* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Phloem-Specific Expression of Tyrosine/Dopa
Plant physiology wikipedia , lookup
Plant ecology wikipedia , lookup
Plant secondary metabolism wikipedia , lookup
Plant reproduction wikipedia , lookup
Evolutionary history of plants wikipedia , lookup
Plant use of endophytic fungi in defense wikipedia , lookup
Plant breeding wikipedia , lookup
Plant morphology wikipedia , lookup
Perovskia atriplicifolia wikipedia , lookup
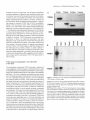
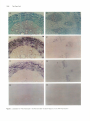
The Plant Cell, Vol. 7, 1811-1821, November 1995 O 1995 American Society of Plant Physiologists Phloem-Specific Expression of Tyrosine/Dopa Decarboxylase Genes and the Biosynthesis of lsoquinoline Alkaloids in Opium Poppy Peter J. Facchini’ and Vincenzo De Luca lnstitut de Recherche en Biologie Végétale, 4101 rue Sherbrooke Est, Département de Sciences Biologiques, Universite de Montréal, Montréal, Québec, H1X 262 Canada Tyrosineklopa decarboxylase (TYDC) catalyzes the formation of tyramine and dopamine and represents the first steps in the biosynthesis of the large and diverse group of tetrahydroisoquinoline alkaloids. Opium poppy accumulates morphine in aerial organs and roots, whereas sanguinarine, which is derived from a distinct branch pathway, accumulates only i n roots. Expression of the TYDC gene family in opium poppy was investigated i n relation to the organ-specific biosynthesis of these different types of alkaloids. Members of the TYDC gene family are classified into two groups (represented by TYDCl and TYDCP) and are differentially expressed. In the mature plant, TYDCP-like transcripts are predominant i n stems and are also present in roots, whereas TYDCl-like transcripts are abundant only i n roots. I n situ hybridization analysis revealed that the expression of TYDC genes i s developmentally regulated. TYDC transcripts are associated with vascular tissue i n mature roots and stems but are also expressed in cortical tissues at earlier stages of development. Expression of TYDC genes i s restricted t o metaphloem and to protoxylem i n the vascular bundles of mature aerial organs. Localization of TYDC transcripts i n the phloem i s consistent with the expected developmental origin of laticifers, which are specialized internal secretory cells that accompany vascular tissues in all organs of select species and that contain the alkaloid-rich latex in aerial organs. The differential expression of TYDC genes and the organ-dependent accumulation of different alkaloids suggest a coordinated regulation of specific alkaloid biosynthetic genes that are ultimately controlled by specific developmental programs. INTRODUCTION Few plants possess the combined historic and contemporary importance, notoriety,and mystique of the opium poppy. Among the first civilizations to cultivate poppy were the Sumerians, inhabitants, at the end of the third millennium B.C., of what is now Iraq. The Sumerian name for opium poppy was hul gil or “plant of joy”(Brownstein, 1993), suggesting that the opium extracted from its seed capsules was used as a narcotic. Pharmaceutical applications of opium, or opium derivatives, have occurred throughout human history. In 1500 B.C. the Ebers Papyrus (Brownstein, 1993) described it as a remedy to prevent the excessive crying of children. In 1898, with the synthesis and widespread availability of 0,O-diacetylmorphine (heroin), it was used as a “cough suppressant.” Today, worldwide use of opium is estimated to be in excess of one million kilograms annually (Roberts, 1988). lsolation in 1806 of the principal active ingredient in opium, a weak base named morphine after the Greek god of dreams, also marked the beginning of study in the field of plant alkaloid chemistry and biosynthesis. 