsal edge of the keel of
the lower pharyngeal and is directed posteriorly to insert within the tissue that is
ventromedial to the opening of the pocket.
Only this tiny muscle was present in Nicholsina in which the pharyngeal pocket is continuous with the sphincter esophagi when
it interconnects the tooth plates. The other
small bundle crosses from the tendon of
the transversus ventralis part 2 to attach
within the epithelium anterior to the orifice.
The long and narrow fleshy origin of the
pharyngocleithralis externus (Figs. 3, 7) is
from the medial edge of the cleithrum
anteroventral to the medial notch. The
fibers are directed dorsomedially and insert
on the ventral surface of the anterior portion of the lower pharyngeal. The insertion of this flat, parallel-fibered muscle is
narrower than the origin. In Nicholsina this
muscle is a parallel-fibered strap and originates more ventrally on the cleithrum than
in Scarus.
The cone-shaped pharyngocleithralis
internus (Fig. 7) has a small tendinous origin from the posterior end of the medial
flange of the cleithrum anterior to the
medial notch just posterior to the pharyngocleithralis externus. The fibers are
directed medially and anteromedially. The
muscle fans out to a large fleshy insertion
on the posteroventral portion of the keel
of the lower pharyngeal. This muscle is a
thin, parallel-fibered strap in Nicholsina.
The sternohyoideus muscle is a complex
assemblage of fibers and myocommata that
327
PARROTFISH PHARYNGEAL JAWS
originates from the anteroventral cleithrum. These fibers meet in the midline with
fibers from the opposite side and insert by
fibrous tissue on the urohyal. From the lateral surface of the connective tissue cover
of the ventromedial mass is a thin sheet of
fibers which are continuous with the
hypaxial musculature. A narrow tendon
extends dorsally from the cover of this portion and inserts on the ventral end of the
third hypobranchial (Fig. 6C). Winterbottom (1974) names this the sternobranchialis.
DISCUSSION
The slurry of fine material that reaches
the gut of parrotfishes is the product of the
massive pharyngeal apparatus which acts
on the ingested organic and inorganic
material. Extraordinary dentition and
bone-muscle systems are required to
accomplish a task for which we would
require a mortar and pestle. The adaptation may allow the parrotfish to exploit not
only macro-algae, but endolithic algae and
bacteria as well (Ogden and Lobel, 1978;
Gobalet, 1980).
Board (1956) recognized the function of
the pharyngeal valve, supported by the first
epibranchial (Fig. 4) to be the movement
of ingested material from the gill rakers of
the floor of the pharynx caudally toward
the pharyngeal jaws. The first levator
externus and the first adductor are positioned to act antagonistically to raise and
lower the valve. Separation of the first
adductor from the ceratobranchial-epibranchial joint enhances its mechanical
advantage. The transversus dorsalis anterior part 1 is in position to retract the valve.
The second levator internus probably
moves the pad out of the way during valve
retraction and the pharyngohyoideus may
raise the floor of the pharynx during transport of food as in wrasses (Liem and Sanderson, 1986). The dorsal portion of the
first gill arch is separate here from the other
arches as it is in wrasses (Liem and Sanderson, 1986) and embiotocids (Liem,
1986). Some peristaltic movement may be
used to transport material caudad as in the
carp (Sibbing et al., 1986) because muscle
fibers are found in the pharyngeal pad.
L.Ph
FIG. 8. Diagrammatic representation of the adductors of the pharyngeal jaws of Scarus, lateral view.
Abbreviations: Ad5, fifth branchial adductor; EB4,
fourth epibranchial; L.Ext.4, fourth levator externus;
L.Ph., lower pharyngeal; L.Post.L., levator posterior
lateralis; L.Post.M., levator posterior medialis; Tr.V.II,
part two of transversus ventralis; U.Ph., upper pharyngeal.
Five pairs of muscles are positioned to
power the grinding pharyngeal jaws (Fig.
8). This is in striking contrast to generalized percoids where transport, not mastication, is the function of this part of the
pharynx (Liem, 1973). In scarids the pharyngeal adductors are the fourth levator
externus, both portions of the levator posterior, the fifth adductor, and the transversus ventralis part 2. The incorporation
of the transversus ventralis part 2 in the
adductor complex is a derived characteristic.
The mass of the five adductors is over
six times that of the homologous muscles
in a generalized percoid (Gobalet, 1980)
where they are not even functional adductors (Liem, 1973).
