* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Mechanisms of increase in cardiac output during acute
Coronary artery disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Jatene procedure wikipedia , lookup
Myocardial infarction wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
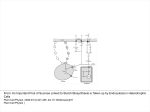
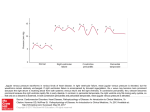
J Appl Physiol 111: 407–411, 2011. First published June 2, 2011; doi:10.1152/japplphysiol.01188.2010. Mechanisms of increase in cardiac output during acute weightlessness in humans Lonnie G. Petersen,1 Morten Damgaard,2 Johan C. G. Petersen,1 and Peter Norsk1 1 Department of Biomedical Sciences, Faculty of Health Sciences, University of Copenhagen, Copenhagen; and 2Department of Clinical Physiology and Nuclear Medicine, Hvidovre University Hospital, Hvidovre, Denmark Submitted 8 October 2010; accepted in final form 2 June 2011 regional blood flow; blood pressure; vascular resistance; gravity GRAVITY CONSTANTLY STRESSES the cardiovascular system in upright humans by diminishing venous return. It decreases stroke volume (SV) and thus cardiac output (CO) and, through the baroreflex system, induces arteriolar constriction and an increase in heart rate (HR) to counteract a fall in blood pressure (4, 21, 30). Compared with the upright 1-G position, sudden entry into weightlessness (0 G) causes a redistribution of blood from the lower vascular beds to the central circulation (4, 29), leading to an increase in venous return, and thus in SV and CO, and, through stimulation of the baroreflexes, to arteriolar vasodilatation and a decrease in HR (18, 21, 23, 29). Our laboratory has previously observed that, in upright seated humans, short-term 0 G created by parabolic airplane flights increases CO by as much as 1.8 l/min, which corresponds to some 0.6 liters over the 20-s period of 0 G (21). Bailliart et al. (2) estimated that, in standing humans, some 165 ml of fluid disappeared from each leg during 20 s of 0 G. These and our observations collectively suggest that the contribution of blood from the legs to the acute 0-G-induced increase in CO amounts to some 50%. In another study from our laboratory, Address for reprint requests and other correspondence: L. G. Petersen, Dept. of Biomedical Sciences, Faculty of Health Sciences, Univ. of Copenhagen, Blegdamsvej 3, DK-2200 Copenhagen, Denmark (e-mail: [email protected]). http://www.jap.org we have, however, observed that water immersion in humans, which is used to simulate the effects of 0 G, increases the diameter of the left atrium to the same degree, whether or not the leg venous vasculature is separated from the rest of the circulation by arterial or venous occlusion (13, 14). This indicates that the majority of the blood translocated from the peripheral to the central circulation during immersion originates from the vascular beds above the legs. In this study, we, therefore, tested the hypothesis that, in upright seated humans, most of the blood redistributed from the lower to the central circulation by 0 G originates from vascular beds above the legs, such as the splanchnic. We also tested the hypothesis that 0 G would increase SV and CO to levels above those of the 1-G supine position, because we have previously observed an increase in transmural central venous pressure in supine subjects, who are suddenly exposed to 0 G (29). Thus we expected 0 G to further increase SV and CO through the Frank-Starling mechanism because of abolishment of the 1-G-induced front to back compression of the thoracic cage (⫹1 Gx). SUBJECTS AND METHODS The protocol was approved by the medical board of the European Space Agency (ESA) and considered acceptable and in compliance with the declaration of Helsinki by