* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Angiotensin-Converting Enzyme Inhibitor–Associated Elevations in
Survey
Document related concepts
Transcript
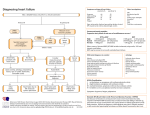
ORIGINAL INVESTIGATION Angiotensin-Converting Enzyme Inhibitor–Associated Elevations in Serum Creatinine Is This a Cause for Concern? George L. Bakris, MD; Matthew R. Weir, MD Background: Reducing the actions of the reninangiotensin-aldosterone system with angiotensinconverting enzyme inhibitors (ACEIs) slows nephropathy progression in patients with or without diabetes. Post hoc analyses of many ACEI-based clinical trials demonstrate the greatest slowing of renal disease progression in patients with the greatest degree of renal insufficiency at study initiation. However, many physicians fail to use ACEIs or angiotensin receptor blockers in patients with renal insufficiency for fear that either serum creatinine or potassium levels will rise. Objective: To determine if limited initial reduction in either glomerular filtration rate (GFR) or elevation in serum creatinine levels, associated with ACEI or angiotensin receptor blocker use, results in long-term protection against decline in renal function in patients with renal insufficiency. Methods: We reviewed 12 randomized clinical trials that evaluated renal disease progression among patients with preexisting renal insufficiency. Six of these studies were multicenter, double-blinded, and placebo controlled, with the remainder being smaller randomized studies with a minimum 2-year follow-up on renal function. These investigations evaluated patients with and without diabetes or systolic heart failure. Average duration of follow-up for all studies was 3 years. Trials were examined in the context of changes in either serum creatinine levels or GFR in the group randomized to an ACEI From the Rush University Hypertension/Clinical Research Center, Department of Preventive Medicine, Rush Presbyterian–St Luke’s Medical Center, Chicago, Ill (Dr Bakris), and Division of Nephrology, Department of Medicine, University of Maryland Medical Center, Baltimore (Dr Weir). T (N = 1102). Sixty-four percent of these individuals (705/ 1102) had renal function data at both less than 6 months and at the end of the study. Results: Most trials demonstrated that patients with pre- existing renal insufficiency manifested an acute fall in GFR, a rise in serum creatinine, or both. Those randomized to an ACEI with a serum creatinine level of 124 µmol/L or greater ($1.4 mg/dL) demonstrated a 55% to 75% risk reduction in renal disease progression compared with those with normal renal function randomized to an ACEI. An inverse correlation was observed between the amount of renal function loss at baseline and the subsequent rate of annual decline in renal function following randomization to an antihypertensive regimen that contained an ACEI. Conclusions: A strong association exists between acute increases in serum creatinine of up to 30% that stabilize within the first 2 months of ACEI therapy and long-term preservation of renal function. This relationship holds for persons with creatinine values of greater than 124 µmol/L (.1.4 mg/dL). Thus, withdrawal of an ACEI in such patients should occur only when the rise in creatinine exceeds 30% above baseline within the first 2 months of ACEI initiation, or hyperkalemia develops, ie, serum potassium level of 5.6 mmol/L or greater. Arch Intern Med. 2000;160:685-693 HE SIXTH REPORT of the Joint National Committee (JNC VI) states that there are specific indications for angiotensin-converting enzyme inhibitor (ACEI) use as part of a regimen to lower blood pressure to levels less than 130/85 mm Hg in patients with diabetes mellitus.1 Moreover, ACEIs should also be part of the “antihypertensive cocktail” used to achieve this same lower level of blood pressure reduction in individuals with renal insufficiency.1 However, data from the Third National Health and NuARCH INTERN MED/ VOL 160, MAR 13, 2000 685 trition Examination Survey III (NHANES III) demonstrate that only half of the 53% of hypertensive patients treated actually achieve the blood pressure goal of less than 140/90 mm Hg.1 From these data, it is estimated that about 3% to 5% of patients achieve a blood pressure goal of less than 130/85 mm Hg, as recommended by the JNC VI report for persons with renal insufficiency or diabetes. The failure to achieve these blood pressure goals may be due, in part, to physician indifference, fear or ignorance, or patient noncompliance with medication therapy.2 Moreover, this WWW.ARCHINTERNMED.COM ©2000 American Medical Association. All rights reserved. MATERIALS AND METHODS We reviewed randomized clinical trials that focused on renal disease progression and had ACEI therapy as one of its arms. Studies included in the evaluation met the following criteria: (1) were randomized to either ACEI-based therapy or blood pressure control using an ACEI as part of the therapeutic armamentarium; (2) had a minimum follow-up period of 2 years; (3) had the majority of participants with greater than 25% loss of their renal function at baseline, regardless of cause; and (4) had blood pressure goals of less than 140/90 mm Hg. Twelve randomized clinical trials met these criteria. Six of these studies were multicenter, doubleblinded, and placebo controlled, with the remainder being smaller randomized studies with a minimum follow-up on renal disease progression of 2 years. All studies evaluated renal disease progression among patients with and without diabetes or systolic heart failure. Mean ± SD duration of follow-up for all studies was 3.2 ± 0.3 years. Trials were examined in the context of changes in either serum creatinine level or GFR in the group randomized to or receiving an ACEI (N = 1102). Sixty-four percent of these individuals (705/1102) had renal function data at both less than 6 months and at the end of the study. Data were assessed using the achieved blood pressures and changes in renal function of a given trial. Serum creatinine values, collected at time periods of less than 6 months and at trial end, were used. If serum creatinine values were not available, then GFR data are reported. Trends in the changes of serum