* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Program of and Mechanisms of Fertilization in the Echinoderm
Survey
Document related concepts
Transcript
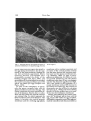
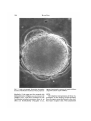
MER. ZOOL., 15:507-522 (1975). The Program of and Mechanisms of Fertilization in the Echinoderm Egg DAVID EPEL Marine Biology Research Division, Scripps Institution of Oceanography, University of California, San Diego, La folia, California 92037 SYNOPSIS. Current research on the mechanisms of sperm-egg fusion, the block to polyspermy, and metabolic activation are described. A cinemicrographic analysis of fertilization reveals that fusion of sperm and egg occurs between non-motile gametes, indicating that the flagellar motion of sperm is not required. The block to polyspermy is reviewed, emphasizing recent work on the role of cortical granule protease in altering sperm receptors of the vitelline layer. Metabolic activation or derepression at fertilization is highly regulated and occurs in a definite sequence. The primary event appears to be release of intracellular Ca2+. The timing of metabolic derepression is different in starfish oocytes. Here, a part of the derepression occurs during maturation and another part at fertilization. The contact between sperm and egg marks a turning point between life and death. Life if a successful fertilization occurs, since the resultant sperm-egg interaction will initiate the programmed development of a new individual. Death in the absence of such interaction, since the unfertilized egg will soon degenerate. For studies of fertilization, the gametes of echinoderms, especially those of sea urchins, have long provided an ideal model system. The major reasons for this are the ease of in vivo fertilization and development, the large amounts of available material, and the presence of a prolonged reproductive season. In this paper, I will describe three aspects of fertilization as learned from studies on sea urchin eggs. These are (i) the mechanisms of sperm attachment and sperm entry into the egg, (ii) the biochemical mechanisms for the block to polyspermy, and (iii) the mechanism of the programmed metabolic activation or metabolic derepression that occurs at fertilization. Finally, we will compare these studies on sea urchins to the gametes of other organisms. This article reports on research done in collaboration with Dr. Edward J. Carroll, Jr., Dr. Margaret Houk, Dr. Richard Steinhardt, Ms. Mia Tegner, and Dr. Victor Vacquier and supported by grants from the National Science Foundation and Population Council. A BRIEF OVERVIEW Ultrastructural aspects of the changes in sperm and egg and the fusion of the sperm-egg plasma membranes that accompany fertilization have been reviewed by a number of authors (recent reviews by Colwin and Colwin, 1967; Anderson, 1968; Austin, 1968; Millonig, 1969) and are also discussed by Summers et al. (1975). Briefly, fertilization can be viewed as a sequence of four successive membrane fusions. TYxfirst fusion takes place when the sperm makes contact with overlying egg jelly. This contact can be envisaged as a receptor-effector system which results in the fusion of sperm membranes and resultant extrusion of the acrosomal process (Dan, 1970). If this extrusion occurs in the immediate vicinity of the egg, the tip of this process will attach in a species-specific fashion to an extracellular layer of the egg, the vitelline layer. This attachment, seen in the scanning electron micrograph shown in Figure 1, is apparently to a sperm receptor protein of the vitelline layer. Aketa and his colleagues have recently isolated this protein from sea urchin eggs and find that (i) sperm attach to films of this protein in a species-specific fashion, (ii) the binding requires calcium, and (iii) antibodies to this protein will prevent fertilization (Aketa et al., 1972). It has long been realized that many ex- 507 508 DAVID EPEL FIG. 1. Scanning electron micrograph of sperm attached to vitelline layer of 5. purpuratus egg. (Courtesy of M. Tegner.) cess or supernumerary sperm also attach to the egg during insemination, but the magnitude of this supernumerary binding has not been previously appreciated. Recent scanning electron micrographs have dramatically revealed the extent of this binding (Tegner and Epel, 1973), and quantitation of the attachment has revealed that under saturating conditions up to 1500 sperm can attach per egg (Vacquier and Payne, 1973). In spite of this redundancy of sperm, only one sperm normally fuses with the plasma membrane and enters the egg. This fusion constitutes the second membrane/usion of fertilization. Shortly after or coincident with this fusion the cortical reactions are initiated (Fig. 2), which represent the third membrane fusion of fertilization. This fusion is between the cortical granule membrane and the overlying egg plasma membrane with a resultant exocytosis and extrusion of the cortical granule contents into the space between the overlying vitelline layer and the plasma membrane (see, e.g., Millonig, 1969). In eggs of Strongylocentrotus purpuratus, this fusion of corti- cal granule membrane and cell plasma membrane takes about 20 sec to propagate over the 75/i diameter egg (Paul and Epel, 1971) and results in the formation of a mosaic membrane with an 80% increase in the surface area of the egg. The contents of the granules are also involved in elevating (Vacquier et al., 1972a) and structuralizing (Bryan, 1970) the vitelline layer as it transforms into the fertilization membrane. During and following the cortical reaction, the "fertilizing" sperm fuses with the egg, rotates 180° within the egg cytoplasm, and begins a migration toward the egg pronucleus. Coincident with this movement is a PROGRAM OF FERTILIZATION plosmo membrane vilellme later attachment bj protease • sensitive bond FIG. 2. Representation of fertilization process of sea urchin egg, showing sperm detachment and cortical reactions. The vitelline layer contains at least two protease-sensitive sites, a sperm attachment site and a plasma membrane-vitelline layer attachment or connective. Many sperm attach to the layer but only one sperm, the fertilizing sperm, initiates the cortical reactions. This results in the propagated fusion of cortical granule membrane with plasma membrane and the release of the cortical granule constituents. One cortical granule protease alters sperm receptors so that supernumerary sperm detach; a second detaches connectives between plasma membrane and vitelline layer. Swelling or hydration of hydrophilic macromolecules elevates the now detached vitelline layer which is thus transformed into the fertilization membrane. swelling and "hydration" of the nucleus, activation of DNA synthesis and finally fusion of the two pronuclei. This fourth fusion of fertilization, which occurs about 20 min after insemination of the S. purpuratus egg, can be considered the end of