the Ethics Committee of Copenhagen (KF 07, 09/26/2006). Eight healthy volunteers [age ⫽ 35 yr (range 26 – 48 yr); weight ⫽ 75 kg (range 52–94 kg); height ⫽ 179 cm (range 156 –192 cm); and male-to-female ratio ⫽ 5/3] participated in the study. All fulfilled the general health criteria required to obtain a Joint Aviation Administration class II physical medical flight certificate and had given their written, informed consent. Subjects with any disease or abnormality were excluded. On the day of flight, the test subject had a light breakfast 1.5–2 h before the experiment was carried out. No medication against motion sickness was administered. All of the subjects had participated in at least one previous parabolic flight campaign. The study was carried out in connection with two ESA parabolic flight campaigns: nos. 42 and 44 in Bordeaux, France. The parabolic maneuvers were conducted in a modified Airbus A-300. Each campaign consisted of 3 consecutive days of flight, and, on each day, 30 parabolic flight maneuvers were carried out in series of 5, leaving a few minutes of 1 G in between each parabolic maneuver and 5–15 min of 1 G in between each series of 5 parabolas. Each parabolic maneuver consisted of three 20-s phases by following a parabolic trajectory: an acceleration phase, where the G force gradually increased from 1.0 to 1.8; a weightless phase (0 G), where the aircraft suddenly entered a free-fall condition, and finally a deceleration phase, where the G force abruptly reached 1.8 and thereafter gradually faded back to 1.0. Experimental procedure. In the airplane, ambient temperature varied between 19 and 23°C and humidity between 12 and 17% at a cabin pressure of 850 millibars. 8750-7587/11 Copyright © 2011 the American Physiological Society 407 Downloaded from http://jap.physiology.org/ by 10.220.33.5 on June 16, 2017 Petersen LG, Damgaard M, Petersen JCG, Norsk P. Mechanisms of increase in cardiac output during acute weightlessness in humans. J Appl Physiol 111: 407– 411, 2011. First published June 2, 2011; doi:10.1152/japplphysiol.01188.2010.—Based on previous water immersion results, we tested the hypothesis that the acute 0-Ginduced increase in cardiac output (CO) is primarily caused by redistribution of blood from the vasculature above the legs to the cardiopulmonary circulation. In seated subjects (n ⫽ 8), 20 s of 0 G induced by parabolic flight increased CO by 1.7 ⫾ 0.4 l/min (P ⬍ 0.001). This increase was diminished to 0.8 ⫾ 0.4 l/min (P ⫽ 0.028), when venous return from the legs was prevented by bilateral venous thigh-cuff inflation (CI) of 60 mmHg. Because the increase in stroke volume during 0 G was unaffected by CI, the lesser increase in CO during 0 G ⫹ CI was entirely caused by a lower heart rate (HR). Thus blood from vascular beds above the legs in seated subjects can alone account for some 50% of the increase in CO during acute 0 G. The remaining increase in CO is caused by a higher HR, of which the origin of blood is unresolved. In supine subjects, CO increased from 7.1 ⫾ 0.7 to 7.9 ⫾ 0.8 l/min (P ⫽ 0.037) when entering 0 G, which was solely caused by an increase in HR, because stroke volume was unaffected. In conclusion, blood originating from vascular beds above the legs can alone account for one-half of the increase in CO during acute 0 G in seated humans. A Bainbridge-like reflex could be the mechanism for the HR-induced increase in CO during 0 G in particular in supine subjects. 