creatinine values or GFR are reported. In addition, changes in serum potassium values were assessed at baseline and study end. These are reviewed in the context of changes in renal function. failure to achieve adequate blood pressure control halted the previously observed trend in reduction of cardiovascular mortality and subsequently increased morbidity from myocardial infarction, heart failure, stroke, and renal disease.1 Another group that may require reductions in arterial pressure to less than 130/85 mm Hg to slow the rate of renal disease progression are African Americans.3,4 Many such patients experience a transient rise in serum creatinine levels after treatment with an ACEI or angiotensin receptor blocker (ARB) is started or after blood pressure is adequately reduced. A rise in the serum creatinine level consequently leads to physician reticence to stay the course with a given therapy. This action subsequently results in failure to maintain adequate blood pressure goals. Thus, some patients are deprived of known strategies that delay progression of renal disease. It is noteworthy that clinical trials in both heart failure and progression of renal disease support the concept that ACEIs reduce cardiovascular mortality and renal disease progression.5-19 However, the dose of ACEIs used in these trials was, on average, 2 to 3 times higher than those used by the average practicing physician treating similar ARCH INTERN MED/ VOL 160, MAR 13, 2000 686 types of patients. Despite these trial outcomes, lower doses of ACEIs are used in everyday practice, a trend not explainable by data from clinical trials. It likely reflects, however, concern about safety and tolerability of higher ACEI doses. Physicians should be aware that at these lower doses, ACEIs might not provide the same positive outcomes in preservation of renal function as noted in clinical trials. This reduced benefit from lower doses of ACEIs may relate to either a failure of blood pressure control or the relative degree of ACE inhibition itself. Some of the main reasons for the failure to achieve adequate drug dosing relate to “emotion-based” rather than evidence-based” medicine. Physicians recall that there are increased side effects as doses increase. While this is true for older antihypertensive agents, it is not true for ACEIs or ARBs. Specifically, cough and angioedema are not dose related and not observed with use of ARBs.15,20 Doserelated changes in serum creatinine and potassium levels do occur with ACEIs, however, and are predictable in many cases. These ACEI-associated changes may serve to be diagnostic as well as therapeutic. However, many physicians, including nephrologists, view a rise in serum creatinine level as a contraindication for ACEI use. The most common cause of an acute rise in serum creatinine level, following inhibition of the renin angiotensin system (RAS), results from a decreased effective arterial blood volume. This is due to hypoperfusion secondary to volume depletion from overaggressive diuresis, low cardiac output seen in heart failure, or both.21 In these clinical settings, the reduced pressure head from the afferent arteriole further lessens the already reduced intraglomerular pressure imposed by ACEIs. Thus, the compensatory elevation of single-nephron glomerular filtration rate (GFR) seen in renal insufficiency and diabetes is reduced in an almost additive fashion when hypoperfusion and ACE inhibition coexist.21,22 One of the major reasons for this renal hemodynamic effect is a loss of the kidney’s ability to autoregulate pressure through the nephron.22-24 Consequently, there is a direct relationship between blood pressure and GFR, whereas under normal circumstances there is a sigmoidal relationship. This is discussed later in this article. In addition, achievement of recommended lower blood pressure goals, ie, less than 125/75 mm Hg may also raise serum creatinine levels, regardless of the type of antihypertensive agent used.1 This is especially prominent in persons with a long history of markedly elevated blood pressure and renal insufficiency.25 Last, bilateral renal artery stenosis might also be a cause of elevated serum creatinine levels following initiation of ACEI therapy. It occurs, however, with a much lower frequency and should be considered in patients with extensive atherosclerotic cardiovascular disease or who smoke. It should also be considered in individuals in whom rehydration has not stabilized or serum creatinine reduced toward normality within a few weeks. Factors to consider when an acute rise in serum creatinine level is observed are • decreased effective circulating volume (most common); • advanced age (.65 years) with and without preexisting lipid abnormalities; and WWW.ARCHINTERNMED.COM ©2000 American Medical Association. All rights reserved. Inhibition of the RAS blunts the maximal capacitance of organs such as the heart and kidney to respond to increased metabolic and physical demands. Clinical trial data support the observation that a small reduction in GFR or rise in serum creatinine level markedly slows renal disease progression. An analogous example in the cardiovascular system is the relationship between heart rate and cardiovascular events. Data support the concept that a reduction in heart rate, by b-blockers, reduces the incidence of cardiovascular events. Thus, we argue that an initial, limited, rise in serum creatinine level is beneficial within the context of preserving renal function. RESULTS GENERAL TRENDS The clinical studies reviewed indicate that a limited elevation in serum creatinine, ie, 30% or less above baseline, following initiation of therapy with an ACEI or ARB was seen in patients with renal insufficiency who achieved their blood pressure goal.1,8-19,25,26 Moreover, an increase in serum creatinine level, if it occurs, will happen within the first 2 weeks of therapy initiation (Figure 1). Assuming normal volume and sodium intake, a rise in serum creatinine that follows ACEI or ARB therapy should stabilize within 2 to 4 weeks (Figure 1). Moreover, if a significant change in serum creatinine level has not been observed within the first month of therapy and blood pressure has been adequately controlled, the