the fertilization period. MECHANISMS OF SPERM FUSION AND ENTRY INTO THE EGG The mechanism of sperm entry into the egg has long rivetted the curiosity of embryologists. The important ultrastructural studies of the Colwin's (1967) reveal that sperm and egg plasma membranes fuse and suggest that fusion perse could provide the motive force for incorporating the sperm into the egg cytoplasm. Recent studies have revealed the presence of actin-like filaments within the acrosomal process (Tilney et al., 1973; Jessen et al., 1973). This suggests that contraction of an actomyosin-like system may be involved in carrying the sperm into the egg. Direct observations on the behavior of the sperm during sperm-egg fusion are remarkably limited. This results in large :; part from the difficulty of observing fusion i 509 and entry, since the process is so rapid and since only one of the many attached sperm will ultimately fuse with the egg. Cinemicrographic analysis had not proved useful, primarily because the eggs freely move when bombarded by sperm. J. Dan (1950), using the then new phase contrast microscope, briefly noted that the fertilizing sperm stops moving as soon as it makes contact with the egg. Collaborating with Naomi Epel, we recently completed a cinemicrographic study of fertilization, which was made possible by the technique of Steinhardt et al. (1971) of immobilizing eggs on a thin film of protamine sulfate. Our movies reveal that the fertilizing sperm becomes immobilized just before beginning its entry into the egg cytoplasm. Analysis of these films shows that each attached sperm (both the fertilizing sperm and supernumerary sperm) continuously moves around the axis of its attached acrosomal process. About 15 to 20 sec after the initial attachment, one sperm, which will be the fertilizing sperm, ceases this movement and literally stands up straight. Almost immediately thereafter the fertilization membrane begins to rise around this sperm and the other or supernumerary sperm are lifted away with the elevating fertilization membrane. Using single-frame analysis we have measured the rate of entry of the fertilizing sperm into the egg cytoplasm. Two types of behavior were observed and are illustrated in Figure 3. The upper curve depicts the case where no detectable inward movement was apparent for several seconds after motility had ceased. The lower curve depicts the case where the sperm began to move into the egg as soon as motility had ceased. Analysis of six cases reveals that, once initiated, the rate of entry is 5 to 11 microns per minute. Phase contrast microscope observations on living eggs indicate that the tail of the fertilizing sperm remains immobile during sperm entry. Figure 4 depicts three possible hypotheses accounting for entry of an immobilized sperm at a rate of 5 to 11 microns per minute. First, entry could be passive, the incorporation being simply a sequel to fusion as when two contiguous oil droplets fuse. In 510 DAVID EPEL ROLE OF THE CORTICAL GRANULE COMPONENTS IN THE BLOCK TO POLYSPERMY SECONDS AFTER MOVEMENT CEASES FIG. 3. T w o examples of rate of movement of the non-motile fertilizing sperm into the L. pictus egg. Measurements were m a d e from movies, using a single-frame projector with a surveying instrument to measure length of the sperm at the indicated times. this case (Models, Fig. 4), entry is simply the assumption of a most probable thermodynamic configuration. A second mechanism could result from actin-like filaments in the sperm or egg interacting with cytoplasmic or cortical myosin of the egg or sperm, leading to contraction and resultant sperm entry (Model B, Fig. 4). As a third mechanism, entry could occur via movement of the plasma membrane itself. Recent work has shown that the plasma membrane of many cells is fluid and in constant motion, moving at a rate of about 5 microns per minute (Edidin, 1972). If the site of sperm-egg fusion provides a directed source for membrane motion, such that membrane fluidity is directed towards the area of fusion, then such movement would incorporate the sperm into the egg as the egg membrane fuses with the sperm membrane (Model C, Fig. 4). A decision among these hypotheses cannot yet be made. However, comparative observations suggest a uniform mechanism of sperm entry among different eggs. First, membrane fusion always occurs. Second, actual entry may be between immobile gametes; in mammals, for example, motility of the fertilizing sperm also ceases during entry (Austin, 1961). All organisms have some mechanism to prevent multiple sperm entry. In eggs of most organisms this "block to polyspermy" occurs at the level of sperm egg-fusion, although in some species the block occurs at the level of fusion of the male and female pronucleus (see Austin, 1968). The consequences of polyspermy are usually lethal. In these cases, the chromosomes are segregated randomly and development aborts in early embryogenesis. Previous work on sea urchins has suggested the existence of two types of blocks. One is fast and incomplete, presumably occurring within the first few seconds of sperm-egg contact (Rothschild and Swann, 1952). The other is slow and complete, and is temporally and experimentally associated with the cortical reactions (reviewed by Allen, 1958). Isolation of cortical granules and cortical granule products Studies on the late block have been considerably advanced by the development of methods for obtaining and studying the cortical granule components and their relationship to blocking polyspermy. Two basic procedures are available: (i) direct isolation of the cortical granules as cell particles or (ii) collection of the "fertilization product" or "cortical exudate" following fertilization or artificial parthenogenesis. The first approach has been especially developed by passive filaments from spurn filaments plasma membrane fluidity - movsment e FIG. 4. Three models accounting for incorporation of the non-motile sperm into the egg. Further details in text. PROGRAM OF FERTILIZATION 511 Schuel and his collaborators, using the zonal centrifuge (Schuel et al., 1972). This method yields granules of high purity, but of poor structural integrity. To overcome this latter problem, I have recently developed a simple discontinuous gradient method which yields intact cortical granules, (Fig. 5) but in only 80% purity in terms of organelle content (described in Vacquier et al, 1973). The experimental approach used most in our studies involves characterization of the fertilization product, i.e., the components released by eggs upon fertilization or artificial activation. As noted (Figs. 2, 6), the cortical granule components are normally released into the space between the plasma membrane and the overlying vitelline layer. If the vitelline layer is removed before insemination or activation, the soluble cortical granule components will instead be released into the supernatant sea water (Fig. FIG. 5. Electron micrograph of cortical granules isolated by differential centrifugation procedure. Method described in Vacquier et al. (1973). 