408 ACUTE WEIGHTLESSNESS AND VENOUS RETURN piece from and into a bag containing 1.5 liters of gas mixture of 1% freon-22 (blood-soluble tracer gas), 1% sulfur hexafluoride (SF6, blood-insoluble tracer gas), and 35% oxygen in nitrogen. The fraction of the gases was measured by an infrared photoacoustic gas analyzer in sampled inspired and expired air through a tube connected to the mouthpiece (6). From the disappearance rate of end-expiratory freon-22, pulmonary blood flow was calculated, which is equal to CO (6, 10). SF6 was used to correct for inadequate mixing of air in the lungs with the gas mixture from the rebreathing bag and for estimating changes in breathing volume. At least 5 min passed between each rebreathing in each subject to ensure adequate clearance of tracer gases from the blood (8). Breathing was timed so that the subjects exhaled, held their breath very shortly, and, on entering 0 G, pushed a switch to commence rebreathing. By afterwards comparing the temporal profiles of the changes in G and the disappearance rate of freon-22, care was taken to ensure that rebreathing had, in fact, taken place only during the desired G phase. MAP and HR were measured continuously by an infrared photoplethysmographic technique (Portapress) in an index finger, and the presented values are averages of the full 20 s at each level of G, with (1 G or 0 G) or without (1.8 G) simultaneous rebreathing. The hydrostatic reference level for MAP measurements during 1 G and 1.8 G was chosen at the fourth intercostal space, when the subjects were seated, and at the midaxillary line, when they were supine. A height sensor between the Portapress cuff on the finger and the reference point was used for this purpose. Data analysis and statistics. SV was calculated by dividing CO by HR, and total peripheral vascular resistance (TPR) by dividing MAP by CO. All data are presented as means ⫾ SE. A one-way ANOVA (SigmaStat 2.03) for repeated measurements was performed to evaluate the effects of each intervention on each variable. Statistical significant differences between selected mean values (P ⬍ 0.05) were Fig. 1. Schematic drawing of the parabolic trajectory in combination with the experimental protocol, with emphasis on cuff inflation (CI) and measurements of cardiac output, mean arterial pressure, and heart rate during the different phases of the flight. CR, cuff release. Shaded bar, CI; striped bar, rebreathing; solid bar, mean arterial pressure and heart rate. J Appl Physiol • VOL 111 • AUGUST 2011 • www.jap.org Downloaded from http://jap.physiology.org/ by 10.220.33.5 on June 16, 2017 On each day of flight, two subjects were investigated, spending the first 20 parabolic maneuvers in the seated and the last 10 in the supine position. Mean arterial pressure (MAP) and HR were recorded continuously, and CO measurements were performed during 20-s periods, during either 1 G or 0 G, and alternating between the two subjects, thus maximizing the total number of measurements performed in each of the subjects and allowing them to rest during every other parabola. Measurements were performed during the following conditions (Fig. 1): seated in between the series of parabolic maneuvers while flying straight and level at 1 G, with or without bilateral thigh-cuff inflation (CI) of 60 mmHg, and allowing for at least 5 min of rest following the previous parabolic maneuver. We also performed the measurements during 0 G, with and without CI, inflating the cuffs immediately before the 1.8-G parabolic phase and maintaining cuff pressure and venous occlusion throughout 0 G (0 G ⫹ CI). Furthermore, we also released the cuff pressure (CR ⫽ cuff release) immediately upon entering 0 G (0 G ⫹ CR). The sequence of the procedures (1 G, 0 G, 0 G ⫹ CI, and 0 G ⫹ CR) was randomized between the subjects and performed once, twice, or three times in each subject, and the data thereafter were averaged for each procedure. Finally, with the subjects supine throughout the parabolic maneuver, we likewise measured MAP and HR continuously and CO during 1 