probability of an acute rise in serum creatinine level following this period is unlikely. There are 3 exceptions to this statement: (1) initiation of or increasing the dose of currently prescribed diuretics; (2) initiation by either the patient or physician of a nonsteroidal anti-inflammatory drug; or (3) development of volume depletion from nondiuretic-induced causes such as gastroenteritis. ACEI-ASSOCIATED IMPACT ON NATURAL HISTORY OF RENAL DISEASE Blood pressure reduction to levels less than 140/90 mm Hg clearly slows renal disease progression regardless of the class of antihypertensive agent used.1 Many clinical trials, however, demonstrate additional slowing of renal disease progression when ACEIs are used as part of the “antihypertensive cocktail.”1,8-19,25 This is especially true for diabetic nephropathy. The effects of ACEIs on serum creatinine values and renal function from clinical trials in patients with renal insufficiency are summarized in the Table. Two representative, long-term studies in patients with diabetic nephropathy illustrate that the initial reduction in GFR, following initiation of ACE inhibition, reverted ARCH INTERN MED/ VOL 160, MAR 13, 2000 687 Creatinine, µmol/L (mg/dL) • baseline serum creatinine level of 124 µmol/L or greater ($1.4 mg/dL), either alone or in association with (1) diabetes, (2) heart failure, or (3) achievement of low blood pressure goal, ie, less than 125/75 mm Hg when previously elevated to levels greater than 180/110 mm Hg for long periods of time. 256 (2.9) 239 (2.7) 221 (2.5) 203 (2.3) 186 (2.1) 168 (1.9) 150 (1.7) 133 (1.5) 115 (1.3) 97 (1.1) 80 (0.9) 62 (0.7) A ACEI or ARB Started B C Baseline 1 2 3 4 Weeks Figure 1. Possible changes in serum creatinine levels in individuals with normal renal function with volume depletion, heart failure, or bilateral renal artery stenosis started on therapy with an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) (A); individuals with abnormal renal function started on therapy with an ACEI or ARB, without conditions noted in case A (B); and individuals with normal renal function started on therapy with an ACEI or ARB (C). to rates not significantly different from baseline 1 month after ACE inhibition was withdrawn.26,30 In a follow-up study by Hansen et al,26 42 hypertensive diabetic subjects received ACEIs for an average period of 6 years. In the ACEI group there was a clear initial reduction in GFR by 3% to 9% below baseline at this time. Overall, mean arterial pressure ranged from 105 to 109 mm Hg. However, at the end of 6 years when therapy with ACEIs was stopped, GFR increased by an average of 6.1% over a period of 1 month. This supports the concept that initial or persistent GFR declines are reversible despite prolonged ACEI use. We also noted that patients with diabetic nephropathy given the ACEI lisinopril had from a 1% to 9% fall in GFR at 1 month following treatment initiation (Figure 2).30 The range of mean arterial pressure achieved in this study was 99 to 105 mm Hg. After an average of 5 years of ACEI therapy, patients were withdrawn from the ACEI treatment and clonidine was substituted to maintain blood pressure control. The GFR returned to levels not different from baseline within 1 month of ACEI termination, despite similar blood pressure control (Figure 2). This study further supports the concept that while GFR may be reduced acutely, one can markedly blunt the rate of progression of renal disease with ACEIs. Moreover, these initial ACEIassociated declines in GFR are reversible and partially independent of systemic arterial pressure. Proteinuria has been recently implicated as not only a risk factor associated with renal disease progression but also a culprit in its genesis.8,31 Two recent meta-analyses clearly support the concept that ACEIs should be part of the antihypertensive regimen to lower blood pressure. This is because ACEIs lower proteinuria and markedly slow nephropathy progression, a relationship that is most pronounced in patients with renal insufficiency (Figure 3).32,33 Results of these studies, together with the results of other clinical trials that used ACEI therapy, support the concept that achievement of lower blood pressure goals preserves renal function.9-11,25 Moreover, these studies support the notion that patients with the greatest degree of proteinuria also garner the greatest benefit from blood pressure reduction when ACEIs are used (Figure 3). WWW.ARCHINTERNMED.COM ©2000 American Medical Association. All rights reserved. Long-term Outcome of Renal Function in Clinical Trials in Persons With Renal Disease: Impact of ACEI Therapy* Study Diabetic subjects Captopril Trial27 Bakris et al8 Lebovitz et al13 Nielsen et al28 Bjorck et al16 Nondiabetic subjects AIPRI Trial29 REIN Trial10 Zucchelli et al17 Hannedouche et al14 MDRD Trial25 Ihle et al19 Kamper et al18 D Renal Function‡ N† Duration of Follow-up, y Achieved MAP, mm Hg ,6 mo Trial End 207 18 28 21 40 3 5 3 3 2.2 105 98 104 112 102 ? −9.47 (GFR)§ ? −3.97 (GFR)§ −3.8 (GFR) −0.15 (Cr clear) −0.02 (Cr clear) −6.3 (GFR) −7.1 (GFR) −2.0 (GFR) 300 78 32 52 255 3 3.5 3 3 3 36 35 2 2.2 100 106 100 105 105 94 101 99 +26 (Cr) ? ? ? −5.7 (GFR) −14.4 (GFR) −0.42 (GFR) −3 (GFR) +31 (Cr) −6.3 (GFR) −0.04 (Cr Clear) −4.8 (GFR) −3.8 (GFR) −2.9 (GFR) −0.7 (GFR) −2.4 (GFR) *ACEI indicates angiotensin-converting enzyme inhibitor; MAP, mean arterial pressure; AIPRI, Angiotensin-Converting Enzyme Inhibition in Progressive Renal Insufficiency; REIN, Ramipril Efficacy in Nephropathy; and MDRD, Modification of Dietary Protein in Renal Disease. †Number of patients randomized to an ACEI in a given trial. Note that for the last 3 trials listed in the table, although many of these patients with a glomerular filtration rate (GFR) of 13 to 24 mL/min received an ACEI, they were not randomized to this class. They were randomized to a MAP level of either 102 to 107 mm Hg or less than 92 mm Hg. ‡GFR is expressed as milliliters per minute; creatinine clearance (Cr Clear), milliliters per second; and serum creatinine (Cr), micromoles per liter. To convert creatinine clearance values to milliliters per minute, divide by 0.01667; to convert serum creatinine to milligrams per deciliter, divide by 88.4. §These