512 DAVID EPEL FP IN NORMAL EGG \t molecular weight FPentrapped in FP IN 0EMEMBRAN6TE0 EGG soluble FP molecules in seawoter fertilization space FIG. 6. Diagram depicting principle of obtaining the fertilization product (FP) from sea urchin eggs. In the normal egg, the FP is extruded into the space between the fertilization membrane and egg (perivitelline space or fertilization space). If the precursor of the fertilization membrane is removed beforehand, the FP is extruded directly into the surrounding sea water. 6) and can be collected after centrifugal removal of the eggs. A non-enzymatic procedure for removing the vitelline layer has proved especially useful (Epel et al., 1970). A third procedure is to extract and solubilize the cortical granule components from intact eggs by suspension of the eggs in an isoosmotic non-electrolyte media. In such media, the eggs are parthenogenetically activated and the cortical granule contents are released. Since there are no divalent cations, the components are released in a soluble form. This procedure, first suggested by Moore (1949) has been further developed by Kane and Stephens (1969). Analysis of the fertilization product has led to the description and characterization of two enzymes, a /3, 1-3 glucanase (Epel et al., 1969; Muchmore et al., 1969) and a trypsin-like protease (Vacquier et al., 1972a). Both of these enzymes have also been localized in the cortical granules (Schuel et al., 1972; Vacquier et al., 1973). Bryan (1970) has also described and characterized a structural protein of the granules, probably involved in the "hardening" or structuralization of the fertilization membrane. /3, 1-3 glucanase The biological role of this enzyme is unclear, but it does not appear to be involved in the block to polyspermy. Indeed, the activity of this enzyme is not concerned solely with fertilization, since only 40% of the enzyme is released following fertilization of 5. purpuratus eggs (Epel et al., 1969). An even smaller proportion of the glucanase is released from L. pictus eggs. The remaining glucanase is contained within small cytoplasmic granules (Epel et al., 1969) and is secreted slowly during cleavage such that approximately 90% of the remaining enzyme is released into the supernatant sea water by the time of hatching (Epel and Vacquier, unpublished). A possible role of the glucanase is in the formation of the hyaline layer as it forms after fertilization. The major protein of this layer, hyalin, is contained in the cortical granules and released at fertilization (Kane and Stephens, 1969). Recent studies suggest that hyalin is continuously secreted during early development (Kane, 1974). Since the glucanase is also released at fertilization and continuously secreted, it may be involved in the processing or attachment of hyalin to cell surface. Comparative studies on the glucanase indicate its presence in at least six genera of sea urchins including Allocentrotus fragilis, Arbacia punctulata, S. purpuratus, andL. pictus (Epel, unpublished), Toxopneustes roseus, Tripneustes depressus, and Arbacia incisa (Vacquier, unpublished). Surprisingly, the glucanase is not present in eggs of the sea urchin Echinometra vanbrunti (Vacquier, unpublished) and the sand dollar, Dendraster excentricus (Vacquier, 1971). It is also not present in eggs of the starfish Patiria miniata (Houk and Epel, unpublished). These data suggest that there may be considerable diversity centering around the fertilization reactions. Although the end result is the same, the exact molecular mechanisms may vary. Protease Lundblad (1954) first described the presence of several proteases in sea urchin eggs and noted marked changes in activity following insemination. He postulated that one of these protease activities was involved with the cortical reactions and elevation of the fertilization membrane. Several years ago we showed that a trypsin-like protease activity was contained in the fertilization product (Vacquier et al., 1972a) and subsequent experiments revealed that this ac- PROGRAM OF FERTILIZATION tivity could be localized in the cortical granules (Schuel et al., 1973; Vacquier et al., 1973). The protease is trypsin-like on the basis of its specificity towards synthetic substrates, pH optimum, and inhibition by natural and synthetic trypsin inhibitors (Vacquier et al., 1972a). The biological role of this enzyme has been approached in two different ways. The first determined the effects on fertilization of prior incubation of the eggs in the crude fertilization product or in the purified protease. The second determined the effects on fertilization when eggs were fertilized in the presence of protease inhibitors. Using these approaches, we can ascribe two actions to the protease. These are (i) elevation of the vitelline layer as it transforms into the fertilization membrane and (ii) detachment of supernumerary sperm. Our results indicate that both of these actions are a part of the block to polyspermy. 513 bonds are hydrolyzed during the prior incubation in protease but that this hydrolysis, by itself, is not adequate to elevate the vitelline layer. We postulate that other components of the cortical granules are required for elevation. These may be the sulfated mucopolysaccharides of the cortical granules. If hydrophilic, these macromolecules would hydrate when the cortical granule contents are exposed to sea water. This swelling would then elevate the detached vitelline layer. These experiments also reveal that the fertilization product protease is highly specific, certainly much more specific than bovine pancreatic trypsin. Treatment of eggs with pancreatic trypsin will remove the vitelline layer (e.g., Berg, 1967; Epel, 1970), whereas treatment with the fertilization product protease only alters the vitelline layer-plasma membrane connectives. Alteration of sperm receptors. As noted ear- lier, the vitelline layer contains proteinaElevation of thefertilization membrane: Two ceous sperm receptor sites. During normal different types of evidence indicate that the fertilization, the fertilizing sperm must first protease action is involved in fertilization attach to one of these receptors, pass membrane elevation. First, if eggs are in- through the vitelline layer, fuse with the seminated or artificially activated in the plasma membrane and thence enter the presence of trypsin inhibitors, the fertiliza- egg. The vitelline layer receptors, however, tion membrane does not elevate properly. are not essential since the vitelline layer can Instead the vitelline layer remains attached be removed and fertilization can still occur to the plasma membrane at many points (e.g., Runnstrom, 1966). Also, one can reand the surface of the egg looks like a bub- move the fertilization membrane after ferbly rosette (Fig. 7) (Vacquier et al., 19726). tilization