* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PLA MICROGRANULES USED AS CARRIERS OF ACTIVE
Survey
Document related concepts
Transcript
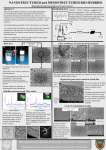
PLA MICROGRANULES USED AS CARRIERS OF ACTIVE SUBSTANCES PREPARED BY EMULSIFICATION IN CHITOSAN SOLUTION Jacek Balcerzak, Maria Mucha Technical University of Lodz, Faculty of Process and Environmental Engineering, ul. Wolczanska 213, 90-924 Łódź, Poland E-mail: [email protected] Abstract The aim of research was the preparation of poly(lactide) and chitosan composite of spherical structures by emulsyfication of both polymers solutions. This resulted in fabrication of PLA microstructures (mPLA) suspended in chitosan acetate. Obtained through coacervation, hybrid structures in the form of beads were freeze dried, which led to final composite matrices. The interactions between oposite charged biopolymers in the composite was confirmed by SEM analysis. Studies of share distribution of mPLA microstructures as well as kinetics of beads swelling and ibuprofen release to the gastro-intestinal track environment were conducted. Key words: chitosan, poly(lactide), emulsification, composite, prolonged drug release. Progress on Chemistry and Application of Chitin and Its ..., Volume XIV, 2009 101 J. Balcerzak, M. Mucha 1. Introduction Medicines of the prolonged release of an active substance (CRS) are being nowadays preferably introduced into the market by pharmaceutical companies. Such drugs ensure the maintenance of the effective therapeutic doses of the biologically active substances therefore elimination of the patient’s exposure to inappropriate concentrations of the medicine in an organism. The ideal carriers of active substances in the system of the controlled release are biopolymers which are susceptible to the gradual erosion (hydrolytic degradation) in biologically ocurring water solutions [1]. The products of this degradation are absorbed and metabolized by within the organism. Chitosan (CS), a carbohydrate polymer, the product of chitin deacetylation, due to a number of properties, such as, biocompatibility and biodegradability, has found a range of applications in medicine. Polylactide (PLA) is also a biodegradable polymer. It presents good mechanical properties and is mainly used in packaging industry or medicine [1, 2]. Opposed to chitosan, PLA can dissolve in organic solvents (e.g. ethyl acetate, dichloromethane). The product of its hydrolytic degradation in the water environment is a natural metabolite – lactic acid. The basic problem in preparing the biopolymer matrices as a drug carriers of the prolonged erosion is a rapid release of a considerable amount of an active substance in the initial phase of the process. This adverse phenomenon is a so-called “burst effect”. To minimize the burst effect the attempts are made to generate the hybrid CRS matrices, composed of two polymers of various physical and chemical properties, constituting a kind of protection for each other and a double barrier of a drug release. In the study, the preparation of hybrid beads made of polylactide and chitosan, containing a model active substance - ibuprofen (IBU) by emulsification followed by an evaporation of an organic solvent, is presented [3]. The Authors discuss the distribution of the obtained mPLA structures size. Apart from the analysis of composite beads morphology, the investigations of their swelling was carried out and the preliminary results of the IBU release kinetics to the solutions simulating the environment of the gastrointestinal tract were discussed [4, 5]. 2. Materials and methods Chitosan (CS) of the deacetylation degree of 73.3% and molecular weight Mw = 4.5∙105 Da was produced at the Sea Fisheries Institute in Gdynia. The second component of polymer composite beads was polylactide (PLA) bought as a granulate from Cargill Dow Polymers LLC company. The polymer solvents were 1% acetic acid and ethyl acetate respectively. Ibuprofen (IBU), RS-2-(-4-isobuthyl-phenyl)-propionic acid of the molecular mass 206.28 g/mol was used, as a model drug of low solubility in water solutions. Pure ibuprofen powder was bought from Cardinal Pharma Trade, Poland. Poly vinyl(alcohol) (PVAL), molecular weight 0.72∙105 Da, produced by POCH Gliwice was a 102 Progress on Chemistry and Application of Chitin and Its ..., Volume XIV, 2009 PLA Microgranules Used as Carriers of Active Substances Prepared by