values were converted to the annual decline rates by converting the GFRs obtained at or before 4 months. Note also that with the exception of 1 study all rates of GFR decline are slower at study end, especially in those with average blood pressures below 130/85 mm Hg. 90 125 85 80 ∗ BP, mm Hg GFR, mL/min 115 ∗ ∗ ∗ 105 75 ∗ 70 65 60 95 Baseline 1 mo 6y 1 mo Off ACEI + Clonidine Baseline 1 mo 6y 1 mo Off ACEI + Clonidine Figure 2. Effects of an angiotensin-converting enzyme inhibitor (ACEI) on creatinine clearance in 23 patients with type 2 diabetes mellitus who received therapy for an average of 5.6 years. Glomerular filtration rate (GFR) returns toward baseline with good blood pressure (BP) control maintained by treatment with clonidine. Asterisk indicates P,.05 compared with baseline. Bars indicate SD. THERAPEUTIC INDEX OF RAS BLOCKADE What is the level of renal insufficiency above which an ACEI or ARB loses its therapeutic index (risk-benefit ratio)? The available data from the randomized clinical trials reviewed demonstrate that ACEIs slow progression of renal disease in both diabetic and nondiabetic subjects. This is likely due to important blood pressure– dependent and –independent effects. Moreover, this effect is most pronounced among those with preexisting renal insufficiency. The data, however, are limited to ARCH INTERN MED/ VOL 160, MAR 13, 2000 688 persons with serum creatinine values up to 265 µmol/L (3.0 mg/dL). This includes studies of patients who have lost more than 75% of their renal function. Thus, no definitive statement can be made about renal outcomes or the therapeutic index of ACEIs in patients with profound renal insufficiency, ie, GFR less than 30 mL/min. It should also be appreciated that, regardless of serum creatinine value, manifestations of renal failure, as assessed by standard laboratory tests, are not apparent until the GFR is well below 30 mL /min (Figure 4). This lower level of GFR may be apparent when the WWW.ARCHINTERNMED.COM ©2000 American Medical Association. All rights reserved. ACEI Benefit 0 (Based on Serum Creatinine Level) –2 ∗ ∗ 120 ∗ –8 –10 –12 80 Age >65 y or Weight <49.5 kg 60 Lab Abnormalities of Renal Failure Start 40 ACEI Benefit GFR, mL/min –6 –14 General Population 100 (Based on GFR) ∆ GFR, mL/min –4 20 Initial GFR (<92 mm Hg MAP) Final GFR (<92 mm Hg MAP) Dialysis –16 0-250 250-1000 † 1000-3000 Proteinuria, mg/d Figure 3. Changes in glomerular filtration rate (GFR) among patients with GFR between 13 and 24 mL/min from the Modification of Dietary Protein in Renal Disease trial as a function of the different levels of proteinuria. Asterisk indicates P,.05 compared with initial fall in GFR; dagger, P,.05 compared with initial GFR from less proteinuric groups. MAP indicates mean arterial pressure. Bars indicate SD. serum creatinine level is as low as 141 µmol/L (1.6 mg/dL) (Figure 4). It should also be noted, that even in this GFR range, ACEIs have been shown to preserve renal function.10,17-19,25 It appears from the collective data that the patients with the greatest degree of renal insufficiency garner the greatest protection from renal disease progression in whatever trial is examined. This is further evidenced by data from the Captopril Trial, 11,12 in which patients whose serum creatinine values were greater than 177 µmol/L (2.0 mg/dL) derived the greatest benefit from ACE inhibition. In this trial, ACEI or placebo were added to a standard antihypertensive regimen to lower blood pressure to less than 140/90 mm Hg. The ACEI group had a 74% risk reduction in doubling of serum creatinine and a 75% risk reduction in the incidence of death, dialysis, or transplantation, compared with the placebo group.11,12 Conversely, patients with a serum creatinine value of less than 88.4 µmol/L (1.0 mg/dL), and similar degrees of blood pressure reduction with the ACEI, experienced only a 4% risk reduction in doubling of serum creatinine or incidence of death, dialysis, or transplantation. Seven separate trials have examined the natural history of renal disease among patients with nondiabetic renal insufficiency, with a range of serum creatinine values between 133 and 265 µmol/L (1.5-3.0 mg/dL) (GFR range, 17-55 mL/min) (Table). Patients in these trials were randomized to receive antihypertensive therapy that contained either placebo or an ACEI to achieve blood pressure control. In the Angiotensin-Converting Enzyme Inhibition in Progressive Renal Insufficiency (AIPRI) trial,29 patients with a serum creatinine level greater than 177 µmol/L (.2.0 mg/dL) had a 66% risk reduction in renal disease progression. This in contrast to those with a serum creatinine level less than 177 µmol/L (,2 mg/dL) who had a 38% risk reduction.9,29 Likewise in the Ramipril Efficacy in Nephropathy (REIN) trial, patients who had serum creatinine values greater than ARCH INTERN MED/ VOL 160, MAR 13, 2000 689 0 88 (1) 177 (2) 265 (3) 354 (4) 442 (5) 530 (6) 619 (7) 707 (8) 796 (9) Serum Creatinine, µmol/L (mg/dL) Figure 4. Relationship of glomerular filtration rate (GFR) decline to change and serum creatinine level. Persons older than 65 years and adults weighing less than 49.5 kg have much lower GFRs for a given level of serum creatinine compared with the usual reference population. Laboratory (lab) manifestations of renal failure start to occur when the GFR is about 30 mL/min. This may occur at serum creatinine values as low as 177 µmol/L (2 mg/dL). These changes include mild anemia and elevations in serum phosphorus levels. As GFR falls below 20 mL/min, additional problems occur, such as acidosis and a tendency toward hyperkalemia. The lighter shaded area indicates the area of shown protection from clinical trials and the darker shaded area indicates the range of where dialysis is usually required. ACEI indicates agiotensin-converting enzyme inhibitor. 