and 0 G. CI was achieved by manual inflation of a standard cuff of appropriate size (21 ⫻ 53 cm) around each upper thigh to 60 mmHg above ambient pressure. This pressure was chosen as the best compromise between ensuring occlusion of leg venous return while causing minimal disruption of arterial flow both during 1 G and when the hydrostatic pressure gradients were augmented during 1.8 G (11). The cuff pressure during CI was registered electronically by a transducer and was 59.0 ⫾ 0.3 mmHg. Measurements. CO was measured by a noninvasive rebreathing technique (Pulmonary Function System, ESA), described in detail elsewhere (6, 8, 10). Measurements were performed by having the subject breathe at a rate of 18 ⫾ 3 breaths/min through a mouth- ACUTE WEIGHTLESSNESS AND VENOUS RETURN evaluated by a post hoc multiple-range test (Newman-Keuls) or a paired, two-sided t-test. RESULTS Seated. In the seated position, 0 G increased CO by 1.7 ⫾ 0.4 l/min from a 1-G value of 7.3 ⫾ 0.7 l/min to a 0-G value of 9.0 ⫾ 0.9 l/min (P ⬍ 0.001, Fig. 2). This increase was attenuated during 0 G ⫹ CI, where CO only increased by 0.8 ⫾ 0.4 l/min from a 1-G ⫹ CI value of 7.0 ⫾ 0.7 l/min (n ⫽ 8) to a 0-G ⫹ CI value of 8.3 ⫾ 1.0 l/min (n ⫽ 7) (P ⫽ 0.026), which was significantly less than when the cuffs were not inflated (P ⫽ 0.028, Fig. 2). Releasing the 60-mmHg cuff pressure upon entering 0 G (0 G ⫹ CR) did not increase CO further compared with when the cuff pressure was maintained 409 DISCUSSION Fig. 2. Cardiac output, stroke volume, and heart rate in seated subjects during 1 G, 0 G, 1 G ⫹ CI (venous CI of 60 mmHg), 0 G ⫹ CI, and 0 G ⫹ CR (venous CI during 1.8 G, followed by cuff pressure release on initiation of 0 G). bpm, Beats/min. The bars represent means ⫾ SE of n ⫽ 8, except for during 0 G ⫹ CI, where n ⫽ 7. *Significant difference compared with 1 G, if not otherwise indicated. J Appl Physiol • VOL The main findings of this study are that vascular beds above those of the legs can alone account for the 0-G induced increase in SV and for some 50% of the increase in CO, because, during 0 G ⫹ CI, SV was increased to its maximum, corresponding to the flat portion of the Starling curve. Thus reestablishing venous return from the legs during 0 G did not cause any further increase in SV, but did surprisingly cause an increase in HR, leading to a significant 50% further increase in CO. Hence, we suggest that the increase in CO during acute 0 G in seated humans consists of two factors, which are both induced by hydrostatic redistribution of blood from the more peripheral, caudad vasculature to the central circulation: 1) an increase in SV to the flat portion of the Starling curve, which can be obtained solely by translocation of blood from the splanchnic to the cardiopulmonary circulation; and 2) an additional increase in HR, which causes the remaining increase in CO and could be explained by a Bainbridge-like reflex, because HR was higher 111 • AUGUST 2011 • www.jap.org Downloaded from http://jap.physiology.org/ by 10.220.33.5 on June 16, 2017 throughout the parabolic maneuver (8.4 ⫾ 0.9 vs. 8.3 ⫾ 1.0 l/min, Fig. 2). 0 G increased SV to a similar extent independently of whether the cuffs were inflated or not, from a 1-G value of 96 ⫾ 15 ml to a 0-G value of 110 ⫾ 15 ml (P ⬍ 0.001), and from a 1-G ⫹ CI value of 93 ⫾ 14 ml to a 0-G ⫹ CI value of 111 ⫾ 16 ml (P ⬍ 0.001), and finally from a 1-G value of 96 ⫾ 15 ml to a 0-G ⫹ CR value of 106 ⫾ 15 ml (P ⬍ 0.001, Fig. 2). Thus the attenuated increase in CO during 0 G ⫹ CI compared with when the thigh cuffs were not inflated was entirely caused by a lower HR (Fig. 2). The lower CO during 0 G ⫹ CR vs. during 0 G was caused by a combination of a slightly lower SV and HR, which, however, were not