Emulsification in Chitosan Solution stabilizer in the emulsification process. The coacervation of chitrosan was carried out in the sodium tripolyphosphate (TPP) (Sigma Aldrich Chemie GmbH Steinheim, Germany) water solution. The preparation of mPLA-IBU/CS composite beads comprised three stages. The polylactide microsctructures (mPLA) encapsulating ibuprofen were made by emulsification in a 1% CS solution in acetic acid with the addition of the stabilizer –PVAL. The organic solvent was further evaporated under the lowered pressure. The subsequent phase of beads preparation was coacervation of chitosan drops containing mPLA suspension in a TPP polyanion solution. The obtained composite beads were subjected to lyophilization which yielded dry porous polymer structures with the encapsulated model active substance. 2. 1. Emulsification with an organic solvent evaporation. The basic stages of the emulsification process are presented in Figure 1. The dispersed phase of emulsion (O/W) was the solution of PLA and ibuprofen (25% mass of polylactide) in ethyl acetate. Ibuprofen is very well soluble in such organic solvents as ethyl acetate, dichloromethane or chloroform [6]. The continuous water phase was a chitosan solution in 1% acetic acid with the addition of PVAL as the emulsion stabilizer. The organic phase to the water phase ratio was as 1:4. The emulsion was prepared using ultrasounds of 21.5 kHz frequency (Figure 1.A). The emulsification in a viscous chitosan solution of low pH was justified. The viscosity of the emulsion continuous phase affects the size of organic phase drops and, in turn, the microstructures obtained from the dispersed phase. It was confirmed [7] that the increase in viscosity of water phase in O/W emulsion results in a decrease of microstructures size and narrowing their distribution. The acitity of the continuous phase affects the drug loading due to the dependence of the active substance solubility on the solution pH. The IBU solubility in water solutions rises with an increase of medium pH and is equal to 0.036 mg/ml at pH=1.4 and 6.14 mg/ml at pH=7.4, respectively [6]. With the aim to increase the effectiveness of the encapsulation of ibuprofen in the polymer structures obtained from an organic phase drops, it is essential that the continuous water phase pH is low (pH of 1% acetic acid is 3.05). The prepared emulsion was then pumped through the nozzle to the excess ( in relation to the complete miscibility of ethyl acetate with water) volume of 1% CS in acetic acid. 1 cm3 of ethyl acetate dissolves completely in 9 cm3 of water [8] as a result, the diffusion of ethyl acetate from the dispersed droplet phase to the continuous phase occured (Figure 1.B) leading to the precipitation of polyactide insoluble in water, in the form of porous microstructures with encapsulated ibuprofen (Figure 1.C). Crucial phenomenon of emulsification of the PLA in ethyl acetate solution in the CS solution is electrostatic interactions between two polymers. The mPLA microstructures possess a surface negative charge which disable their coagulation [8]. On the other hand, chitosan, in the acidic solutions, occurs in the form of a polycation due to the amino group Progress on Chemistry and Application of Chitin and Its ..., Volume XIV, 2009 103 J. Balcerzak, M. Mucha protonation. The electrostatic interactions between the polymers lead to the formation of chitosan shells on the polylactide microstructures. Figure 1. Main stages emulsification with solvent evaporation process; A) emulsification inducted by ultrasonds; B) organic solvent diffusion; C) porous mPLA microspheres precipitation followed by electrostatic attractions with CS. The process of ethyl acetate diffusion to water phase results in the occurrence of considerable amount of the organic solvent in this phase. Due to the fact that ethyl acetate is an undesireable component of the system it was evaporated during 10 hours in the vacuum chamber (Artvac) under the pressure of 0.15 bar and at the temperature of 50 °C. 2.2. Coacervation of mPLA-IBU/CS The solution of CS with the suspended mPLA microstructures containing ibuprofen was added dropwise under high pressure through the nozzle (f = 0.8 mm) to 1% sodium tripolyphosphate (TPP) water solution (TPP) of pH = 9.6. This compound dissociates in the solution into the Na+ and P3O105- ions. With the contact of chitosan dissolved in acetic acid with anions of P3O105- and the