* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Nitrogen Cycle
Survey
Document related concepts
Human microbiota wikipedia , lookup
Microorganism wikipedia , lookup
Bacterial cell structure wikipedia , lookup
Magnetotactic bacteria wikipedia , lookup
Phospholipid-derived fatty acids wikipedia , lookup
Bacterial morphological plasticity wikipedia , lookup
Transcript
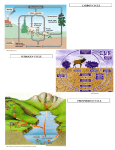
General Ecology (BIO 160) The Nitrogen Cycle Dr. Jim Baxter Sacramento State THE NITROGEN CYCLE As a component of proteins, enzymes, nucleic acids and light-‐harvesting pigments, such as chlorophyll, nitrogen (N) is a key element in biological organisms. In plants, total N ranges from between 1-‐4% of dry mass. Plants take up N from the soil as either nitrate (NO3-‐) or ammonium (NH4+); most plants take up N as nitrate. The nitrogen cycle (see figure below) is characterized by many transitions in the oxidation states of N, with the primary reservoir of N being in the atmosphere as N2 gas. This form of N is not available to plants, except via N-‐fixing bacteria. Nitrogen gas is also fixed and can become available to plants by lightening conversion and industrial fertilizer manufacturing. The process of denitrification returns N to the atmosphere. Although N is typically thought of as a nutrient that is beneficial to biological organisms, it may also be a pollutant (e.g., derived from combustion reactions). Atmospheric N2 (~79% of air) is a large but relatively inert reservoir, accessible only to a few bacterial forms. Living and dead organic material (e.g., proteins, nucleic acids, humus) is also a fairly large reservoir, but smaller than that in the atmosphere. Inorganic N salts (e.g., NO3-‐, NH4+) are very small, rapidly cycled reservoirs. These salts are present in soils and aquatic environments and are the forms on N that are available for uptake by biological organisms. N2 N2O N fixation NH4+ NO AmmonifiAssimil N cation ation it ri fi -‐ c NO2 Biomass -‐ NO2 atio R-‐NH 2 n Assimilation R = organic molecule NO3-‐ The Nitrogen Cycle Steps in the nitrogen cycle Nitrogen fixation: This is the way N becomes available to organisms from its gaseous atmospheric source (N2). The process of N fixation compensates for losses of available N by denitrification. It is an energy-‐demanding process (i.e., ~150 Kcal/mole N2). 1 General Ecology (BIO 160) The Nitrogen Cycle Dr. Jim Baxter Sacramento State Free-‐living N fixers – Azotobacter, Azospirillum, Clostridium are all free-‐living heterotrophic bacteria. Because of the high energy demand of the process, these bacteria do not fix a large amount of N (1-‐3 kg N/ha/yr). Some Cyanobacteria (blue-‐green algae) also fix nitrogen. Because they are photosynthetic, they can fix up to 50-‐100 kg N/ha/yr. Symbiotic N fixers – Rhizobium bacteria occur in root nodules of leguminous plants (Fabaceae family). These bacteria may fix up to 150-‐200 kg N/ha/yr. There are also many other non-‐leguminous symbiotic N fixing associations (e.g., alder trees with actinomycetes). Assimilation: This step involves the uptake of N (either as NH4+ or NO3-‐) into biological organisms. Ammonification: This is the liberation of N from dead organic matter. It is a bacterial process that occurs in both terrestrial and aquatic environments. R-‐NH2 NH4+ Nitrification: This is a two-‐step bacterial process whereby ammonium is first oxidized to nitrite and to nitrate. The bacteria that perform these reactions are called nitrifiers and they are chemosynthetic. Step I. NH4+ NO2-‐ (Performed by Nitrosomonas, Nitrosocystis) Step II. NO2-‐ NO3-‐ (Performed by Nitrobacter, Nitrocystis) Most higher plants N in NO3-‐ form. However, NO3-‐ leaches out of soils rapidly, while NH4+ is held by ion exchange forces to clay particles in the soil. Denitrification: Denitrification occurs in anaerobic environments (e.g., flooded soil and anoxic sediments). It depletes available N and may threaten the ozone layer by production of N20 (nitrous oxide). NO2-‐ NO N20 N2 2