1 To whom correspondence should be addressed. Current address: Department of BiologicalSciences, University of Calgary, 2500 University Drive N.W., Calgary, Alberta, T2N 1N4 Canada. Morphine belongs to the largest and most diverse class of naturally occurring alkaloids,which includes compoundsbased on the tetrahydroisoquinoline nucleus. In total, some 40 isoquinoline alkaloids, representing many different structural types, have been isolated from opium (Preininger, 1985).Opium consists of the dried latex exuded from unripe seed capsules of opium poppy. The latex represents the multinucleate cytoplasm of fused cells, known as laticifers, that comprise a highly specialized internal secretory system (Fairbairn and Kapoor, 1960; Nessler and Mahlberg, 1977, 1978, 1979). Mature laticifers possess numerous isoquinoline alkaloid-rich (Fairbairn and Djote, 1970; Fairbairn et al., 1974; Rush et al., 1985) and membrane-boundvesicles that are derived from dilations of the endoplasmic reticulum (Nessler and Mahlberg, 1977, 1978, 1979). In addition to the phenanthrene alkaloids, which include the analgesics morphine and codeine, other important classes of tetrahydroisoquinoline alkaloids found in opium poppy include the benzylisoquinolines, such as the vasodilator papaverine and the antispasmodic noscapine, and the benzophenanthri- dines, such as the antibiotic sanguinarine (Phillipson, 1983; Preininger, 1985). Classic radiotracer experiments and modern applications of plant cell culture technology have contributed to our knowledge 1812 The Plant Cell of the chemistry and enzymology of specific tetrahydroisoquinoline branch pathways. For example, the unique biosynthetic pathways from the common branch point intermediate(S)-reticuline to either morphine (reviewed in Brochmann-Hanssen, 1985) or sanguinarine (reviewed in Kutchan and Zenk, 1993) are well established (Figure 1). Many of the enzymes that catalyze these multistep pathways have also been characterized (Zenk, 1985; Gerardy and Zenk, 1993; Kutchan and Zenk, 1993). However, despite extensive work on alkaloid chemistry and the characterization of many biosynthetic enzymes, the molecular mechanisms that regulate the activity of key enzymes and control the synthesis of specific alkaloids are not well understood. The precise chemistry and the nature of enzymes involved in the early steps of isoquinoline alkaloid biosynthesis are less well characterized. Most tetrahydroisoquinoline alkaloids are derived from (S)-norcoclaurine (Stadler et al., 1987, 1989), which is formed via the condensation of dopamine and 4-hydroxyphenylacetaldehyde(Figure 1). Both precursors originate from L-tyrosine, or ~-3,4-dihydroxyphenylalanine(dopa) in the case of dopamine, with the initial and potentially rate-limiting steps catalyzed by a tyrosineldopa decarboxylase (TYDCldopa decarboxylase; EC 4.1.1.25; Figure 1). Key regulatory functions are often associated with enzymes, such as phenylalanine ammonia-lyase (EC 4.3.1.5; Hahlbrock and Scheel, 1989), that operate at the interface of primary and secondary metabolism. A similar regulatory function has been suggested for TYDC and dopa decarboxylase (Marques and Brodelius, 1988; Kawalleck et al., 1993). Despite almost 200 years of intensive research on the chemistry and biosynthesisof morphine and related alkaloids, much remains to be learned about the basic biology of alkaloid biogenesis in poppy and other plants. For example, it has long been known that isolated latex can convert radioactive amino acid and amine precursors into phenanthrene alkaloids (Fairbairn and Wassel, 1964a, 1964b; Fairbairn et al., 1968; Fairbairn and Djote, 1970). In addition, enzymes of both general metabolism (Antoun and Roberts, 1975) and alkaloid biosynthesis, including TYDC (Roberts, 1971, 1974; Roberts and Antoun, 1978; Roberts et al., 1983), have been detected in poppy latex. However, little is known about the relationships between plant development, cellular differentiation, alkaloid biosynthetic gene expression, and accumulation Of specific alkaloids in opium poppy. In this study, we examined the differential and tissue-specific expression of the TYDC gene family (Facchini and De Luca, 1994) as a putative marker for the localization and developmental regulation of tetrahydroisoquinoline alkaloid biosynthesis in opium poppy. To determine the precise cell-specific localization of TYDC genes, we used in situ RNA hybridization techniques with probes specific for two differentiallyexpressed subgroups of WDC genes (representedby WDC7 and WDCP). We demonstrated that TYDC gene expression becomes developmentally restricted to the vascular tissues in stems and roots. WDC7-like transcripts are most abundant in root phloem, whereas TYDCP-like transcripts are predominant in the ............ .