hydroxyl group OH- one observes the deprotonation of the biopolymer NH3+ groups which induces the coacervation of chitosan [9, 10]. The mPLAIBU/CS composite beads of mean diameter 1.5 mm, obtained in this process, are presented in Figure 2.A. 2.3. Freeze-drying of mPLA-IBU/CS In order to obtain dry mPLA-IBU/CS composite beads of natural porous structure ,the freeze-drying of the material in an ALPHA 2-4 lyophilisator (Christ) was carried out. The process was conducted using a at the following parameters: T = –30 °C, p = 0.630 mbar (Tequlibrium = –25 °C). The conditions of freeze-drying were selected in such a way that the phenomena of shrinking and destroying the structure of strongly swollen chitosan material by ice were minimized. The mPLA-IBU/CS beads after freeze-drying are shown in Figure 2.B. 104 Progress on Chemistry and Application of Chitin and Its ..., Volume XIV, 2009 PLA Microgranules Used as Carriers of Active Substances Prepared by Emulsification in Chitosan Solution Figure 2. Photographs of mPLA-IBU/CS beads. A) after coacervaction; B) after freeze-drying. 3. Results and discussion 3.1. Morphological studies of mPLA-IBU microstructures - Optical microscopy The microscopic investigations of mPLA polylactide microstructures obtained in the process of emulsification with an organic solvent evaporation were carried out. Figure 3. Dimension of PLA microspheres analysis; A), B) microphotographs of mPLA suspension in chitosan acetate; C) dependence of percentage share of mPLA microparticles on their diameters. The microphotographs of the mPLA suspension in the CS solution obtained using a polarizing and interference microscope (PZO Biolar PI) equipped with a camera are presented in Figure 3.A and 3.B. The crystals of IBU were not noticed which allows one to conclude that the model drug occurs in the polymer matrix in a molecular form. Progress on Chemistry and Application of Chitin and Its ..., Volume XIV, 2009 105 J. Balcerzak, M. Mucha Using software for the computer image analysis (Microscan v. 1.5) the distribution of microstructure size was investigated. The sample analysed contained 312 mPLA microparticles. The values of the mPLA diameters were categorized into classes and, then, the percentage share of medians of particular diameter classes was determined. The histogram (Figure 3.C) was defined by the log-normal distribution curve P(f), according to Equation (1). (ln(f ) − mf )2 1 ⋅ exp − P(f ) = A ⋅ (1) 2 ⋅ σ f2 f ⋅σ f ⋅ 2 ⋅π where: A = 1.95 – the constant; f - the mPLA diameter in mm; sf = 0.55 and mf = 1.65 – the parameters of the log-normal distribution. From the size distribution of mPLA (Figure 3.C) one may deduce that the microstructures of diameters from 1 mm to 17 mm were observed. In fact, an insignificant number of particles reached the diameters over 20μm. Approximately, 60% of all microstructures have diameters from the range of 2 to 6 μm. On the other hand, mPLA particles of size up to 8 μm constitute ¾ of population. Such a narrow distribution of mPLA diameters in comparison with the results obtained by the authors in the previous researches [11] is the result of the emulsification in the viscous chitosan solution. 3.2. Morphological studies of mPLA-IBU/CS beads - SEM The analysis of the surface structure and the cross-section of mPLA-IBU/ CS composite beads was carried out using a scanning electron microscopy (SEM) (Jeol JSM – 5500LV) at the voltage of 10 kV. The presented microphotographs (Figure 4) confirm the thesis of the interactions between polylactide and chitosan. The porous mPLA microstructures were embedded into the structure of CS beads. Chitosan acts as a shell material for polylactide microparticles with encapsulated ibuprofen. Additionally, in the microphotographs of the mPLA-IBU/CS cross-section (Figures 4.C and 4.D) one may observe the porous inner cell structure and the external compact wall of the bead. 3.3. Swelling kinetics of mPLA-IBU/CS beads In order to investigate the beads ability of water sorption the gravimetric examination of the swelling kinetics in distilled water during 4 hours was conducted. After mentioned period of time, any significant change of the swollen particles mass was not noticed. The beads swelling degree α in distilled water was determined according to the dependence (2). a = (mw - ms)/ms . 