The extraordinary hypertrophy of the
pharyngeal muscles has led to compensatory changes in the neurocranium (Fig. 2).
The anterior and deep subtemporal fossae
serve as the site of origin of the fourth
levator externus. The lateral occipital fossa
and the small fossa on the posterior face
of the pterotic serve as the sites of origin
of the levator posterior. Subtemporal fossae exist in other fish, but never multiply
as here (Patterson, 1975). Among wrasses
the origin of the levator posterior alone
may be expanded (Yamaoka, 1978; Wainwright, 1987).
In Scarus the expansion of the origin of
the fourth levator externus has encroached
upon that region of the prootic from which
328
KENNETH WALTER GOBALET
B
AOB
FIG. 9. A. Diagrammatic representation of the pharyngeal apparatus of Scarits, caudal view. B. Vectorial
representation of horizontal component of branchial
adductors of Scarus. Abbreviations: Ad5, fifth branchial adductor; EB4, fourth epibranchial; Fm, medial
component of force; L.Ph., lower pharyngeal; LPostL,
levator posterior lateralis; LPostM, levator posterior
medialis; RDors, rectractor dorsalis; U.Ph., upper
pharyngeal.
the first three levatores externi and two
levatores interni originate. The ventral
spike of the prootic has probably formed
in response to selection to enlarge the
fourth levator externus while maintaining
the origin of the other levators. The levators originate from the prootic spike, and
the anterior portion of the fourth levator
externus originates from the prootic medial
to the spike. In generalized percoids, trunk
musculature would attach to much of the
skull that in scarids accommodates the pharyngeal jaw adductors. The small fossa of
the levator posterior lateralis on the posterior face of the pterotic is unique to scarids. Myocommata are present within the
fourth levator externus, levator posterior,
and fifth adductor, making all these muscles complex and multipinnate. In constrained spaces pinnate muscles have distinct advantages over parallel-fibered
muscles (Hildebrand, 1988).
The robust fourth epibranchial is a key
feature of the pharyngeal mill of wrasses
(Yamaoka, 1978) and parrotfishes. Both the
fifth adductor and the levator posterior lateralis attach to its expanded lateral wing
(Fig. 9 A). The medial edge of the wing lies
directly dorsal to the insertion of the fifth
adductor on the muscular process of the
lower pharyngeal. Consequently, the vectors of the actual and effective forces of
this medial portion of the adductor are the
same, a mechanically favorable arrangement. The lateral edge of the wing is positioned lateral to the muscular process of
the lower pharyngeal. Medially-directed
components of the vectors of force of the
levator posterior lateralis and of the lateral
portion of the fifth adductor will press the
fourth epibranchial against the upper pharyngeal and hold it in position (Fig. 9B).
This anatomical arrangement suggests that
the fifth adductor and the levator posterior
lateralis function as a single massive unit
with the included fourth epibranchial
transducing forces from the vertical to the
horizontal plane.
Forces of contraction of the transversus
ventralis part 2 also act on the fourth epibranchial through the joint between the
fourth ceratobranchial and epibranchial
(Fig. 8). The transversus ventralis part 2
attaches to the anterior portion of the keel
of the lower pharyngeal, and the fourth
ceratobranchial-fourth epibranchial joint
is on the anterior part of the lower tooth
plate. The keel may give the muscle a
mechanical advantage in its role as an
adductor of the lower pharyngeal. The
simultaneous contraction of the transversus ventralis and the levator posterior lateralis would adduct the anterior edge of
the lower pharyngeal.
The pharyngocleithralis externus is
positioned to abduct the anterior edge of
the lower pharyngeal. The pharyngocleithral joint could act as the fulcrum for
a first-class lever powered by the action of
the transversus ventralis part 2 or other
muscles, but this interpretation by Liem
(1973) for cichlids is unlikely to apply to
parrotfish due to the weakness of the ligaments around the joint. The pharyngocleithral joint and the pharyngocleithralis
internus probably function only to stabilize
PARROTFISH PHARYNGEAL JAWS
the lower pharyngeal. Except during rapid
escape responses scarids and wrasses propel themselves using their pectoral fins
(Randall, 1967). The constant use of the
pectoral fin suggests that through the
pharyngocleithral joint, the pectoral girdle
may stabilize the lower pharyngeal or even
assist in the grinding process. The hypaxial
musculature has this role in the carp (Sibhmgetal, 1986).