177 µmol/L (.2.0 mg/dL) and more than 3.0 g/d of proteinuria had a 62% risk reduction in renal disease progression.10,34 Post hoc analyses of data from the Modification of Dietary Protein in Renal Disease Trial (MDRD)25 also suggest that those with the lowest GFR had the largest reduction in GFR in the presence of an ACEI. These data, together with data from previous trials, support the concept of a bell-shaped curve with regard to the acute effects of ACE inhibition on serum creatinine levels. That is, at normal levels of serum creatinine there is little to no effect on the change in serum creatinine value although GFR is reduced 5% to 20%. With relatively greater degrees of renal dysfunction, there is a loss of renal reserve as well as autoregulatory ability of the kidney. This results in a greater acute fall in GFR following ACE inhibition that becomes more clinically evident. HYPERKALEMIA Several factors contribute to the development of hyperkalemia in the presence of ACEI use. Some of the more common factors include a diet high in fruit, especially dried fruit, and vegetable intake or use of salt substitute. A reduction in aldosterone production as well as concomitant use of nonsteriodal anti-inflammatory agents and/or reduced potassium clearance secondary to reduced GFR, ie, less than 20 mL/min are also common contributory factors. Data from the clinical studies reviewed as well as others suggest that elevations in serum potassium levels that follow ACEI initiation limit the use of ACEI in most patients with concomitant renal or cardiac disease. A case-controlled study of outpatients followed WWW.ARCHINTERNMED.COM ©2000 American Medical Association. All rights reserved. Serum Creatinine >133 µmol/L (>1.5 mg/dL) Long-Acting ACEIs Heart Failure Serum Creatinine <133 µmol/L Concomitant Use of Diuretics 0 1 2 3 4 5 6 7 Relative Risk Figure 5. Risk factors with relative risk (and 95% confidence interval) for developing hyperkalemia from use of angiotensin-converting enzyme inhibitors (ACEI). Adapted from Reardon and Macpherson.35 up in a general medicine clinic with baseline serum potassium levels of greater than 5.1 mmol/L while receiving ACEI therapy illustrates this point. 35 The ACEIs evaluated in this trial included some with dual modes of excretion (fosinopril), and some with a long half-life (lisinopril). Only 194 (11%) of 1818 patients reviewed developed further increases in serum potassium levels. Thirty-seven of the 194 patients had potassium levels of 5.6 mmol/L or greater. Moreover, only 3 (1.5%) of the 194 had potassium levels of 6 mmol/L or greater. The most relevant factor for predicting hyperkalemia was a baseline serum creatinine level of 144 µmol/L (1.6 mg/dL) or greater. Other factors that predicted development of hyperkalemia are shown in Figure 5. Note that only 15 (0.8%) of 1818 patients required termination of the ACEI due to hyperkalemia. Other clinical trials that used ACEIs for blood pressure control in patients with diabetic or nondiabetic renal disease (serum creatinine levels, 133-265 µmol/L [1.5-3.0 mg/dL]) further support the notion that serum potassium levels increase by 0.4 to 0.6 mmol/L; such elevations in serum potassium levels are self-limited. 9,10,12-15 In the AIPRI trial, the average increase in serum potassium was 0.5 mmol/L.9 Only 5 (1.7%) of the 300 patients in the benazepril hydrochloride group and 3 (1.0%) of the 283 patients in the placebo group developed serum potassium levels of 6 mmol/L or greater. In the REIN trial, there were only 2 (1.2%) of 166 stop points due to hyperkalemia; one patient was taking an ACEI, the other was taking placebo.10 In the Captopril Trial, hyperkalemia defined as serum potassium level of 6 mmol/L or greater was only observed in 3 (1.4%) of the 207 patients in the group randomized to captopril. No hyperkalemia was observed in the 202 patients receiving placebo.12,36 Taken together these studies demonstrate a very low risk (,2%) of hyperkalemia with drugs that block the RAS when used in patients with moderate to severe renal impairment. This low incidence of hyperkalemia is further exemplified by the results of clinical trials in patients with heart failure. A recent trial of elderly patients with heart failure evaluated the risk for development of either functional renal insufficiency or ARCH INTERN MED/ VOL 160, MAR 13, 2000 690 hyperkalemia between an ACEI, captopril, or an ARB, losartan potassium.15 The incidence of persisting functional renal insufficiency was approximately 10.5% in each group. Fewer than 2% of the more than 700 patients required drug discontinuation. The risk for hyperkalemia (potassium .6 mmol/L) was low in the captopril group (5/370 [1.4%]), and even lower in the losartan group (2/352 [0.57%]). Other smaller randomized studies have also observed this lower incidence of hyperkalemia with use of angiotensin receptor antagonists.37,38 These studies evaluated the effects of ACEIs and ARBs on potassium balance and the RAS among patients with renal insufficiency; this includes one crossover study that compared each drug class in the same group of patients.38 In these studies, the average increase in serum potassium, at the highest dose of an ARB, ranged from 0.05 to 0.3 mmol/L; the average increase when given an ACEI was approximately double this value. COMMENT The available clinical evidence suggests that the use of drugs that block the RAS are appropriate for patients with renal insufficiency. Moreover, ACEIs are specifically indicated for use in patients with renal insufficiency by the JNC VI.1 They have proven therapeutic benefits, particularly in patients with renal insufficiency (ie, serum creatinine level 133-265 µmol/L [1.5-3.0 mg/dL]). This class of drugs may also provide renoprotective effects that are non–blood pressure dependent when used as part of combination antihypertensive therapy in patients with more advanced renal disease. Drugs that block the RAS might have diagnostic benefits as well. This is exemplified by increases in serum creatinine of more than 25% as indicative of diminished effective arterial blood volume from volume contraction and/or anatomical renal artery disease. An increase in serum potassium may be reflective of increased intake or concomitant use of nonsteroidal anti-inflammatory drugs or salt substitutes. These clinical situations can and should be remedied. Once marked elevations in serum creatinine are present and renal reserve is lost (serum creatinine level 265-309 µmol/L [3.0-3.5 mg/dL] in persons aged .50 years and with normal body habitus), the unique benefits of ACEIs may not exceed that of achieving the