- Llsc , ROOTS e (R-CHJ v ROOTSISHOOTS Figure 1. Biosynthesis of Morphine, Codeine, and Sanguinarine in Opium Poppy. The proposed biosynthetic pathway from L-tyrosine to the common tetrahydroisoquinoline intermediate (S)-norcoclaurineand the branch pathways from (S)-reticulineto the benzophenanthridine alkaloid sanguinarine and the phenanthrenealkaloids codeine and morphine. The sites of action of tyrosine/dopa decarboxylase (TYDCIDODC)and berberine bridge enzyme (BBE) are indicated. metaphloem of the mature stem. The low leve1 of TYDC gene expression in carpels contrasts sharply with the abundance of alkaloid-rich latex that can be extracted from seed capsules. These data suggest that carpelslcapsules are sites of alkaloid accumulation and that stems and roots are more likely the sites of alkaloid biosynthesis. The major alkaloids found in aerial organs were morphine and noscapine, whereas sanguinarine was found at high levels only in roots. Thus, the.differentia1 expression of TYDC genes in stems and roots and the accumulation of specific alkaloids in these organs suggest that different developmental programs may regulate the biosynthesisof specific secondary products. RESULTS The TYDC Gene Family Exhibits Differential and Organ-Specific Expression Four members of the TYDC gene family in opium poppy have been cloned and characterized previously (Facchini and De Luca, 1994). Full- and partial-length cDNA and genomic clones for cTY DC1, cTY DC2, cTY DC3, and TYDC4 were separated by agarose gel electrophoresis, and two identical blots were probed independently with radiolabeled cTYDCl and Localization of Alkaloid Biosynthetic Genes CTYDC2 as shown in Figure 2A. The stringent hybridization conditions depicted in Figure 2A were identical to those used for all other nucleic acid hybridizations reported in this study. In previous work, we showed that cTYDCI and TYDC4 share >84% nucleotide sequence identity. Similarly, cTYDC2 and cTYDCS share >90% nucleotide sequence identity in regions that overlap. In contrast, cTYDCI and cTYDC2, as representatives of the two groups, share <73°/o nucleotide sequence identity. Thus, the cTYDCI and cTYDC2 probes revealed only genes with a minimum sequence identity of ~85% (Figure 2A). The differential and organ-specific expression of TYDC genes in mature opium poppy plants is shown in Figure 2B. The predominant site of expression for TYDC7-like genes is the root, although much lower levels of expression were also detected in stems. In contrast, rYDC2-like genes are expressed predominantly in stems, but significant expression was also detected in roots. Lower levels of TVDCZ-like transcripts were observed in total RNA from sepals 1 day before anthesis and from carpels 1 day after anthesis. No signals were detected with the cTYDCI or CTYDC2 probes in total RNA isolated from the leaf blade (minus midrib), anthers, or petals collected 1 day after anthesis. The length of rVDC2-like transcripts in both roots and sepals appeared to be slightly shorter than those in stems and carpels (Figure 2B), suggesting further differential and organ-specific expression of the TYDC gene family in opium poppy. 1813 TYDC1 TYDC2 TYDC3 TYDC4 TYDC1 TYDC2 DNA £ g s i •s3 <8a (3| < 2 •^ B TYDC1 DC $ <D — CO ff TYDC Genes Are Expressed in All Internodes of the Stem The localization of abundant TYDC transcripts in stems and roots suggested that these organs are potential primary sites fortetrahydroisoquinoline alkaloid biosynthesis in opium poppy. Poppy has a predominantly fibrous root system, with relatively few roots >1 to 2 mm in diameter emanating from the central axis. The observed pattern of expression in small fibrous roots (<1 mm in diameter) and larger roots (5 to 10 mm in diameter) was identical (data not shown). 7YDC transcript levels along the length of the stem at successive internodes are shown in Figure 3. Expression of 7~YDC7-like genes was observed from the first to sixth internode and rapidly declined in subsequent internodes to the base of the stem. At least two 7YDC7-like transcripts that differ in size by several hundred nucleotides were detected. The larger transcript(s) appeared to be similar in size to the major 7VDC7-like transcript(s) occurring in the root. Levels of rVOC2-like transcripts decreased gradually in successive internodes of the stem. Low levels of rVDC2-like transcripts were detectable even in the final internodes just above the initiation of root tissue. The relative abundance of TVDC transcripts in roots and carpels is shown for comparison. Furthermore, 7YDC7-like transcript levels