and "refertilize" such eggs This suggests that the vitelline layer is at- (Sugiyama, 1951; Tyler etal., 1956). These tached to the plasma membrane by results, therefore, indicate that a sperm reprotease-sensitive bonds and that the pro- ceptor system also is present on the plasma membrane of the egg. tease detaches these linkages. Several lines of evidence indicate that the The second type of evidence comes from recent experiments of Carroll on the effects fertilization product protease alters the of incubating unfertilized eggs in the pur- sperm receptor sites on the vitelline layer as ified fertilization product protease. If such a part of the block to polyspermy. First, if eggs are artificially activated in the pres- eggs are incubated in either the crude ference of trypsin inhibitors, normal fertiliza- tilization product or a purified protease tion membranes are elevated. Conversely, preparation, they are rendered unfertilizaif the eggs are not exposed to the purified ble. The vitelline layer is structurally alprotease but are activated in the presence tered, and sperm do not attach to this layer of trypsin inhibitors, the fertilization mem- and do not activate or fertilize the eggs brane does not elevate properly and is (Vacquier et al., 19726, 1973). If, however, blebbed and bubbly (Carroll and Epel, these non-fertilizable eggs are now incubated in pronase so that the altered vitelline 1975). Our interpretation of these experiments layer is removed, the eggs can then be feris that the vitelline layer-plasma membrane tilized by sperm (Vacquier et al., 1973). 514 DAVID EPEL FIG. 7. Light micrograph illustrating incomplete eggs are inseminated in presence of trypsin inhibitors elevation of fertilization membrane when S. purpuratus (lmgm/ml soybean trypsin inhibitor). Similarly, if the eggs are first treated with dithiothreitol or trypsin so as to remove the vitelline layer, and then incubated in the fertilization product protease, there is no effect on fertilizability (Vacquier et al., 1973). The simplest interpretation of these experiments is that during normal fertilization, the sperm must first attach to the vitelline layer receptor sites. This attachment PROGRAM OF FERTILIZATION permits penetration of the layer and subsequent fusion of the sperm membrane with the underlying cell membrane. When eggs are incubated in the highly specific fertilization product protease, this protease alters only the sperm receptors of the vitelline layer; it does not remove the vitelline layer to expose the underlying plasma membrane. Following incubation in fertilization product protease, therefore, the modified but still intact vitelline layer acts as a physical barrier between sperm and plasma membrane receptor. Recent results indicate the presence of two proteases in the fertilization product. One is specific for the sperm receptors and the other is involved in vitelline layer elevation through alteration of plasma membrane-vitelline layer connectives (Carroll and Epel, 1975). This proteolytic alteration of sperm receptor sites has a functional role in the prevention of polyspermy. Normally, the supernumerary sperm attached to the vitelline layer become detached as the membrane elevates (Tegner and Epel, 1973; Vacquier and Payne, 1973). This detachment does not occur in the presence of protease inhibitors. Rather, sperm remain attached to both elevated and non-elevated areas of the vitelline layer. Associated with insemination in the presence of trypsin inhibitors is an increased incidence of polyspermy (Vacquier et al., 19726, 1973). This suggests that the supernumerary sperm remaining attached to the vitelline layer can still fuse with the egg. Even under these conditions, however, only a small percentage of the attached sperm are successful in fusing with the egg (Tegner, 1973, and unpublished). This may be because not all sperm binding sites are equivalent or because not all sperm are functional (Tegner and Epel, 1973) or because sperm can enter eggs only in those areas where the vitelline layer and plasma membrane remain in tight apposition (Longo and Schuel, 1973). Observations on hamster eggs reveal that a sperm attachment-detachment sequence also occurs in this organism (Hartmann and Gwatkin, 1971) and that a protease released from the egg is involved in sperm detachment and the block to polyspermy. Barros and Yanagamachi (1971) have shown that a 515 cortical granule product results in loss of the sperm binding sites on the zona pellucida. Furthermore, the action of this cortical granule product on sperm detachment is prevented if the exudate is incubated in trypsin inhibitors (Gwatkin et al., 1973). In making comparative observations, it should be emphasized that the sequence of sperm attachment and detachment is not always apparent by direct microscopic observation of fertilization. With sea urchin eggs, for example, attachment is quite apparent, but the detachment phase is less dramatic and takes place over a period of several minutes. However, detachment is quite obvious when the eggs are fixed at various times after insemination in aldehyde fixatives. When such eggs are examined, sperm are found attached only to areas of the vitelline layer which have not yet elevated. Since detachment does not occur when eggs are fertilized in the presence of protease inhibitors, the protease action must alter the sperm-vitelline layer bond such that the attachment is no longer retained in the aldehyde fixatives. METABOLIC ACTIVATION OR METABOLIC DEREPRESSION During oogenesis, the developing oocyte is synthetically active, utilizing exogenous substances provided by its mother for growth. At some point, probably around maturation or ovulation, the egg becomes independent of these exogenous substrates and becomes metabolically quiescent or repressed. The exact extent of this repression is unclear. In the sea urchin oocyte as compared with the fertilized egg, the activities of respiration, transport, protein and RNA synthesis are considerably reduced and DNA synthesis is completely turned off. Numerous studies on sea urchin eggs have shown that fertilization "derepresses" metabolism and activates new metabolic pathways (for reviews see Monroy, 1965, 1973). These studies reveal a definite temporal sequence or program of metabolic derepression (Epel et al., 1969). The timetable or program for the S. pur- 516 DAVID EPEL puratus embryo is indicated in Figure 8. Changes for which definite times are known are indicated on the right; those for which times are tentative are indicated on the left. It is apparent that there are two distinct clusters of changes, a large number of "early reactions," including all those changes occurring in the first 60 sec after insemination and a number of "late reactions," beginning about 300 sec after insemination. The "early changes" begin at 3 sec, with alterations in the egg plasma membrane resulting in an influx of Na + ion and a consequent "fertilization