statistically significant. During 1.8 G, HR increased from 80 ⫾ 6 to 89 ⫾ 4 beats/min (P ⫽ 0.017). This increase was abolished by CI (83 ⫾ 4 beats/min, Table 1), and, during the subsequent 0 G ⫹ CI, HR decreased to 76 ⫾ 6 beats/min, which was significantly lower than when CI was not applied during the preceding 1.8 G (Fig. 2). During 0 G ⫹ CR, inflation of the cuffs again abolished the 1.8-G induced increase in HR (Fig. 2, Table 1). However, when the cuff pressure was released upon entering 0 G (0 G ⫹ CR), HR was not decreased and was significantly higher compared with 0 G ⫹ CI (P ⫽ 0.03, Fig. 2). MAP decreased during 0 G from a 1-G seated value of 99 ⫾ 6 to 95 ⫾ 6 mmHg (P ⬍ 0.05). This decrease was still present during 0 G ⫹ CI and 0 G ⫹ CR (Table 1). Because MAP decreased during 0 G, while CO increased, TPR decreased from 14.2 ⫾ 1.1 to 11.2 ⫾ 1.1 mmHg·l⫺1·min (P ⬍ 0.001, Table 1). 0 G ⫹ CI still caused a decrease in TPR to 12.3 ⫾ 1.4 mmHg·l⫺1·min (P ⬍ 0.001, Table 1), which, however, was significantly less compared with when the venous return from the legs was intact (P ⬍ 0.017). The intervention of 40 s of CI without variations in G (1 G vs. 1 G ⫹ CI) showed a decrease in MAP from 99 ⫾ 6 to 96 ⫾ 5 mmHg (P ⬍ 0.05), whereas no other cardiovascular variables were affected (Table 1, Fig. 2). Supine. In the supine position, 0 G increased CO by 0.8 ⫾ 0.3 l/min, from 7.1 ⫾ 0.7 to 7.9 ⫾ 0.8 l/min (P ⫽ 0.037, Table 2). This was caused entirely by an increase in HR from 73 ⫾ 5 to 79 ⫾ 5 beats/min (P ⬍ 0.001, Table 2), leaving SV unchanged. 410 ACUTE WEIGHTLESSNESS AND VENOUS RETURN Table 1. CO, SV, HR, MAP, and TPR during same conditions as described in Fig. 2 and additionally during 1.8 G, 1.8 G ⫹ CI, and 1.8 G ⫹ CR CI Seated 1G CO, l/min SV, ml HR, beats/min MAP, mmHg TPR, mmHg 䡠 l⫺1 䡠 min 7.3 ⫾ 0.7 96 ⫾ 15 80 ⫾ 6 99 ⫾ 6 14.2 ⫾ 1.1 1.8 G 89 ⫾ 4 95 ⫾ 5 0G 1G 9.0 ⫾ 0.9* 110 ⫾ 15* 84 ⫾ 6 95 ⫾ 6* 11.2 ⫾ 1.1* 7.0 ⫾ 0.7 93 ⫾ 14 79 ⫾ 6 96 ⫾ 5* 14.4 ⫾ 1.3 1.8 G 83 ⫾ 4 94 ⫾ 5 CR 0G 1G 8.3 ⫾ 1.0* 111 ⫾ 16* 76 ⫾ 6† 95 ⫾ 7* 12.3 ⫾ 1.4*† 7.3 ⫾ 0.7 96 ⫾ 15 80 ⫾ 6 99 ⫾ 6 14.2 ⫾ 1.1 1.8 G 85 ⫾ 4 96 ⫾ 5 0G 8.4 ⫾ 0.9* 106 ⫾ 15* 82 ⫾ 5† 91 ⫾ 5* 11.6 ⫾ 1.2* Values are means ⫾ SE; n ⫽ 8 subjects except for 0 G ⫹ CI, which is based on n ⫽ 7. CO, cardiac output; SV, stroke volume; HR, heart rate; MAP, mean arterial pressure; TPR, total peripheral vascular resistance; 1.8 G ⫹ CI, 1.8 G combined with venous thigh cuff inflation; 1.8 G ⫹ CR, 1.8 G followed by release of the cuff inflation immediately after completion of 1.8 G. Significant difference compared with *1 G during the same intervention, and †0 G without cuffs: P ⬍ 0.05. Table 2. CO, SV, HR, MAP, and TPR during 1.8 G, 1.8 G, and 0 G in the supine position Supine 1G CO, l/min SV, ml HR, beats/min MAP, mmHg TPR, mmHg 䡠 l⫺1 䡠 min 7.1 ⫾ 0.7 101 ⫾ 15 73 ⫾ 5 91 ⫾ 5 13.5 ⫾ 1.2 1.8 G 71 ⫾ 5† 92 ⫾ 4 0G 7.9 ⫾ 0.8* 104 ⫾ 15 79 ⫾ 5* 92 ⫾ 4 12.5 ⫾ 1.3 Values are mean ⫾ SE; n ⫽ 8 subjects. Significant difference compared with *1 G and †0 G: P ⬍ 0.05. J Appl Physiol • VOL Because, however, the effect of the 1.8-G phase was abolished during both 0 G ⫹ CI and 0 G ⫹ CR, thus ensuring identical pre-0-G conditions, the higher HR in the latter condition may be explained by an additional venous return from the legs to the central circulation, activating a Bainbridge-like reflex. The notion of a Bainbridge-like reflex is supported by our observations, when the subjects were supine, where the increase in CO during 0 G was purely a result of an unexpected increase in HR of 6 ⫾ 5 beats/min. This increase might theoretically have been initiated by a Bainbridge-like reflex, as previously suggested in studies concerning acute central volume loading by head-down tilt (5, 28), or by intravenous infusion (3, 22), and/or by mechanisms intrinsic to the heart, such as direct stretch of the sinus node (25), because our laboratory has previously shown that the transmural central venous pressure increases during 0 G in supine humans (29). This is also in accordance with previous water immersion observations by our laboratory (17), where HR was more suppressed during immersion to the midchest than to the neck, despite that central transmural filling pressures were higher during neck immersion. It may be argued that hyperventilation during the rebreathing maneuver, which was performed at 1 G and 0 G, but not during 1.8 G, may have affected the cardiovascular parameters (1, 12). However, active hyperventilation has not been shown to have a significant effect on MAP (16), nor does breathing rate or breathing volume change CO (8, 15, 16, 19). Furthermore, it has been reported that HR does not change during short-term hyperventilation (7, 19, 27). Thus existing evidence does not support that cardiovascular variables are significantly affected by the rebreathing maneuver itself. The unchanged SV during 0 G, when the subjects were supine, was also somewhat unexpected because of our laboratory’s previously observed increase in cardiac distension pressures (29), which, through the Frank-Starling mechanism, should have increased SV. Apparently this was not the case. One explanation could be that, in the 1-G supine position, the relationship between SV and preload is on the upper flat portion of the Starling curve, so that an increase in cardiac preload has no further effect on SV. Conclusion. Our results indicate that vascular areas above those of the legs in seated humans can alone account for some 50% of the increase in CO during acute 0 G induced by parabolic flights. Furthermore, our results surprisingly indicate that the remaining increase in CO during 0 G is caused by an accelerated HR for which the origin of blood cannot be 111 • AUGUST 2011 • www.jap.org Downloaded from http://jap.physiology.org/ by 10.220.33.5 on June 16, 2017 during 0 G and 0 G ⫹ CR than during 0 G ⫹ CI. Whether this suggestion is correct remains, however, to be further explored. In the supine position, the increase in CO during 0 G is caused by a higher HR leaving SV unchanged. Thus an increase in transmural central venous pressure and preload (29) induced by abolishment of the front to back thoracic compression is probably the cause for an augmented venous return and a Bainbridge-like increase in HR. Possible mechanisms. Whether the cardiac preload during 0 G was diminished by occlusion of venous return from the legs cannot be deducted from this study with certainty. Regardless, however, of whether cardiac preload was affected or not by CI, SV increased to the same extent during 0 G, with or without occlusion. Thus blood from the leg vasculature was not a necessity for increasing SV during 0 G. Acute weightlessness did not decrease HR to below the 1-G control value, as expected compared with effects of simulation experiments, such as an upright-to-supine body posture change, head-down tilt, or head-out water immersion (4, 9, 13, 14, 17, 26, 28, 29). One might speculate that a possible mechanism for the lack of a decrease in HR during 0 G could be that the preceding 1.8 G stimulated HR by activation of the sympathetic nervous system through inhibition of the baroreflexes, and that this activation counteracted the effects of the subsequent short-term 0-G phase. Support for this view is the fact that 1.8 G ⫹ CI did not increase HR (Table 1) and that it was lower during the subsequent 0 G ⫹ CI (Fig. 2 and Table 1). The thigh cuffs in this way could have acted as a modified anti-G suit, shifting the venous hydrostatic indifference point