recommended level of blood pressure reduction alone (Figure 4). Thus, in a person with preexisting renal insufficiency, aggressive blood pressure control itself, in the absence of ACE inhibition, may lead to a rise in serum creatinine level. This is secondary to a loss of renal reserve and autoregulatory ability when blood pressure falls. Consequently, the nephron fails to maintain the required perfusion pressure to sustain GFR in the remnant “functional” nephrons.39,40 The mechanisms that portend increases in serum creatinine following ACEI therapy are discussed. Inhibition of the RAS by either an ARB or ACEI leads to a reversible reduction in intraglomerular pressure in most nephrons.22,41 In the case of preexisting renal insufficiency, however, fewer functional nephrons, “remnant WWW.ARCHINTERNMED.COM ©2000 American Medical Association. All rights reserved. ARCH INTERN MED/ VOL 160, MAR 13, 2000 691 150 140 GFR (mL/min) or HR (beats/min) nephrons,” are present and thus function at a relatively higher baseline pressure to maintain stable renal function.39,40 Under these circumstances, if RAS activity is reduced, the resultant reduction in intraglomerular pressure is proportionally greater in these remnant nephrons. Thus, the fewer the functional nephrons (higher serum creatinine level) the greater the likelihood that GFR will decrease when RAS activity is reduced. This reduction in renal function may not be reflected as a fall in GFR, however, unless blood pressure falls substantially, ie, at least to levels well below 140/90 mm Hg.34,42,43 The change in GFR under these circumstances depends on the amount of autoregulatory ability preserved by the kidney. If autoregulation is not present, then the GFR will change in direct relation to the level of blood pressure.43,44 The degree of blood pressure reduction necessary to see this effect is variable and depends on preexistent level of renal function. Another reflection of how ACEIs may protect against declines in renal function may be their effect on “renal reserve.” Angiotensin-converting enzyme inhibitors blunt the rise in renal blood flow and GFR that follow a protein load.45 This may be another harbinger of how this class of antihypertensive agents protects against the chronic decline in renal function. The nephron responds to factors such as increased protein intake with an elevation in GFR. This is referred to as “renal reserve” since it reflects the ability of the kidney to increase its clearance rate in the presence of higher urea genesis. This increase in GFR is due to afferent glomerular arteriole dilation in response to various amino acids.45 Many investigators have described a blunted rise in GFR in the presence of an ACEI in both animal models of renal insufficiency and in people with renal dysfunction following protein loading.45-48 The blunted rise in GFR, in part, secondary to persistent efferent arteriolar dilation that results from decreased angiotensin II effects, thus inhibits one mechanism by which the nephron compensates to heightened urea loads. This is analogous to the b-blocker effect on the heart’s ability to increase its rate in response to increased metabolic demands. From these data, it becomes axiomatic that the protective effect of a given antihypertensive agent relates to its ability to blunt a given organ’s response to a heightened work demand. This assertion is based on the observation that these classes of agents have been shown to reduce cardiovascular mortality.5-7 A simple way to conceptualize the benefits of ACEIs to the kidney is through an analogy with cardiac function. We propose that ACEIs reduce the maximal response of a given nephron to excrete metabolic waste products by reducing its baseline GFR. This is analogous to the effects of b-blockers that reduce baseline heart rate and thus blunt the maximal increase in heart rate and blood pressure during excerise (Figure 6). Thus, by decreasing the maximal effort of the myocardium and improving coronary flow, it is not surprising that these drugs are associated with a reduction in cardiac mortality in secondary prevention trials.49 When b-blocker therapy is stopped, HR Response to Exercise–Normal 130 GFR Response to Protein Load–Normal 120 110 100 90 Reduced Baseline HR Response to Exercise–b-Blockers GFR Response to Protein Load–ACEI 80 70 60 Reduced Baseline 50 0 100 % Effect Figure 6. Representative changes in heart rate (HR) or glomerular filtration rate (GFR) in response to exercise or protein loading in the presence and absence of a given angiotensin-converting enzyme inhibitor (ACEI). heart rate and myocardial work increase. One could argue that ACEIs in much the same way reduce the level of baseline function of individual “functional nephrons,” and thus preserve nephron function. The aforementioned data from various clinical trials clearly support this concept9,10 (Table). Angiotensin-converting enzyme inhibitors also affect the morphology of the heart, kidney, and vasculature over time. They are known to have antifibrogenic effects on various organs including the vasculature and thereby help to preserve organ function.50-52 Their effects are secondary to inhibition of various cytokines including transforming growth factor b, collagen IV, and others. 51 Thus, these agents may provide additional protection against renal and cardiovascular disease progression by mechanisms independent of their blood pressure–lowering effects. This is exemplified by restoration of endothelial function by ACEIs in the Trial on Reversing ENdothelial Dysfunction (TREND).52 Angiotensin-converting enzyme inhibitors have a proven benefit in delaying progression of diabetic renal disease and are recommended by the JNC VI for use in patients with diabetes and renal insufficiency. Moreover, data from clinical trials in both diabetic and nondiabetic renal disease demonstrate that if an elevation in serum creatinine occurs, it stabilizes quickly and does not progressively worsen. Moreover, this reduction in GFR is reversible. Thus, no patient should be denied a long-term trial of an ACEI because of a preexisting elevation in serum creatinine level or one that increases up to 30% above baseline and stabilizes within 2 to 3 weeks. A