action potential" (Steinhardt et al., 1971). Sperm entry and cortical reactions begin at about 25 to 30 sec (Paul and Epel, 1971). About 3 sec later, the cytoplasmic activation of NAD kinase begins (Epel, 1964a), followed about 15 sec later by a large and transient burst in respiration rate (Epel, 19646). It is postulated that during this early period there also occurs the rapid block to polyspermy (Swann and Rothschild, 1952), a release of intraTENTATIVE PLACEMENT DEFINITE IN PROGRAM PLACEMENT IN PROGRAM C o " release-ropid polyspermy block--; Glucose-6-P0, Dehydrog Shift No' influx — Cortical reaction — NAD kinase extracellular protease .gluconase fertilization membrane block to polyspermy Respiration Two- P- Dependent alterations of plasma membrane K'- conductance protein synthesis omino ocid, PO^ transport Qdenyl cyclase nucleoside transport cellular calcium (Mazia, 1937; Steinhardt and Epel, 1974), and a translocation of glucoses-phosphate dehydrogenase from a particulate to a soluble form (Isono and Yasumasu, 1968). The "late changes" begin about 5 min after fertilization and include plasma membrane changes resulting in development of potassium conductance (Steinhardt et al., 1971) and transport systems for amino acids (Epel, 1972), phosphate (Whiteley and Chambers, 1966), and nucleosides (Piatigorsky and Whiteley, 1965). There is also initiated a large increase in protein synthesis (Epel, 1967) and a change in the intracellular location of adenyl cyclase (Castaneda and Tyler, 1968). However, there are no fertilizationassociated changes in cyclic AMP (Yasumasu et al., 1973). Thus far, there are no definite changes known to take place during the "dark" period between 1 and 5 min. Energy requiring steps are needed for establishing potassium conductance (Steinhardt et al., 1972) and amino acid transport (Epel, 1972), but the nature and exact timing of these steps is unclear. In the following section, I will first describe some recent research, done in collaboration with Richard Steinhardt of the University of California, Berkeley, that indicates that an increase in free calcium may be close to the primary event of fertilization (Steinhardt and Epel, 1974). I will then analyze this derepression by examining the interrelationships between three of the socalled late events, K+-conductance, protein synthesis, and amino acid transport (Epel et al., 1974). Increases in free Ca2+ as a primary event In 1937, Mazia showed that intracellular free calcium increased after fertilization of Arbacia punctulata eggs. Was this change simply a result of activation? Or was it a Pronucltor fusion 20 polyodenylation j DNA synthesis primary cause of activation? If primary, ol m RNA I one should be able to derepress metabolism 90 *• I Division by increasing the intracellular content of FIG. 8. Timetable or program of post-fertilization free Ca2+. One means of accomplishing this changes in eggs of 5. purpuratus at 17°C. Events for which times are known are on the right; events for is with ionophorous antibiotics, which abolish the selective permeability of memwhich times are not exactly known are on the left. s1 517 PROGRAM OF FERTILIZATION branes to ions. These can be specific for monovalent cations, such as valinomycin, or specific for divalent cations, such as A23187 (see Pressman, 1973, for review). Steinhardt and I observed that when A23187 was applied in micromolar amounts to unfertilized sea urchin eggs, the membrane conductance changes and cortical reactions were activated just as in normal fertilization. Was A23187 acting as a full parthenogenetic agent? We examined a large number of the metabolic and structural changes that normally accompany fertilization and found that all of these are activated similar to the normal activation by sperm. These include respiration, synthesis of protein, DNA, and chromosome condensation. Indeed, the rate of activation was faster than other more classical, parthenogenetic agents. For example, activation by thymol or KC1 results in a delay in DNA synthesis (von Ledebur-Villiger, 1972) which is not seen with A23187. However, we have noted that developmental abnormalities begin around the time of the first mitotic division. Monasters develop, but the eggs do not divide. This may reflect toxic effects of the ionophore resulting from aberrations in Ca2+ metabolism, nonspecific side effects, or the need for secondary parthenogenetic treatments, such as incubation in hypertonic media. What cation is being affected by the ionophore and is thus activating the eggs? The two major divalent cations of sea water are calcium and magnesium. To determine whether the ionophore was affecting the transport of either of these ions into the egg, we measured cortical activations, respiration, and protein synthesis in ionically substituted media. To our surprise, we found that the composition of the external environment made no difference. Eggs could be activated in Ca2+-free or Mg2+free sea water, even in 0.55 M KC1 containing 2 mM EGTA. These results suggest that the ionophore might be acting through release of divalent cations within the cell. Calcium and magnesium are also the major divalent cations within the cell. When we estimated the content of free and bound Ca2+ and Mg2+, we found that the bulk of Ca2+ was bound but available for release, whereas Mg 2+ was always "free." We estimated the content of free and bound cations by preparing homogenates in various media and analyzing Mg2+ and Ca2+ in the 37,000 g pellet and supernatant. We found that most of the Mg2+ was in the supernatant, and hence "free," whereas most of the calcium was sedimentable and hence "bound." In some media, however, the "free" Ca 2 + content was high. These findings indicate that although Ca2+ is normally sequestered, it is available for release. We postulate that the ionophore is most likely affecting calcium distribution within the egg and that the release of this calcium from some intracellular store results in the activation of the egg. The store of Ca2+ may be a Ca2+-binding protein (Nakamura and Yasumasu, 1974). DEPENDENT AND INDEPENDENT DEREPRESS1ON Irrespective of whether or not Ca2+ release is the primary trigger leading to derepression, we can experimentally determine whether metabolic activations of fertilization are connected in series in a cause-effect (dependent) relationship or whether they are unconnected (independent) (Fig. 9). We have examined this question, looking at three "late" responses: K+-conductance, protein synthesis, and Na + -dependent amino acid transport. As noted, all of these changes begin at about 5 min after insemination. Our analysis, however, indicates no causal interconnections between any of Dependent pathway model -A — B— C— D Independent pathway model A —B -C -E FIG. 9. Two models accounting for the metabolic derepression occurring upon fertilization. 