In wrasses (Liem and Sanderson, 1986),
embiotocids (Liem, 1986), and cichlids
(Liem, 1978), the upper pharyngeal rocks
in a horizontal figure-8 through a cycle of
double contact with the lower pharyngeal.
The upper pharyngeal rocks on the apophysis of the basicranium during this cycle.
Such a motion is clearly impossible in scarids because the basipharyngeal groove of
the braincase and the condyle of the upper
pharyngeals are elongate and only slightly
recurved. The upper pharyngeals must act
as a single unit because of interconnecting
cruciate ligaments and interdigitating
teeth. The synovial joint between the
fourth epibranchials and the upper pharyngeals restricts the upper pharyngeals to
the midline. The only possible motion
appears to be slight arching during protraction by the third levator internus and
transversus dorsalis posterior and retraction by the fourth obliquus dorsalis and
retractor dorsalis (Fig. 10).
If the figure-8 pattern of labroids (sensu
Lauder and Liem, 1983) is evolutionary
conserved, protraction of the upper pharyngeal by the third levator internus and
transversus dorsalis posterior would be
accompanied by elevation and protraction
of the lower pharyngeal by the levator
externus 4, supplemented by the fifth
adductor, levator posterior lateralis and
transversus ventralis part 2. Retraction of
the upper pharyngeal would be caused by
the action of the fourth obliquus dorsalis
and retractor dorsalis muscles. Elevation of
the lower pharyngeal would additionally
utilize the levator posterior medialis. Such
motions would result in simultaneous forward and backward movement of both jaws.
Since milling rather than crushing is the
action of the jaws, holding one element
stationary while the other moved would
329
FIG. 10. Diagrammatic representation of the protractors and retractors of the upper pharyngeals of
Scarus, lateral view. Abbreviations: E.B.4, fourth epibranchial; L.Int.III, third levator internus; L.Ph.,
lower pharyngeal; Obi. D.I V, fourth obliquus dorsalis;
R.Dors., retractor dorsalis; Tr.D.P., transversus dorsalis posterior; U.Ph., upper pharyngeal.
seem more effective in pulverizing the foodsubstrate combination. The system would
thus parallel that found in the carp (Sibbing, 1982). Anatomy suggests that the elevation of the lower pharyngeal by its
adductors overall has an anterior component. This is reflected in the trabeculae of
the lateral wall of the parasphenoid above
the basipharyngeal joint. Here the trabeculae are in parallel with the tendon of the
fourth levator externus. The same is true
of the internal trabeculae of the upper pharyngeal.
Trabeculae of bone form along lines of
stress (Murray, 1936). The upper pharyngeal has the shape of an I-beam, a shape
suited to resisting stresses in a single plane
(Hildebrand, 1988). The configuration of
trabeculae within the bone suggests that a
considerable force is exerted on the lower
pharyngeal which is transmitted through
the upper pharyngeal to the neurocranium. The fourth obliquus dorsalis and
retractor dorsalis are in positions to stabilize the upper pharyngeal against protraction by the fourth levator externus.
The role of these muscles may be to hold
the upper pharyngeal in place while the
lower pharyngeal is elevated and protracted. The third levator internus and
transversus dorsalis posterior may resist
motion of the upper pharyngeal while the
lower pharyngeal is retracted. Greater
330
KENNETH WALTER GOBALET
excursion of the upper pharyngeal may be
effected during swallowing.
In contrast with generalized percoids, all
five pairs of adductors and the protractors
and retractors of the upper pharyngeal are
red, an indicator of substantial endurancerelated use by these muscles (Gobalet,
1980). This is expected considering the
nature of the food consumed.
The anatomical foundations for determining the function of the parrotfish pharyngeal apparatus have been established.
The inference on the function serves as a
model to be tested experimentally in the
future with cineradiography and electromyography.
Gregory, W. K. 1933. Fish skulls: A study of the
evolution of natural mechanisms. Trans. Amer.
Phil. Soc. 23(2):75-481.
Hiatt, R. W. and D. W. Strasburg. 1960. Ecological
relationships of the fish fauna on coral reefs of
the Marshall Islands. Ecol. Monogr. 30(l):65129.
Hildebrand, M. 1988. Analysis of vertebrate structure,
3rd ed. John Wiley and Sons.