100% (2) where: mw – the beads mass after water sorption [mg]; ms – the mass of dry beads [mg]; The mPLA-IBU/CS composite beads revealed considerably smaller ability of water sorption than beads made of pure chitosan (Figure 5). The CS beads sample attained the value of the swelling degree greater than 400% after 3.5 h. On the other hand, the swelling degree of mPLA-IBU/CS, after 4h of swelling reached the value of 250%. The 106 Progress on Chemistry and Application of Chitin and Its ..., Volume XIV, 2009 PLA Microgranules Used as Carriers of Active Substances Prepared by Emulsification in Chitosan Solution incorporation of hydrophobic mPLA microstructures into CS beads causes a decrease of the ability of water sorption, due to their durability in the water environment. Figure 4. SEM microphotographs of mPLA/CS bead; A) the overwiev; B) the surface, C), D) the cross-section. Figure 5. Swelling kinetics of obtained beads; A) CS, B) mPLA-IBU/CS (PLA to CS as ratio as 1 : 5). Progress on Chemistry and Application of Chitin and Its ..., Volume XIV, 2009 107 J. Balcerzak, M. Mucha 3.4. IBU release kinetics from mPLA-IBU/CS beads The Polish Pharmacopea [12] defines the spectrophotometric way of ibuprofen determination in a solution. The UV spectrum of a model drug - IBU used depicts an occurrence of characteristic bands at the wavelengths: 272, 264 and 222 nm. The measurements of IBU release kinetics from mPLA-IBU/CS beads were performed in the media of pH equal to 1.4 and 7.2 (hydrochloric acid and phosphate buffer respectively) at the temperature of 37 °C. The change of UV spectra during release process with a marked analysed band of IBU is demonstrated in Figure 6. The increase of IBU concentration at the pH = 7.2 is reflected by the absorbance increase in the band at 222 nm. The measurements of absorbance – the height of the peak at 222 nm in the case of the release to medium of pH = 1.4 was ambiguous. The characteristic IBU band indicated a shift in the direction of shorter wavelength (the maximum at λ = 219 nm). Moreover, the UV spectrum in the area of the analysed wavelength changed its character. The noticeable maximum of the band became, during the process, gradually replaced by the point of the band inflexion. On the basis of the 222 nm or 219 nm band absorbance, the IBU concentrations in the analysed release media using the model curves were calculated. The obtained results of the relative amount of IBU released in time (ft) were described by a two-stage model proposed by Gallagher and Corrigan [13], developed for the release of a drug not chemically bounded to its polymer carrier. The so-called “burst effect” occures at the onset of the release process and its course is characterised by the first order kinetic curve. After this stage, the diffusion-mediated IBU release through the pores of the polymer matrix accompanied with its hydrolytic degradation occurs. The model suggested by Gallagher and Corrigan is defined by Equation (3). e k 2 ⋅(t − t 2 max ) f t = f t1 + f t 2 = f B ⋅ 1 − e − k1 ⋅t + ( f t max − f B ) ⋅ k 2 ⋅(t − t 2 max ) 1+ e ( ) (3) where: ft = Ct/C0 – the relative amount of drug release in time; (Ct – the concentration), regarding the total drug concentration in the polymer matrix (C0); ft1 – the relative amount of drug released in the I stage (the first order kinetics); ft2 – the relative amount of drug released in the II stage; fB –the total relative amount of the released drug in the I stage; ftmax – the maximal rate of the released drug ; k1, k2 –the kinetic constants for both stages of the release; t2max – the time at which the maximal rate of the drug release is accomplished in the II stage. The values of the Equation (3) parameters of are summarized in Table 1. Table 1. Obtained values of equatuin (3) parameters; where R2 - correlation coefficient. 108 pH R2 fB k1, h-1 ftmax k2, h-1 t2max, h 1.4 0.987 0.207 7.54 0.413 0.461 5.29 7.2 0.992 0.202 15.4 0.238 0.557 4.04 Progress on Chemistry and Application of Chitin and Its ..., Volume XIV, 2009 PLA Microgranules Used as Carriers of Active Substances Prepared by Emulsification in Chitosan Solution Figure 6. Release kinetics of IBU from mPLA-IBU/CS beads; A)medium pH = 1.4, B) medium pH = 7.2. The curves defined by Equation (3) describe the experimental data (Figure 6) accurately. During the first hour of the process considerable amount of the model drug (within the “burst effect”) was released. After this stage one may notice a subsequent mechanism of the release, mediated by swelling and the