518 DAVID EPEL them (Epel et al., 1974). To examine the relationship between potassium conductance and protein synthesis, we abolished conductance by acidifying the sea water around the eggs. This alters the plasma membrane such that K+conductance does not take place (Steinhardt and Mazia, 1972). Even so, protein synthesis was activated under these conditions. Tupper (1974) has come to similar conclusions, using barium ion to prevent K+-conductance. There is also no apparent relationship between protein synthesis and amino acid transport. This is best seen in experiments where the metabolism of the egg is partially activated by incubation in sea water containing 1 mM NH4C1 or NH4OH, pH 9. This treatment has been shown to activate a number of metabolic changes, such as K+conductance, DNA synthesis and chromosome condensation (Steinhardt and Mazia, 1972; Mazia and Ruby, 1974; Mazia, 1974) and polyadenylation of RNA (Wilt and Mazia, 1974). Not initiated are the cortical reactions and Na + -conductance (Steinhardt and Mazia, 1972). We have found that although protein synthesis and K+conductance are initiated, Na + -dependent amino acid transport is not (Epel et al., OUTSIDE 1974). This indicates that although these three events are normally initiated coincidentally, they are not causally interconnected. Assuming release of intracellular Ca2+ is the primary activator, it may act as a master switch initiating a series of independent events. Indeed, the "early" events may all be dependently linked, whereas the "late" events are independent of each other (Epel et al., 1974). Some reactions are, of course, causally interconnected and I have indicated some of these as a flow diagram in Figure 10. In this diagram, the fertilization changes are depicted with reference to changes within the plasma membrane of the egg and the resultant intracellular and extracellular consequences. We hypothesize that the primary effect resulting from the interaction of the fertilizing sperm with the plasma membrane is the release of a store of intracellular Ca2+. This Ca2+ release affects both cytoplasm and plasma membrane. Possibly, the increased free Ca2+ induces No?-conductance. This Na+-conductance could be related to the hypothetical early block to polyspermy. Ca2+ is required for exocytosis and membrane fusion (e.g., Poste and Allison, 1973), and the release of intracellular Ca2+ could [late polyspermy I block I |spwm receptors I hydrophilic substances protease] PLASMA MEMBRANE INSIDE |dewtoproent"| EARLY CHANGES FIG. 10. A hypothetical flow diagram of fertilization, showing changes and possible causal relationships oc- LATE CHANGES curring in plasma membrane, cytoplasm and vitelline layer. 519 PROGRAM OF FERTILIZATION initiate cortical granule fusion. (Indeed, in long after the completion of meiosis. In the order to isolate intact cortical granules, other echinoderm classes, fertilization and chelating agents must be included in the meiosis (or maturation) are tightly coordihomogenizing media.) Increased cytoplas- nated. Does a similar program of metabolic mic Ca2+ could activate the transient res- derepression occur in these organisms as in piratory burst (Epel, 1969; Nagazawa et the sea urchin? Is derepression initiated by aL, 1970) and also activate the enzyme NAD maturation? Or by fertilization? kinase (Epel, 1967; Blomquist, 1973) and Margaret Houk and I have recently glycogen phosphorylase (Shoger et al., examined these questions, using eggs of the 1973). The resultant increase in NADP and starfish, Patiria miniata. We found that the glucose-6-phosphate would release starfish oocyte is similar to the sea urchin glucose-6-phosphate dehydrogenase from egg in that a major derepression in rate of the particulate to supernatant fraction respiration (Houk, 1974) and protein syn(Isono and Yasumasu, 1968). Changes in thesis (Houk and Epel, 1974) takes place. It this enzyme, plus the increased levels of is dissimilar in that the major changes occur NADP and glucose-6-phosphate could during maturation: fertilization results in maintain increased respiration of the eggs, no change in protein synthesis (Houk and which is primarily through the pentose Epel, 1974) and only a transient increase in phosphate pathway (Krahl, 1956). It is un- respiration (Houk, 1974). clear how protein synthesis is activated at Figure 11 shows the per cent incorporathe translational level, but activation of a tion of amino acid into protein at various cytoplasmic protease may be important times after initiation of maturation by (e.g., Monroy et al., 1965; Mano and 1-methyladenine. As seen, the level inNagano, 1970), perhaps to remove an in- creases twofold by 40 min and fivefold by 2 hibitory protein from the ribosomes, such hr. This protein synthesis is required for as described by Metafora et al. (1971). completion of meiosis; if protein synthesis Eventually, steps in the program result in is inhibited with pactamycin, meiosis is arinitiation of DNA synthesis, cleavage and rested at metaphase or anaphase. Fertilizadevelopment. tion has no effect on the rate of protein synthesis. This can be seen in Figure 12, which depicts an experiment measuring METABOLIC DEREPRESSION IN OTHER ORGANISMS protein synthesis after insemination. Ca2+ release (ionophore effects) in eggs of other organisms. Does this general picture of metabolic derepression, as described for sea urchins, hold for other organisms? Assuming that activation of eggs by A23187 results from a release of intracellular calcium, an increase in this cation may be the primary event in activation of all eggs. So far, we have found ionophore activation in eggs or oocytes of five different organisms including starfish (Patiria miniata), tunicates (Ciona intestinalis), amphibians (Xenopus laevis), and hamsters (Mesocricetus auratus) (Steinhardt et al., 1974). 40 80 120 160 200 MINUTES AFTER 1-MA STIMULATION Metabolic derepression during maturation and fertilization of starfish oocytes Fertilization of the sea urchin egg occurs FIG. 11. Incorporation of amino acid increases during 1-methyladenine-induced maturation of starfish (Patiria miniata). O, oocytes exposed to 1-methyladenine; A, unactivated oocytes. (Data of Houk and Epel, 1974). 520 DAVID EPEL A similar activation of protein synthesis during maturation has previously been described in amphibians (Smith and Ecker, 1970). Indeed, the parallels between starfish and amphibians are striking. In both, protein synthesis increases in response to a hormonal stimulus, and in both this protein synthesis is necessary for the completion of meiosis. In both, there is no increase in protein synthesis after fertilization. Information about the metabolic changes occurring during maturation and fertilization is lacking for the echinoderm classes other than the asteroids and echinoids. If maturation is also under hormonal control in these groups, their metabolic activation may be more similar to the starfish than to the sea urchin. Based on this limited comparative data, it is probable that in organisms where maturation and fertilization are closely coordinated, as in asteroids, amphibians, and other vertebrates, a partial derepression occurs during maturation and this derepression is completed at fertilization. Conversely, if maturation occurs long before fertilization as in the echinoids, the eggs are ovulated in a metabolically repressed state and are completely derepressed by fertilization. Since ionophore A23187 activates 60 120 MINUTES AFTER FERTILIZATION FIG. 12. Incorporation of amino acid is not altered by fertilization of P. miniata eggs. A, Unfertilized eggs; O, fertilized eggs. (Data of Houk and Epel, 1974.) both classes of eggs, the common event at fertilization may be an increase in the intracellular content of calcium. REFERENCES Aketa, K., K. Onitake, and H. Tsuzuki. 