Hobson, E. S. 1974. Feeding relationships of teleostean fishes on coral reefs in Kona, Hawaii. NOA A
U.S. Nat. Fish. Bull. Mar. Fish. Ser. 72(4):9151031.
Kaufman, L. and K. F. Liem. 1982. Fishes of the
suborder Labroidei (Pisces: Perciformes): Phylogeny, ecology and evolutionary significance.
Breviora Mus. Comp. Zool. 472:1-19.
Lauder, G. V. and K. F. Liem. 1983. The evolution
and interrelationships of actinopterygian fishes.
Bull. Mus. Comp. Zool. 150:95-197.
Liem, K. F. 1973. Evolutionary strategies and morACKNOWLEDGMENTS
phological innovations: cichlid pharyngeal jaws.
Syst. Ecol. 22:425-441.
My thanks to Karel Liem whose research
Liem, K. F. 1978. Modulating multiplicity in the
stimulated this project, to Milton Hildefunctional repertoire of the feeding mechanism
brand for support and Steve Strand for
in cichlid fishes. J. Morph. 158(3):323-360.
field assistance.
Liem, K. F. 1986. The pharyngeal jaw apparatus of
the Embiotocidae (Teleostei): A functional and
evolutionary
perspective. Copeia 2:311-323.
REFERENCES
Liem, K. F. and P. H. Greenwood. 1981. A functional approach to the phylogeny of pharyngoAerts, P. 1982. Development of the muscular levator
gnath teleosts. Amer. Zool. 21:83-101.
externus IV and the musculus obliquus posterior
in Haplochromis elegans Trewavas, 1933 (Teleos- Liem, K. F. and S. L. Sanderson. 1986. The phatei: Cichlidae): A discussion on the shift hypothryngeal apparatus of labrid fishes: A functional
esis. J. Morph. 173:225-235.
morphological perspective. J. Morph. 187:143158.
Al-Hussaini, A. H. 1945. The anatomy and histology
of the alimentary tract of the coral feeding fish Lobel, P. S. andj. C. Ogden. 1981. Foraging by the
Scarussordidus (K\unz.). Bull. Inst.d'Egypte Cairo
herbivorous parrotfish Sparisoma radians. Mar.
27:349-377.
Biol. 64:173-183.
Longley, W. H. and S. F. Hildebrand. 1941. SystemAnker, G. Ch. 1978. The morphology of the head
atic catalogue of the fishes of the Tortugas, Flormuscles of a generalized Haplochromis species: 11.
ida. Pap. Tortugas Lab. 34, Carbenie Inst. Wash.
elegans Trewavas 1933 (Pisces, Cichlidae). Neth.
Publ. 535.
J. Zool. 28(2):234-271.
Board, P. A. 1956. The feeding mechanism of the Monod, T. 1951. Notes sur la squelette visceral des
parrotfish Sparisoma cretense (Linne). Proc. Zool.
Scaridae. Bull. Soc. Hist. Nat. Toulouse 86:191Soc. London 127:59-77.
194.
Boas, J. E. V. 1879. Die Zahne der Scaroiden. Zeit- Murray, P. D. F. 1936. Bones, a study of development
and structure of the vertebrate skeleton. University
schrift fur wissenschaftliche Zoologie 22:189-210.
Press, Cambridge.
Claeys, H. and P. Aerts. 1984. Notes on the compound lower pharyngeal jaw operators in Asta- Nelson, G. J. 1967a. Gill arches of some teleostean
fishes of the families Girellidae, Pomacentridae,
lotilapia elegans (Trewavas), 1933 (Teleostei:
Embiotocidae, Labridae, and Scaridae. J. Nat.
Cichlidae). Neth.J. Zool. 34(2):210-214.
Hist. 1:289-293.
Earle, S. A. 1972. The influence of herbivores on
the marine plants of Great Lameshur Bay, with
Nelson, G.J. 19676. Branchial muscles in some genan annotated list of plants. In B. B. Collette and
eralized teleostean fishes. Acta Zool. 48:277-288.
S. A. Earle (eds.), Results of the Tektite Program:
Nelson, G.J. 1969. Gill arches and the phylogeny of
Ecology of coral reef fishes. Nat. Hist. Mus. L.A.
fishes with notes on the classification of verteCounty Science Bulletin 14.
brates. Bull Am. Mus. Nat. Hist. 141:479-552.
Gobalet, K. W. 1980. Functional morphology of the Nelson, J. S. 1984. Fishes of the world, 2nd ed. John
head of parrotfishes of the genus Scarus. Ph.D.
