* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Thermodynamics Chapter 4
Calorimetry wikipedia , lookup
Reynolds number wikipedia , lookup
Heat transfer physics wikipedia , lookup
Conservation of energy wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Internal energy wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Adiabatic process wikipedia , lookup
Non-equilibrium thermodynamics wikipedia , lookup
Thermodynamic system wikipedia , lookup
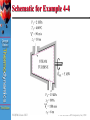
4 CHAPTER Çengel Boles Thermodynamics The First Law of Thermodynamics: Control Volumes Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-1 Velocity Profiles for Flow in a Pipe (fig. 4-6) Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, © The McGraw-Hill Companies,Inc.,1998 Inc.,1998 4-2 Volume Flow Rate Volume flow rate is the volume of fluid flowing through a cross section per unit of time Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-3 Mass Flow, Heat, and Work Affect Energy Content The energy content of a control volume can be changed by mass flow as well as heat and work interactions Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-4 Control Volume May Involve Boundary, Electrical, and Shaft Work (Fig. 4-9) Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-5 Schematic for Flow Work (Fig. 4-10) Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-6 During Steady Flow Process, Volume Flow Rates are not Necessarily Conserved (Fig. 4-19) Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-7 A Water Heater Under Steady Operation . Çengel Boles . . (Fig. 4-21) . Thermodynamics . Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-8 Steady-Flow Devices Operate Steadily for Long Periods (Fig. 4-25) Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-9 Nozzle and Diffuser Shapes Cause Large Changes in Fluid Velocities Nozzles and Diffusers are shaped so that they cause large changes in fluid velocities and thus kinetic energies (Fig. 4-27) Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-10 Schematic for Example 4-2 Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-11 Schematic for Example 4-4 Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-12 Throttling Valve Devices Cause Large Pressure Drops in Fluid (Fig.4-32) Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-13 Ideal Gas Temperature Does Not Change During a Throttling The temperature of an ideal gas does not change during a throttling(h =constant) process since h = h (T) Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-14 T-Elbow Serves as Mixing Chamber for Hot and Cold Water Steams The T-ebow of an ordinary shower serves as the mixing chamber for hot- and cold-water streams. (Fig. 4-35) Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-15 Heat Transfer Via Heat Exchanger Depends on System Selection The heat transfer associated with a heat exchanger may be zero or nonzero depending on how the system is selected Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-16 Schematic for Example 4-9 . Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-17 Rigid Tank Charging From a Supply Line is an Unsteady-Flow Process Charging of a rigid tank from a supply line is an unsteady-flow process since it involves changes within the control volume (Fig. 4-47) Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-18 Temperature of Steam Rises Entering Tank, Flow Energy Converts to Internal Energy The Temperature of Steam rises from 300 to 456°C as it enters a tank as a result of flow energy being converted to internal energy (Fig. 4-54) Çengel Boles o Thermodynamics o Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-19 Enthalpy of a Saturated Vapor at a Given Pressure In a pressure cooker, the enthalpy of the existing steam is Hg@P (enthalpy of the saturated vapor at the given pressure) Çengel Boles Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-20 Çengel Boles Chapter Summary Thermodynamics • A control volume differs from a closed system in that it involves mass transfer. Mass carries energy with it, and thus the mass and energy content of a system change when mass enters or leaves. Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-21 Çengel Boles Chapter Summary • The mass and energy balances for any system undergoing any process can be expressed as Thermodynamics Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-22 Çengel Boles Chapter Summary Thermodynamics • The mass and energy balances for any system undergoing any process can be expressed in the rate form as Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-23 Çengel Boles Thermodynamics Third Edition Chapter Summary • Mass flow through a cross section per unit time is . called the mass flow rate and is denoted m. It is expressed as where = density, kg/m3 (= 1/v) = average fluid velocity normal to A, m/s A = cross-sectional area, m2 WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-24 Çengel Boles Chapter Summary Thermodynamics • The fluid volume flowing through a cross section . per unit time is called the volume flow rate V. It is given by Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-25 Chapter Summary Çengel Boles Thermodynamics • The mass and volume flow rates are related by Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-26 Çengel Boles Chapter Summary Thermodynamics • Thermodynamic processes involving control volumes can be considered in two groups: steadyflow processes and unsteady-flow processes. During a steady-flow process, the fluid flows through the control volume steadily, experiencing no change with time at a fixed position. The mass and energy content of the control volume remain constant during a steady-flow process. Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-27 Çengel Boles Thermodynamics Third Edition Chapter Summary • Taking heat transfer to the system and work done by the system to be positive quantities, the conservation of mass and energy equations for steady-flow processes are expressed as for each exit for each inlet where the subscript i stands for inlet and e for exit. These are the most general forms of the equations for steady-flow processes. WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-28 Chapter Summary • For single-stream (one-inlet--one-exit) systems such as nozzles, diffusers, turbines, compressors, and pumps, the steady flow equations simplify to Çengel Boles Thermodynamics In the above relations, subscripts 1 and 2 denote the inlet and exit states, respectively. Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-29 Çengel Boles Chapter Summary Thermodynamics • During a uniform-flow process, the state of the control volume may change with time, but it may do so uniformly. Also, the fluid properties at the inlets and the exits are assumed to remain constant during the entire process. The conservation of energy equation for a uniformflow process reduces to Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998 4-30 Çengel Boles Chapter Summary Thermodynamics • When the kinetic and potential energy changes associated with the control volume and the fluid streams are negligible, the conservation of energy equation for a uniform-flow process simplifies to Third Edition WCB/McGraw-Hill © The McGraw-Hill Companies, Inc.,1998