suggested paradigm for ACEI use in patients with renal insufficiency is outlined in Figure 7. Note that if chronic increases in serum creatinine level of more than 30% following 4 or more weeks of ACEI therapy occurs, the patient should be evaluated as suggested in Figure 7. This is especially true in patients with more advanced renal disease. This magnitude of reduction in renal function may interfere with other metabolic functions of the kidney, such as vitamin D metabolism, erythropoietin synthesis, and maintenance of acid-base balance (Figure 4). Thus, elevations in WWW.ARCHINTERNMED.COM ©2000 American Medical Association. All rights reserved. ACEI Started Check Electrolytes and Serum Creatinine (1-2 wk) B Serum Creatinine Increased <30% No Electrolyte Issues A Serum Creatinine Unchanged BP in Goal Continue to Titrate Agent Until BP in Goal∗ C Serum Creatinine + Increased ≥50% BP Not in Goal Recheck Serum Creatinine in 2-3 wk Continue ACEI; Add Other Agents to Achieve BP Goal >30% Increase in Creatinine Level Recheck Creatinine + Electrolytes in 2-3 wk Recheck Electrolytes and Serum Creatinine (3-4 wk) Reduce Dose of ACEI by 50% Add Other Agents to BP Goal If Stable, Recheck Annually; If NSAID Started or Hypoperfusion State Develops, Recheck More Frequently Recheck in 4 wk; If Stable, as per A; If Still >30% Rise, Stop ACEI and Achieve BP Control With Other Agents <30% Increase in Creatinine + BP in Goal Proceed as per A <30% Increase in Creatinine + BP∗∗ If Not in Goal–Add Other Agents to Achieve BP Goal Exclude Hypoperfusion States (Volume Depletion and NSAID Use) Captopril Renal Scan or Angiogram to Rule Out Bilateral Renal Artery Stenosis Check Serum Creatinine in 3-4 wk If BP in Goal, Proceed as per A, If >30% Rise See ∗∗ Figure 7. A schematic approach to a patient with renal insufficiency started on therapy with an angiotensin-converting enzyme inhibitor (ACEI). Asterisk indicates blood pressure (BP) less than 130/85 mm Hg for those with renal insufficiency or diabetes; double asterisks, if serum creatinine level increases more than 30%, reduce ACEI dose by 50% and add other BP-lowering agents; plus sign, if serum creatinine rise is greater than 30% and less than 50% within the first month of therapy, causes for hypoperfusion are eliminated, and nonsteroidal anti-inflammatory drugs (NSAIDs) are not given, treat as if bilateral renal arterial disease is present. serum phosphorus or in potassium levels above 5.6 mmol/L should prompt a reduction in ACEI dose, a change to one with dual mode of excretion (eg, trandolapril or fosinopril), or its discontinuance. This is particularly true for patients with New York Heart Association class IV heart failure.20,53 Accepted for publication May 27, 1999. Reprints: George Bakris, MD, Rush Presbyterian–St Luke’s Medical Center, Department of Preventive Medicine, 1700 Van Buren St, Suite 470, Chicago, IL 60612 (e-mail: [email protected]). REFERENCES 1. The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:24132446. 2. Taylor Nelson Healthcare, Epson Survey. London, England: Copyright Cardiomonitor; 1992. 3. Lazarus JM, Bourgoignie JJ, Buckalew VM, et al, for the MDRD Group. Achievement and safety of a low blood pressure goal in chronic renal disease: the Modification of Diet in Renal Disease Study Group. Hypertension. 1997; 29:641-650. ARCH INTERN MED/ VOL 160, MAR 13, 2000 692 4. Weir MR, Chrysant SG, McCarron DA, et al. Influence of race and dietary salt on the antihypertensive efficacy of an angiotensin-converting enzyme inhibitor or a calcium channel antagonist in salt-sensitive hypertensives. Hypertension. 1998; 31:1088-1096. 5. Ball SG, Hall AS, Murray GD. ACE inhibition, atherosclerosis and myocardial infarction—the AIRE Study in practice: Acute Infarction Ramipril Efficacy Study. Eur Heart J. 1994;15(suppl B):20-25. 6. Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial. N Engl J Med. 1992;327: 669-677. 7. Gustafsson F, Kober L, Torp-Pedersen C, et al. Long-term prognosis after acute myocardial infarction in patients with a history of arterial hypertension: TRACE study group. Eur Heart J. 1998;19:588-594. 8. Bakris GL, Copley JB, Vicknair N, Sadler R, Leurgans S. Calcium channel blockers versus other antihypertensive therapies on progression of NIDDM associated nephropathy: results of a six year study. Kidney Int. 1996;50:16411650. 9. Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-convertingenzyme inhibitor benazepril on the progression of chronic renal insufficiency: the Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Disease trial. N Engl J Med. 1996;334:939-945. 10. The GISEN Group. Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349:1857-1863. 11. Hebert LA, Bain RP, Verne D, for the Collaborative Study Group. Remission of nephrotic range proteinuria in type I diabetes. Kidney Int. 1994;46:16881693. WWW.ARCHINTERNMED.COM ©2000 American Medical Association. All rights reserved. 12. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, for the Collaborative Study Group. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456-1462. 13. Lebovitz HE, Wiegmann TB, Cnaan A, et al. Renal protective effects of enalapril in hypertensive NIDDM: role of baseline albuminuria. Kidney Int. 1994;45(suppl 45):S-150–S-155. 14. Hannedouche T, Landais P, Goldfarb B, et al. Randomised controlled trial of enalapril and beta blockers in non-diabetic chronic renal failure. BMJ. 1994;309:833-837. 15. Pitt B, Segal R, Martinez FA, et al. Randomized trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet. 1997;349:747-752. 16. Bjorck S, Mulec H, Johnsen SA, Norden G, Aurell M. Renal protective effect of enalapril in diabetic nephropathy. BMJ. 1992;304:339-343. 17. Zucchelli P, Zuccala A, Borghi M, et al. Long-term comparison between captopril and nifedipine in the progression of renal insufficiency. Kidney Int. 1992;42:452-458. 18. Kamper AL, Strandgaard S, Leyssac PP. Effect of enalapril on the progression of chronic renal failure. Am J Hypertens. 1992;5:423-430. 19. Ihle BU, Whitworth JA, Shahinfar S, Cnaan A, Kincaid-Smith PS, Becker GJ. Angiotensin-converting enzyme inhibition in non-diabetic progressive renal insufficiency: a controlled double-blinded trial. Am J Kidney Dis. 1996;27:489-495. 