Wiley, New York.
Diss., University of California, Davis.
Ogden, J. C. and P. S. Lobel. 1978. The role of
herbivorous fishes and urchins in coral reef comGohar, H. A. F. and A. F. A. Latif. 1959. Morphomunities. Env. Biol. Fish. 3(l):49-63.
logical studies on the gut of some scarid and labrid
fishes. Mar. Biol. St. Al-Chardaqu (Red Sea) Pub. Patterson, C. 1975. The braincase of pholidophorid
10:145-190.
and leptolepid fishes, with a review of the actinop-
PARROTFISH PHARYNGEAL JAWS
terygian braincase. Phil. Trans. Roy. Soc. London Ser. B 269:275-579.
Patterson, C. 1977. Cartilage bones, dermal bones,
and membrane bones, or the exoskeleton versus
the endoskeleton. In S. Andrews, R. S. Miles, and
331
Stiassny, M. L. J. and J. S. Jensen. 1987. Labroid
interrelationships revisited: Morphological complexity, key innovation and the study of comparative diversity. Bull. Mus. Comp. Zool. 151:269319.
A. D. Walker (eds.), Problems in vertebrate evoluSuyehiro, Y. 1942. A study on the digestive system
tion. Linnean Society Symposium Series No. 4:
and feeding habits of fish. Jap. J. Zool. X(l):l77-121.
303.
Quignard, J. P. 1962. Squellette et musculature Tedman, R. A. 1980a. Comparative study of the
cranial morphology of the labrids Choerodon vebranchiale des Labrides. Naturalia Monspeliennustus and Labroides dimitiatus and the scarid Scarsia (Zoologie) 4:125-147.
us fasciatus (Pisces: Perciformes). I. Head skeleRandall, J. E. 1967. Food habits of reef fishes of the
ton. Aust. J. Mar. Freshwater Res. 31:337-349.
West Indies. Stud. Trop. Oceanogr. Miami 5:
Tedman, R. A. 19804. Comparative study of the cra665-847.
nial morphology of the labrids Choerodon venustus
Rognes, K. 1973. Head skeleton and jaw mechanism
and Labroides dimitiatus and the scarid Scarusfasin Labridae (Teleostei: Labridae) from Norweciatus (Pisces: Perciformes). II. Cranial myology
gian waters. Arb. Univ. I. Bergen 1971. Mat.and feeding mechanisms. Aust. J. Mar. FreshNaturv. Series No. 4.
water Res. 31:351-372.
Rosenblatt, R. H. and E. S. Hobson. 1969. Parrotfishes (Scaridae) of the eastern Pacific, with a Verwey.J. 1931. The depth of coral reefs in relation
to their oxygen consumption and the penetration
generic rearrangement of the Scarinae. Copeia
of light in the water. Treubia 13:169-198.
3:434-453.
Schultz, L. P. 1969. The taxonomic status of the Wainwright, P. C. 1987. Biomechanical limits to ecological performance: Mollusc-crushing by the
controversial genera and species of parrotfishes
Caribbean hogfish, Lachnolaimus maximus (Labriwith a descriptive list (family Scaridae). Smithdae). J. Zool. (London) 213(2):283-297.
sonian Contrib. Zool. No. 17.
Sibbing, F. A. 1982. Pharyngeal mastication and food Winterbottom, R. 1974. A descriptive synonymy of
the striated muscles of the Teleostei. Proc. Acad.
transport in the carp (Cyprinus carpio L.): A cineradiographic and electromyographic study. J.
Nat. Sci. Phil. 125, 12:225-317.
Morph. 172:223-258.
Yamaoka, K. 1978. Pharyngeal jaw structure in labrid
Sibbing, F. A.,J. W. M. Osse, and A. Terlouw. 1986.
fish. Pub. Seto Mar. Biol. Lab. 24(4/6):409-426.
Food handling in the carp (Cyprinus carpio L.), its Yamaoka, K. 1980. Some pharyngeal jaw muscles of
Calotomus japonkus (Scaridae, Pisces). Pub. Seto
movement patterns, mechanisms and limitations.
Mar. Biol. Lab. 25(5/6):315-322.
J. Zool. (Lond.) 210:161-203.
Smith, R. L. and A. C. Paulson. 1974. Food transit
times and gut pH in two Pacific parrotfishes.
Copeia 1974(3):796-799.