hydrolytic degradation of the polymer matrix. The final rate of the released ibuprofen (ftmax) achieved after about 20 h (pH = 1.4) and about 10 h (pH = 7.2) is insufficient and equal to 41.7% into pH = 1.4 (Figure 6.A) and 23.8% into pH = 7.2 (Figure 6.B). A low final relative amount of the drug released from the mPLA-IBU/CS composite beads may be explained by the insufficient degradation of the polymer matrix during whole release process or by inproper evaluation of the IBU amount (loading) in the analyzed samples. Some amount of ibuprofen may be lost during the coacervation process (dissolving and diffusion to TPP solution of high pH) and lyophilization (evaporation with liquid absorbed initially by beads). The investigations of IBU loading efficiency in the polymer matrix are currently being carried out. 4. Conclusions In order to obtain a biopolymer composite of mPLA/CS in the form of beads the combination of the emulsification with the evaporation of an organic solvent, coacervation and frreze-drying, were applied. The system of mPLA/CS is designed as a carrier of the active substances of low solubility in water solutions (especially of low pH). The narrow range of mPLA microstructure sizes was achieved due to high viscosity of the O/W emulsion continuous phase (chitosan solution). The analysis of the morphology (SEM microscopy) Progress on Chemistry and Application of Chitin and Its ..., Volume XIV, 2009 109 J. Balcerzak, M. Mucha confirms the porous character of composite beads and the occurrence of the interactions between biopolymers. The measurements of swelling of chitosan beads indicate a decrease of their ability of water sorption as a result of the introduction of hydrophobic mPLA microstructures. The kinetics of the IBU release had a two-stage character. The obtained release results indicate on necessity of increasing the loading efficiency of IBU in composite beads. 5. References 1.Merkli A., Tabatabay C., Gurny R., Heller J.; (1998) Biodegradable Polymers for The Controlled Release of Ocular Drugs. Prog. Polym. Sci. 23, pp. 563-580. 2. Xiong X. Y., Tam K. C., Gan L. H.; (2005) Release kinetics of hydrophobic and hydrophylic model drugs from pluronic F127/poly(lactid acid) nanoparticles. Journal of Controlled Release 103, pp. 73-82. 3.O’Donnell P. B., McGinity J. W.; (1997) Preparation of microspheres by the solvent evaporation technique. Adv. Drug Deliv. Reviews 28, 25-42. 4.Win P. P., Shin-ya Y., Hong K., Kajiuchi T.; (2003) Formulation and characterization of pH sensitive drug carrier based on phosphorylated chitosan (PCS). Carbohydrate Polym. 53, pp. 305-310. 5.Babazadeh M.; (2006) Synthesis and study of controlled release of ibuprofen from the new acrylic type polymers. Int. J. Pharm. 316, pp. 68-73. 6.Wang C., Ye W., Zheng Y., Liu X., Tong Z.; (2007) Fabrication of drug-loaded biodegradable microcapsules for controlled release by combination of solvent evaporation and layer-by-layer self-assembly. Int. J. Pharm. 338, pp. 165-173. 7.Shi P., Li Y., Zhang L.; (2008) Fabrication and Property of Chitosan Film Carrying Ethyl Cellulose Microspheres. Carbohydrate Polym. 72, pp. 490-499. 8.Trimaille T., Pichot C., Elaissari A., Fessi H., Briancon S., Delair T.; (2003) Poly(D,L-lactic acid) Nanoparticle Preparation and Colloidal Characterization. Colloid Polym. Sci. 281, pp. 1184-1190. 9.Bhumkar D. R., Pokharkar V. B.; (2006) Studies on Effect of pH on Cross-linking of Chitosan With Sodium Tripolyphosphate: A Technical Note. AAPS Pharm. Sci. Tech. 7 Article 50. 10.Lin W., Yu D., Yang M.; (2005) pH-sensitive Polyelectrolyte Complex gel Microspheres Composed of Chitosan/sodium tripolyphosphate/dextran Sulfate: Swelling Kinetics and Drug Delivery Properties. Colloids and Surfaces B. Biointerfaces 44, pp. 143-151. 11.Balcerzak J., Mucha M.; (2008) Method For Preparation of Hybrid Spherical Chitosan/Poly(lactic acid) Structures. In Progress on Chemistry and Application of Chitin and Its Derivatives Vol. XIII, pp. 57-64. 12.(2002) Farmakopea Polska wyd. VI, p. 434. 13. Gallagher K. M., Corrigan OI.; (2000) Mechanistic Aspects of The Release of Levamisole Hydrochloride From Biodegradable Polymers. J. Control. Release 69, pp. 261-272. Acknowledgments The authors gratefully thank the Polish Ministry of Science and Higher Education (MNiSW) for the support under Grant no. N N208 2987 33. 110 Progress on Chemistry and Application of Chitin and Its ..., Volume XIV, 2009