* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download here
Taura syndrome wikipedia , lookup
Foot-and-mouth disease wikipedia , lookup
Hepatitis C wikipedia , lookup
Influenza A virus wikipedia , lookup
Neonatal infection wikipedia , lookup
Orthohantavirus wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Marburg virus disease wikipedia , lookup
Canine distemper wikipedia , lookup
Hepatitis B wikipedia , lookup
Henipavirus wikipedia , lookup
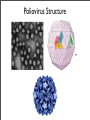
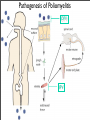
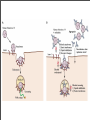
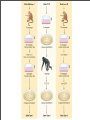
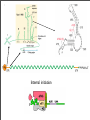
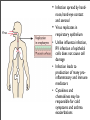
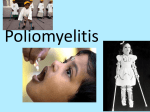
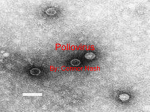
Vincent Racaniello [email protected] www.virology.ws Poliomyelitis Polio (grey), myelon (marrow) = Greek itis (inflammation of) = Latin “A common, acute viral disease characterized clinically by a brief febrile illness with sore throat, headache and vomiting, and often with stiffness of the neck and back. In many cases a lower neuron paralysis develops in the early days of illness” —J.R. Paul, “Poliomyelitis (Infantile Paralysis)”, in A Textbook of Medicine, 1959. Genus Picornaviridae Enterovirus Poliovirus Coxsackieviruses, group A Coxsackieviruses, group B Echoviruses of humans Enteroviruses of humans Enteroviruses, nonhuman Hepatovirus Hepatitis A virus Parechovirus Human parechovirus Rhinovirus Human rhinoviruses Bovine rhinoviruses Cardiovirus EMCV (mengovirus), TMEV Aphthovirus Foot & mouth disease virus Erbovirus Equine rhinitis B virus Kobuvirus Aichi virus Teschovirus Porcine teschovirus 1 Antigenic types 3 23 5 28 4 ~31 2 ~103 ~3 2 ~7 Poliovirus Structure Poliovirus Genome Structure • Egyptian stele, Eighteenth Dynasty (1580-1350 B.C.) • Withered and shortened left leg, foot held in position characteristic of flaccid paralysis • Danish physician Ove Hamburger (1911) concluded deformity was due to infantile paralysis Epidemic poliomyelitis • •First epidemics of poliomyelitis occurred in Sweden: 1868 (14 cases), 1881 (13 cases). • •Rutland, Vermont, 1894 (132 cases) • •New York City, 1907 (750 cases) Polio Research 1908 • Karl Landsteiner isolates poliovirus in monkeys after injection with sterile filtrate from the spinal cord of a boy who had died of polio 1949 • Enders, Weller, Robbins grow poliovirus in cultures of human cells from non-nervous tissue. Replaces the monkey for detecting and studying poliovirus. Nobel Prize, 1954. 1954 Francis clinical trial of Salk's formalin-killed poliovirus (IPV): 1,800,000 children. >50% protection; IPV licensed 12 April 1955. 1955-1960 Paralytic poliomyelitis fell from 20,000 cases/ yr. to 2,500/yr. 1961 Sabin's live, attenuated strains are licensed in the U.S. and replace IPV. 1979 Last case of poliomyelitis (wild type virus) in U.S. 2000 IPV replaces OPV in U.S. Pathogenesis of Poliomyelitis Pathogenesis of Poliomyelitis OPV IPV Poliovirus replication in spinal cord Poliovirus vaccines • Inactivated poliovirus vaccine, IPV – – – – must be injected when properly prepared does not cause disease does not produce intestinal immunity used 1955 - 1961 and 2000 - present in U.S. • Oral poliovirus vaccine, OPV – – – – – easy to administer produces intestinal immunity mutant viruses empirically derived from virulent strains usually reverts during intestinal replication used 1961 - 2000 in U.S. Determinants of attenuation in the Sabin vaccine strains Virus Attenuation determinant P1/Sabin 5’-UTR (480) VP4 (4065) VP3 (3225) VP1 (1106) VP1 (1134) P2/Sabin 5’-UTR (481) VP1 (1143) P3/Sabin 5’-UTR (472) VP3 (3091) Internal initiation CD155 Transgenic Mice Viral growth in mouse brain Viral growth in HeLa cells PD50 472C 9 x 103 pfu 472U >2 x 107 pfu Reversion of P3/Sabin Virus Base at 472 Time of isolation after vaccination Sabin vaccine DM1 DM2 DM3 DM4 DM38 P3/119 U U U U/C C C C 24 h 31 h 35 h 47 h 18 da 3-4 weeks from Evans et al., Nature 314: 548 (1985) Histological lesion score 0.36 ND 1.58 ND 2.48 ND 3.34 Polio Eradication Timeline 1988 WHA Resolution 2000 Stop poliovirus transmission 2005 Certify Global Eradication 2005-2010 Stop polio immunization Polio Eradication Progress 1988 2003 Global cases of poliovirus: 2006: 2001 2007: 1307 2008: 333 Polio eradication • In countries using OPV, only source of polio is the vaccine • Therefore OPV use will cease in posteradication period • The plan to stop vaccination is based on the assumption that there are no nonhuman reservoirs of poliovirus, and circulation of attenuated strains and their derivatives (VDPV) is limited Circulating vaccine-derived polioviruses Problems associated with VDPV • Recent outbreaks of poliomyelitis in Egypt, Dominican Republic/ Haiti, Philippines, Madagascar caused by VDPV • These VDPV strains regained virulence and spread in human populations • Long-term persistence and excretion of VDPVs in immunocompromised persons • These recent outbreaks demonstrate that neurovirulent revertants of OPV can circulate for years (even in immune populations) and cause poliomyelitis Minnesota, October 2005 • Poliovirus type 1 isolated from an unvaccinated, immunocompromised child (7 months old) in an Amish community in Long Prairie, MN • Spread to four other children • No paralytic disease associated with infections • Isolates are VDPVs OPV transmission • Polio outbreaks caused by VAPP strains demonstrate that neurovirulent revertants of OPV can circulate for years (even in immune populations) and cause poliomyelitis • These outbreaks mimic the situation that will occur when OPV usage is halted: circulation of neurovirulent revertants when vaccination coverage drops • In light of this information, we cannot simply stop vaccinating Vaccination against the vaccine • After eradication, immunize globally with IPV (not infectious) – Higher cost than OPV – Not effective in tropical, underdeveloped countries • Careful monitoring of environmental samples for poliovirus Why poliovaccine must be stockpiled after immunization ceases • Virus in research laboratories (mislabeled?) • Stored clinical and environmental samples • Bioterrorism: Synthesis of infectious DNA readily done • Elimination of all sources of poliovirus is impossible; assume an outbreak will occur Which vaccine should be stockpiled? • IPV: noninfectious, no reintroduction of virus into the environment • However, IPV is produced from virulent strains • Poliovirus has escaped from vaccinemanufacturing plants at least twice • mOPV Rhinovirus • • • Typical picornaviruses Cell receptor is either ICAM-1 (91 serotypes) or LDLR (10 serotypes) Cause half of all common colds, the most common infection of humans Why do we care about the common cold? •Adults get 2-4 colds/yr; children 6-8/yr •Yearly costs in US > $20 billion: OTC and Rx medicine, doctor visits, lost work days •Complications include otitis media, sinusitis, serious lower tract infection, particularly in young children, elderly, immunocompromised, and with chronic disorders such as cystic fibrosis •50-80% of all asthma attacks occur with respiratory infections, the majority being RVs; associated with asthma-induced mortality •Upper respiratory tract infections are the most common cause of inappropriate antibiotic use, leading to resistant bacteria •No effective treatments available • • Infection spread by handnose, hand-eye contact and aerosol Virus replicates in respiratory epithelium • Unlike influenza infection, RV infection of epithelial cells does not cause cell damage • Infection leads to production of many proinflammatory and immune mediators • Cytokines and chemokines may be responsible for cold symptoms and asthma exacerbations Prevention of rhinovirus infections • Vaccines are not practical due to large number of viral serotypes • Capsid-binding antiviral drugs (e.g. Pleconaril) • Antiviral drugs against other viral targets (proteinases, RNA polymerase) • Early diagnosis is essential for antiviral therapy - acute infections