cranially, thus counteracting a decrease in preload. This view is supported by results of Montmerle and Linnarsson (20), who found that 70 mmHg of lower body positive pressure during centrifugation of 2 G was sufficient to prevent a decrease in SV. ACUTE WEIGHTLESSNESS AND VENOUS RETURN ACKNOWLEDGMENTS We are grateful to the European Space Agency, in particular Vladimir Pletser and supporting staff, the company Novespace, and all of the subjects who supported and participated in our study. GRANTS This study was supported by Grants 2105-04-0006 and 272-07-0614 from the Danish Research Councils. DISCLOSURES No conflicts of interest, financial or otherwise, are declared by the author(s). REFERENCES 1. Angelone A, Coulter NA Jr. Respiratory sinus arrhythmia: a frequency dependent phenomenon. J Appl Physiol 19: 479 –482, 1964. 2. Bailliart O, Capderou A, Cholley BP, Kays C, Riviére D, Téchoueyres P, Lachaud J, Vaïda P. Changes in lower limb volume in humans during parabolic flight. J Appl Physiol 85: 2100 –2105, 1998. 3. Barbieri R, Triedman JK, Saul JP. Heart rate control and mechanical cardiopulmonary coupling to assess central volume: a systems analysis. Am J Physiol Regul Integr Comp Physiol 283: R1210 –R1220, 2002. 4. Blomqvist CG, Stone HL. Cardiovascular adjustments to gravitational stress. In: Handbook of Physiology. The Cardiovascular System. The Heart. Bethesda, MD: Am. Physiol. Soc., 1983, sect. 2, vol. III, pt. 2, chapt. 28, p. 1025–1063. 5. Bundgaard-Nielsen M, Sørensen H, Dalsgaard M, Rasmussen P, Secher NH. Relationship between stroke volume, cardiac output and filling of the heart during tilt. Acta Anaesthesiol Scand 53: 1324 –1328, 2009. 6. Clemensen P, Christensen P, Norsk P, Grønlund J. A modified photoand magnetoacoustic multigas analyser applied in gas exchange measurements. J Appl Physiol 76: 2832–2839, 1994. 7. Cummin AR, Ivawe VI, Mehta N, Saunders KB. Ventilation and cardiac output during the onset of excercise, and during voluntary hyperventilation, in humans. J Physiol 370: 567–583, 1986. 8. Damgaard M, Norsk P. Effects of ventilation on cardiac output determined by inert gas rebreathing. Clin Physiol Funct Imaging 25: 142–147, 2005. J Appl Physiol • VOL 9. Gabrielsen A, Johansen LB, Norsk P. Central cardiovascular pressures during graded water immersion in humans. J Appl Physiol 75: 581–585, 1993. 10. Gabrielsen A, Videbæk R, Schou M, Damgaard M, Kastrup J, Norsk P. Non invasive measurements of cardiac output in heart failure patients using a new foreign gas rebreathing technique. Clin Sci (Lond) 102: 247–252, 2002. 11. Groothuis JT, Vliet LV, Kooijman M, Hopman MTE. Venous cuff pressure from 30 mmHg to diastolic pressure are recommended to measure arterial inflow by plethysmography. J Appl Physiol 95: 342–347, 2003. 12. Guyton AC, Hall JE. Cardiac arrhythmias and their electrocardiographic interpretation. In: Textbook of Medical Physiology (11th Ed.), edited by Belfus L, Schmitt W, Gruliow R. Philadelphia, PA: Elsevier Saunders, 2006, unit III, chapt. 13, p. 148. 13. Johansen LB, Jensen TUS, Pump B, Norsk P. Contribution of abdomen and legs to central blood volume expansion in humans during immersion. J Appl Physiol 83: 695–966, 1997. 14. Johansen LB, Pump B, Warberg J, Christensen NJ, Norsk P. Preventing hemodilution abolishes natriuresis of water immersion in humans. Am J Physiol Regul Integr Comp Physiol 275: R879 –R888, 1998. 15. Kallay MC, Hyde RW, Fahey PJ, Utell MJ, Peterson BT, Ortiz CR. Effect of the rebreathing pattern on pulmonary tissue volume and capillary blood flow. J Appl Physiol 58: 1881–1894, 1985. 16. Kety SS, Schmidt CF. The effects of active and passive hyperventilation on cerebral blood flow, cerebral oxygen consumption, cardiac output and blood pressure of normal young men. J Clin Invest 25: 107–119, 1946. 