1972. Tryptic disruption of sperm binding site of sea urchin egg surface. Exp. Cell Res. 71:27-32. Allen, R. D. 1958. The initiation of development. Pages 17-66 in W. D. McElroy and B. Glass, eds., The chemical basis of development. Johns Hopkins Univ. Press, Baltimore. Anderson, E. 1968. Oocyte differentiation in the sea urchin Arbacia punctulata, with particular reference to the origin of cortical granules and their participation in the cortical reaction. J. Cell Biol. 37:514-539. Austin, C. R. 1951. Observations on the penetration of sperm into the mammalian egg. Aust. J. Sci. Res. B. 4:581-596. Austin, C. R. 1968. Ultrastructure of fertilization. Holt, Rinehart and Winston, New York. Barros, C, and R. Yanagimachi. 1971. Induction of zona reaction in golden hamster eggs by cortical granule material. Nature (London) 233:268-269. Berg, W. E. 1967. Some experimental techniques for eggs and embryos of marine invertebrates. Pages 767-776 in F. H. Wilt and N. K. Wessells, eds., Methods in developmental biology. T. Y. Crowell Co., New York. Blomquist, C. H. 1973. Partial purification and characterization of nicotinamide adenine dinucleotide kinase from sea urchin eggs. J. Biol. Chem. 248:7044-7048. Bryan, J. 1970. The isolation of a major structural element of the sea urchin fertilization membrane. J. Cell Biol. 44:635-644. Carroll, E. J., and D. Epel. 1975. Isolation and biological activity of the proteases released by sea urchin eggs following fertilization. Develop. Biol. 44:22-32. Castaneda, M., and A. Tyler. 1968. Adenyl cyclase in plasma membrane preparations of sea urchin eggs and its increase in activity after fertilization. Biochem. Biophys. Res. Commun. 33:782-787. Colwin, L. H., and A. L. Colwin. 1967. Membrane fusion in relation to sperm-egg association. Pages 295-368m C. B. Metz and A. Monroy, eds., Fertilization. Academic Press, New York. Dan, J. 1950. Sperm entrance in echinoderms observed with the phase microscope. Biol. Bull. 99: 399-411. Dan, J. 1970. Morphogenetic aspects of acrosome formation and reaction. Advan. Morphogenesis 8:1-40. Edidin, M. 1972. Aspects of membrane fluidity. Pages 15-25 in C. F. Fox, ed., Membrane research: biological membranes. Academic Press, New York. Epel, D. 1964a. A primary metabolic change of fertilization: interconversion of pyridine nucleotides. Biochem. Biophys. Res. Commun. 17:62-68. Epel, D. 19646. Simultaneous measurement of TPHN formation and respiration rollowing fertilization of PROGRAM OF FERTILIZATION 521 the sea urchin egg. Biochem. Biophys. Res. Comat fertilization. Exp. Cell Res. 81:301-311. mun. 17:69-73. Kane, R. E., and R. E. Stephens. 1969. A comparative Epel, D. 1967a. Early biochemical events after fertilistudy of the isolation of the cortex and the role of the zation of sea urchin eggs. Pages 17-35 in R. Deering calcium-insoluble protein in several species of sea and M. Trask, eds., The molecular aspects of deurchin egg. J. Cell Biol. 41:133-144. velopment. NASA publication CR-673, Clearing- Krahl, M. E. 1956. Oxidative pathways for glucose in house for Federal and Scientific Information, eggs of sea urchin. Biochim. Biophys. Acta 20: Springfield, Va. 27-33. Epel, D. 1967A. Protein synthesis in sea urchin eggs: a Longo, F. G., and H. Schuel. 1973. An ultrastructural "late" response to fertilization. Proc. Nat. Acad. Sci. examination of polyspermy induced by soybean U.S.A. 57:899-906. trypsin inhibitor in the sea urchin/1 rbaciapunclulata. Develop. Biol. 34:187-199. Epel, D. 1969. Does ADP regulate respiration following fertilization of sea urchin eggs? Exp. Cell Res. Lundblad, G. 1954. Proteolytic activity in sea urchin 58:312-319. gametes. Almquist and Wiksell, Stockholm. Epel, D. 1970. Methods for removal of the vitelline Mano, Y.,and H. Nagano. 1970. Mechanism of release membrane of the sea urchin eggs. II. Controlled of maternal messenger RNA induced by fertilizaexposure to trypsin to eliminate post-fertilization tion in sea urchin eggs. J. Biochem. (Tokyo) clumping of the embryos. Exp. Cell Res. 61:69-70. 67:611-628. + Epel, D. 1972. Activation of an Na -dependent amino Mazia, D. 1937. The release of calcium in Arbacia eggs acid transport system upon fertilization of sea urupon fertilization. J. Cell Comp. Physiol. 10:291chin eggs. Exp. Cell Res. 72:74-89. 304. Epel, D., B. C. Pressman, S. Elsaesser, and A. M. Mazia, D. 1974. Chromosome cycles turned on in unWeaver. 1969. The program of structural and fertilized sea urchin eggs exposed to NH4OH. Proc. metabolic changes following fertilization of sea urNat. Acad. Sci. U.S.A. 71:690-693. chin eggs. Pages 279-298 in G. Padilla, G. L. Whit- Mazia, D., and A. Ruby. 1974. DNA synthesis turned son, and I. Cameron, eds., The cell cycle: geneon in unfertilized sea urchin eggs by treatment with enzyme relationships. Academic Press, New York. NH4OH. Exp. Cell Res. 85:167-172. Epel, D., A. M. Weaver, and D. Mazia. 1970. Methods Metafora, S., L. Felicetti, and R. Gambino. 1971. The for removal of the vitelline membrane of sea urchin mechanism of protein synthesis activation after fertilization of sea urchin eggs. Proc. Nat. Acad. Sci. eggs. I. Use of dithiothreitol (Cleland reagent). Exp. U.S.A. 68:600-604. Cell Res. 61:64-68. Epel, D., R. A. Steinhardt, T. Humphreys, and D. Millonig, G. 1969. Fine structural analysis of the cortiMazia. 1974. An analysis of the partial metabolic cal reaction in the sea urchin egg: after normal ferderepression of sea urchin eggs by ammonia; the tilization and after electric induction. J. Submicrosc. existence of independent pathways in the program Cytol. 1:69-84. of activation at fertilization. Develop. Biol. 40: Monroy, A. 1965. Chemistry and physiology of fertili245-255. zation. Holt, Rinehart and Winston, New York. Epel, D., A. M. Weaver, A. V. Muchmore, and R. T. Monroy, A. 1973. Fertilization and its biochemical Schimke. 1969. /3-1,3-glucanase of sea urchin eggs. consequences, Addison-Wesley Module in Biology, Release from particles at fertilization. Science No. 7. Addison-Wesley Publishing Co., Reading, 163:294-296. Mass. Gwatkin, R. B. L., D. T. Williams, J. F. Hartmann, and Monroy, A., R. Maggio, and A. M. Rinaldi. 1965. ExM. Kniauz. 