20. Alderman CP. Adverse effects of the angiotensin-converting enzyme inhibitor. Ann Pharmacother. 1996;30:55-61. 21. Toto RD. Renal insufficiency due to angiotensin-converting enzyme inhibitors. Miner Electrolyte Metab. 1994;20:193-200. 22. Anderson S, Rennke HG, Brenner BM. Nifedipine versus fosinopril in uninephrectomized diabetic rats. Kidney Int. 1992;41:891-897. 23. Christensen PK, Hansen HP, Parving HH. Impaired autoregulation of GFR in hypertensive non-insulin dependent diabetic patients. Kidney Int. 1997;52:1369-1374. 24. Bidani AK, Griffin KA, Plott W, Schwartz MM. Renal ablation acutely transforms “benign” hypertension to “malignant” nephrosclerosis in hypertensive rats. Hypertension. 1994;24:309-316. 25. Levey A. Short-term effects of protein intake, blood pressure, and antihypertensive therapy on glomerular filtration rate in the Modification of Diet in Renal Disease Study. J Am Soc Nephrol. 1996;7:2097-2109. 26. Hansen HP, Rossing P, Tarnow L, Nielsen FS, Jensen BR, Parving HH. Increased glomerular filtration rate after withdrawal of long-term antihypertensive treatment in diabetic nephropathy. Kidney Int. 1995;47:1726-1731. 27. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-convertingenzyme inhibition on diabetic nephropathy: the Collaborative Study Group [published erratum appears in N Engl J Med. 1994;330:152]. N Engl J Med. 1993; 329:1456-1462. 28. Nielsen FS, Rossing P, Gall MA, Skott P, Smidt UM, Parving HH. Impact of lisinopril and atenolol on kidney function in hypertensive NIDDM subjects with diabetic nephropathy. Diabetes. 1994;43:1108-1113. 29. Bakris GL. Short- and long-term effects of ACE inhibitors on progression of renal disease. Nephrology. 1997;3(suppl 1):S40. 30. Keane WF, Eknoyan G, for the PARADE Committee. Proteinuria, albuminuria, risk assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004-1010. 31. Remuzzi G, Ruggenenti P, Benigni A. Understanding the nature of renal disease progression. Kidney Int. 1997;51:2-15. 32. Kloke HJ, Branten AJ, Huysmans FT, Wetzels JF. Antihypertensive treatment of patients with proteinuric renal diseases: risks or benefits of calcium channel blockers? Kidney Int. 1998;53:1559-1573. 33. Locatelli F, Carbarns IR, Maschio G, et al. Long-term progression of chronic renal insufficiency in the AIPRI Extension Study: the Angiotensin-Converting Enzyme Inhibition in Progressive Renal Insufficiency Study Group. Kidney Int. 1997; 63:S63-S66. ARCH INTERN MED/ VOL 160, MAR 13, 2000 693 34. Ruggenenti P, Perna A, Benini R, Remuzzi G. Effects of dihydropyridine calcium channel blockers, angiotensin-converting enzyme inhibition, and blood pressure control on chronic, nondiabetic nephropathy. J Am Soc Nephrol. 1998;9: 2096-2101. 35. Reardon LC, Macpherson DS. Hyperkalemia in outpatients using angiotensinconverting enzyme inhibitors: how much should we worry? Arch Intern Med. 1998;158:26-32. 36. Rodby RA, Rohde R, Evans J, Bain RP, Mulcahy WS, Lewis EJ. The study of the effect of intensity of blood pressure management on the progression of type I diabetic nephropathy: study design and baseline patient characteristics, Collaborative Study Group. J Am Soc Nephrol. 1995;5:1775-1781. 37. Gansevoort RT, deZeeuw D, Shahinfar S, Redfield A, de Jong PE. Effects of the angiotensin II antagonist losartan in hypertensive patients with renal disease. J Hypertens. 1994;12(suppl 2):S-37–S-42. 38. Bakris GL, Siomos M, Kasprowicz S, et al. Differential effects of ACE inhibitors and angiotensin-1 receptor blockers on potassium homeostasis in nephropathy [abstract]. Am J Hypertens. 1999;12:36A. 39. Brown SA, Brown CA. Single-nephron adaptations to partial renal ablation in cats. Am J Physiol. 1995;269(5 pt 2):R1002-R1008. 40. Yoshioka T, Shiraga H, Yoshida Y, et al. “Intact nephrons” as the primary origin of proteinuria in chronic renal disease: study in the rat model of subtotal nephrectomy. J Clin Invest. 1988;82:1614-1623. 41. Tarif N, Bakris GL. Angiotensin II receptor blockade and progression of renal disease in nondiabetic patients. Kidney Int. 1997;52(suppl 63):S-67–S-70. 42. Bakris GL, Barnhill BW, Sadler R. Treatment of arterial hypertension in diabetic man: importance of therapeutic selection. Kidney Int. 1992;41:912-919. 43. Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest. 1995;96:793-800. 44. Griffin KA, Picken MM, Bakris GL, Bidani AK. Class differences in the effects of calcium channel blockers in the rat remnant kidney model. Kidney Int. 1999;55: 1849-1860. 45. Tietze IN, Sorensen SS, Ivarsen PR, Nielsen CB, Pedersen EB. Impaired renal hemodynamic response to amino acid infusion in essential hypertension during angiotensin converting enzyme inhibitor treatment. J Hypertens. 1997;15:551560. 46. Herrera J, Rodriguez-Iturbe B. Stimulation of tubular secretion of creatinine in health and in conditions associated with reduced nephron mass: evidence for a tubular functional reserve. Nephrol Dial Transplant. 1998;13:623-629. 47. Bohler J, Woitas R, Keller E, Reetze-Bonorden P, Schollmeyer PJ. Effect of nifedipine and captopril on glomerular hyperfiltration in normotensive man. Am J Kidney Dis. 1992;20:132-139. 48. Jaffa AA, Vio CP, Silva RH, et al. Evidence for renal kinins as mediators of amino acid–induced hyperperfusion and hyperfiltration in the rat. J Clin Invest. 1992; 89:1460-1468. 49. Messerli FH, Grossman E, Goldbourt U. Are beta-blockers efficacious as firstline therapy for hypertension in the elderly? a systematic review. JAMA. 1998; 279:1903-1907. 50. Garber L, Walton C, Brown S, Bakris G. Effects of different antihypertensive treatments on morphologic progression of diabetic nephropathy in uninephrectomized dogs. Kidney Int. 1994;46:161-169. 51. Rabelink TJ, Bakris GL. The renin-angiotensin system in diabetic nephropathy: the endothelial connection. Miner Electrolyte Metab. 1998;24:381-388. 52. Mancini GB, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease: the TREND (Trial on Reversing ENdothelial Dysfunction) study. Circulation. 1996;94:258-265. 53. The CONSENSUS Trial Study Group. Effect of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavia Enalapril Survival Study. N Engl Med J. 1987;316:1429-1435. WWW.ARCHINTERNMED.COM ©2000 American Medical Association. All rights reserved.