17. Larsen AS, Johansen LB, Stadeager C, Warberg J, Christensen NJ, Norsk P. Volume homeostatic mechanisms in humans during graded water immersion. J Appl Physiol 77: 2832–2839, 1994. 18. Lathers CM, Charles JB, Elton KF, Holt TA, Mukai C, Bennett BS, Bungo MW. Acute hemodynamic responses to weightlessness in humans. J Clin Pharmacol 29: 615–627, 1989. 19. Matalon S, Dashkoff N, Nesarajah MS, Klocke FJ, Farfi LE. Effects of hyperventilation on pulmonary blood flow and recirculation time of humans. J Appl Physiol 52: 1161–1166, 1982. 20. Montmerle S, Linnarsson D. Cardiovascular effects of anti-G suit inflation at 1 and 2 G. Eur J Appl Physiol 94: 235–241, 2005. 21. Norsk P, Damgaard M, Petersen L, Gypel M, Pump B, Gabrielsen A, Christensen NJ. Vasorelaxation in Space. Hypertension 47: 69 –73, 2006. 22. Pawelczyk JA, Levine BD. Cardiovascular responses to rapid volume infusion: the human Bainbridge reflex (Abstract). Circulation 92: I656, 1995. 23. Pump B, Videbæk R, Gabrielsen A, Norsk P. Arterial pressure in humans during weightlessness induced by parabolic flight. J Appl Physiol 87: 928 –932, 1999. 24. Rowell LB. Human Cardiovascular Control. New York: Oxford University Press, 1993. 25. Slovut DP, Wenstrom JC, Moeckel RB, Wilson RF, Osborn JW, Abrams JH. Respiratory sinus dysrythmia persists in transplanted human hearts following autonomic blockade. Clin Exp Pharmacol Physiol 25: 322–330, 1998. 26. Stadeager C, Johansen LB, Warberg J, Christensen NJ, Foldager N, Bie P, Norsk P. Circulation, kidney function and volume-regulating hormones during prolonged water immersion in humans. J Appl Physiol 73: 530 –538, 1992. 27. Van De Borne P, Montano N, Narkiewicz K, Degaute JP, Malliani A, Pagani M, Somers VK. Importance of ventilation in modulating interaction between sympathetic drive and cardiovascular variability. Am J Physiol Heart Circ Physiol 280: H722–H729, 2001. 28. Van Lieshout JJ, Harms MPM, Pott F, Jenstrup M, Secher NH. Stroke volume of the heart and thoracic fluid content during head-up and head-down tilt in humans. Acta Anaesthesiol Scand 49: 1287–1292, 2005. 29. Videbæk R, Norsk P. Atrial distension in humans during microgravity induced by parabolic flights. J Appl Physiol 83: 1862–1866, 1997. 30. Watenpaugh DE, Hargens AR. The cardiovascular system in microgravity. In: Handbook of Physiology. Environmental Physiology. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 4, vol. I, chapt. 29, p. 631–674. 111 • AUGUST 2011 • www.jap.org Downloaded from http://jap.physiology.org/ by 10.220.33.5 on June 16, 2017 determined from the results of this study. We suggest that a Bainbridge-like reflex exists in humans, which accounts for some 50% of the increase in CO during acute 0 G. This is supported by our observation that, in supine humans, the increase in CO during 0 G compared with 1 G is only secondary to an increase in HR. Perspectives. It is of fundamental interest in physiology to investigate whether the human cardiovascular system possesses a Bainbridge-like reflex or other intrinsic mechanisms that regulate HR during acute changes in G stress. According to Rowell (24), humans do not possess a Bainbridge reflex, or, at least, it is very weak. This is probably very true, when central filling pressures vary between those in the upright and supine body position, whereas when central filling exceeds that of supine, a Bainbridge or similar reflex may play a role in humans. The results in this study indicate so. Thus acute 0 G during parabolic flights, in particular in supine humans, might be a unique way of studying the existence of such a fundamental mechanism, because cardiac distension can be created without intervening hydrostatic gradients. 411