1973. The zona reactions of hamster and perimentally induced activation of the ribosomes of mouse eggs; production in vitro by a trypsin-like the unfertilized sea urchin egg. Proc. Nat. Acad. Sci. protease from cortical granules. J. Reprod. Fert. ' U.S.A. 54:107-111. 32:259-265. Moore, A. R. 1949. The relation of ions to the appearHartmann, J. F., and R. B. L. Gwatkin. 1971. Alteraance and persistence of the fertilization and hyaline tion of sites on the mammalian sperm surface folmembranes in the eggs of the sea urchin. Amer. lowing fertilization. Nature (London) 234:479-481. Natur. 83:233-246. Houk, M. 1974. Respiration of starfish oocytes during Muchmore, A. V., D. Epel, and R. T. Schimke. 1969. meiosis, fertilization and artificial activation. Exp. Purification and properties of an exo-/3Cell Res. 83:200-206. D(l,3)glucanase from sea urchin eggs. Biochem. Houk, M. S., and D. Epel. 1974. Protein synthesis Biophys. Acta 178:551-560. during hormonally induced meiotic maturation and Nakamura, M., and I. Yasumasu. 1974. Mechanism fertilization in starfish oocytes. Develop. Biol. 40: for increase in intracellular concentration of free 298-310. calcium in fertilized sea urchin egg. A method for Isono, N., and I. Yasumasu. 1968. Pathways of carestimating intracellular concentration of free calbohydrate breakdown in sea urchin eggs. Exp. Cell cium. J. Gen. Physiol. 63:374-388. Res. 50:616. Nakazawa, T., K. Asami, R. Shoger, A. Fujiwara, and Jessen, J., O. Behnke, K. G. Wingstrand, and J. I. Yasumasu. 1970. Ca+2 uptake, H + ejection and Rostgaard. 1973. Actin-like filaments in the acrespiration in sea urchin eggs on fertilization. Exp. rosomal apparatus of spermatozoa of a sea urchin. Cell Res. 63:143-146. Exp. Cell Res. 80:47-54. Paul, M., and D. Epel. 1971. Fertilization-associated Kane, R. 1974. Hyalin release during normal sea urlight-scattering changes in eggs of the sea urchin chin development and its replacement after removal Strongylocentrotus purpuratus, Exp. Cell Res. 65:281- 522 DAVID EPEL 288. Sugiyama, M. 1951. Re-fertilization of the fertilized Piatigorsky, J., and A. H. Whiteley. 1965. A change in eggs of the sea urchin. Biol. Bull. 101:335-344. permeability and uptake of CM-uridine in response Summers, R., B. L. Hylander, L. H. Colwin, and A. to fertilization in Strongyhcentrotus purpuratus eggs. L. Colwin. 1975. The functional anatomy of the Biochim. Biophys. Acta 108:404-418. echinoderm spermatozoon and its interaction with the egg at fertilization. Amer. Zool. 15:523-551. Poste, G., and Allison, A. C. 1973. Membrane fusion. Biochem. Biophys. Acta, Reviews on Biomem- Tegner, M. J. 1973. Protease plays a three part role against polyspermy in sea urchins. Amer. Zool. branes. 300:421-467. 13:1316. (Abstr.) Pressman, B. C. 1973. Properties of ionophores with broad range cation selectivity. Fed. Proc. 32:1698- Tegner, M. J., and D. Epel. 1973. Sea urchin spermegg interactions studied with the scanning electron 1704. microscope. Science 179:685-688. Rothschild, L., and M. M. Swann. 1952. The fertilization reaction in the sea urchin. The block to poly- Tilney, L. G., S. Hatano, H. Ishikawa, and M. Mooseker. 1973. The polymerization of actin: its spermy. J. Exp. Biol. 29:2169-2183. role in the generation of the acrosomal process of Runnstrom,J. 1966. The vitelline membrane and corcertain echinoderm sperm. J. Cell Biol. 59:109-126. tical particles in sea urchin eggs and their function in maturation and fertilization. Advan. Mor- Tupper,J. T. 1974. Inhibition of increased potassium permeability following fertilization of the phogenesis 5:221-325. echinoderm embryo; its relationship to the initiation Schuel, H., W. L. Wilson, R. S. Bressler, J. W. Kelley, of protein synthesis and potassium decompartmenand J. R. Wilson. 1972. Purification of cortical talization. Develop. Biol. (In press) granules from unfertilized sea urchin egg homogenates by zonal centrifugation. Develop. Biol. Tyler, A., A. Monroy, and C. B. Metz. 1956. Fertiliza29:307-320. tion of fertilized sea urchin eggs. Biol. Bull. Schuel, H., W. L. Wilson, K. Chen, and L. Lorand. 110:184-195. 1973. A trypsin-like protease localized in cortical Vacquier, V. D. 1971. The appearance of /3-l,3granules isolated from unfertilized sea urchin eggs glucanohydrolase activity during the differentiation by zonal centrifugation. Role of the enzyme in ferof the gut of sand dollar plutei. Develop. Biol. 26:1tilization. Develop. Biol. 34:175-186. 10. Shoger, R., K. Asami, I. Yasumasu, and A. Fujiwara. Vacquier, V. D., D. Epel, and L. A. Douglas. 1972a. Sea urchin eggs release protease activity at fertiliza1973. Activation of phosphorylase in sea urchin tion. Nature (London) 237:34-36. eggs by Ca+2 and 3'-5'-AMP. Exp. Cell Res. 82:375Vacquier, V. D., and J. E. Payne. 1973. Methods for 383. quantitating sea urchin sperm-egg binding. Exp. Smith, L. D., and R. E. Ecker. 1970. Foundations for Cell Res. 82:227-235. the expression of developmental potential. Pages 355-380 in R. Hanley, ed., Problems in biology: Vacquier, V. D., M. J. Tegner, and D. Epel. 19726. Protease activity establishes the block against polyRNA in development. Univ. Utah Press, Salt Lake • spermy in sea urchin eggs. Nature (London) City. 240:352-354. Steinhardt, R. A., and D. Epel. 1974. Activation of sea urchin eggs by a calcium ionophore. Proc. Nat. Vacquier, V. D., M. J. Tegner, and D. Epel. 1973. Protease released from sea urchin eggs at fertilizaAcad. Sci. U.S.A. 71:1915-1919. tion alters the vitelline layer and aids in preventing Steinhardt, R. A., D. Epel, E. J. Carroll, and R. polyspermy. Exp. Cell Res. 80:111-119. Yanagimachi. 1974. Is calcium ionophore a universal activator for unfertilized eggs? Nature (London) von Ledebur-Villiger, M. 1972. Cytology and nucleic 252:41-43. acid synthesis of parthenogenetically activated sea urchin eggs. Exp. Cell Res. 72:285-308. Steinhardt, R. A., L. Lundin, and D. Mazia. 1971. Bioelectric responses of the echinoderm egg to fer- Whiteley, A. H. and E. L. Chambers. 1966. Phosphate tilization. Proc. Nat. Acad. Sci. U.S.A. 68:2426transport in fertilized sea urchin eggs. II. Effects of metabolic inhibitors and studies on differentiation. 2430. J. Cell Physiol. 68:309-324. Steinhardt, R. A., and D. Mazia. 1972. Development of + K -conductance and membrane potentials in unfer- Wilt, F. H., and D. Mazia. 1974. The stimulation of cytoplasmic polydenylation in sea urchin eggs by tilized sea urchin eggs after exposure to NH4OH. ammonia. Develop. Biol. 37:422-424. Nature (London) 241:400-401. Steinhardt, R. A., S. Shen, and D. Mazia. 1972. Mem- Yasumasu, I., A. Fujiwara, and K. Ishida. 1973. Periodic change in the content of adenosine 3'-5'brane potential, membrane resistance and an cyclic monophosphate with close relation to the cycle energy requirement for die development of potasof cleavage in the sea urchin egg. Biochem. Biophy. sium conductance in the fertilization reaction of echinoderm eggs. Exp. Cell Res. 72:195-203. Res. Comm. 54:628-632.