in the root, as determined by scanning laser densitometry, were at least 250fold higher than those in the stem (Figure 3), whereas TYDC2like transcript levels were at least twofold higher in some parts of the stem than in roots (Figure 3, compare lanes 2 and 10). TYDC2 Figure 2. Specificity of TYDC Probes and Differential Expression of TYDC Genes in Opium Poppy. (A) Each lane contains 100 ng of DNA representing cTYDCI, CTYDC2, cTYDCS, and a fragment of TYDC4. The gel was stained with ethidium bromide before blotting. Blots were hybridized with the 32P-labeled probes indicated at left. (B) RNA gel blot analysis of TYDC transcript levels in various organs from mature plants at anthesis. Each lane contains 15 ng of total RNA fractionated on formaldehyde-agarose gels, transferred to nitrocellulose membranes, and hybridized with 32P-labeled cTYDCI or cTYDC2 probes at high stringency Gels were stained with ethidium bromide before blotting to ensure equal loading of samples. The conditions for hybridization and washing were identical for blots shown in (A) and (B), and blots were developed after a 12-hr film exposure. 1814 The Plant Cell The autoradiograms shown in Figure 3 were exposed fourfold longer than those shown in Figure 2. Although this increased the background of the blot, it improved detection of message levels in several different tissues. With the longer exposure time, low levels of TVDC7- and TYDC2-like transcripts in carpel tissue were clearly detectable, and all parts of the stem appeared to express TYDC genes. TYDC2-Like Genes Are Expressed at Low Levels in Floral Buds before Anthesis The traditional method used to harvest the alkaloid-rich latex of opium poppy involves making incisions in the unripe seed capsule and collecting the white exudate. Results presented in Figures 2B and 3 demonstrate that TYDC transcripts are not abundant in carpels at anthesis. To determine the possible relationship of TYDC gene expression to alkaloid biosynthesis and accumulation, we studied the accumulation of TYDC transcripts in carpels dissected from floral structures at different stages of development, as shown in Figure 4A. The carpels of opium poppy consist of a large stigma and a short style, and each contains many ovules. After pollination, the carpels expand over a period of a few weeks (Figure 4A, stages 3 to 6) to form the large seed capsule containing hundreds of seeds. Incisions made on the surface of the mature, but still unripe seed capsule (stage 6) result in the exudation of considerable amounts of latex. Results shown in Figure 4B demonstrate that the levels of expression of both TYDC1- and TVDC2-like genes continued to decrease as the carpel developed and as the seed capsule matured. The level of 7YDC2-like transcripts is higher in carpels than the level of 7VDC7-like transcripts, as shown in Figures 2B and 3. In previous work (Facchini and De Luca, 1994), we demonstrated that the specific activity of TYDC in carpels of plants at anthesis is much lower than that in stems and roots. A decline in the level of TYDC activity correlates with the decrease in the levels of TYDC transcripts during carpel and seed capsule development (data not shown). Thus, although the laticifers of unripe seed capsules accumulate and store isoquinoline alkaloids, the low level of IVDC expression suggests that this tissue may not be the primary site of alkaloid biosynthesis. TYDC mRNAs Are Localized in the Vasculature of Stem and Root Tissues We examined the spatial distribution of TYDC transcripts in various tissues of opium poppy by in situ hybridization using cTYDCl and cTYDC2 sense and antisense probes, as shown in Figure 5. Transcripts of both TYDC1- and 7YDC2-like genes are widely expressed throughout the phloem and selectively expressed in many fewer cells in the primary xylem (Figure 5B) but are not expressed in the secondary xylem or in the 250- TYDC1 TYDC2 Figure 3. Expression of TYDC Genes in Different Internodal Stem Segments. Each lane contains 15 ng of total RNA fractionated on formaldehydeagarose gels, transferred to nitrocellulose membranes, and hybridized with 32P-labeled cTYDCl or CTYDC2 probes at high stringency. Gels were stained with ethidium bromide before blotting to ensure equal loading of samples. Lanes 1 contain total RNA isolated from the carpel dissected from a flower at anthesis. Lanes 2 to 9 contain total RNA isolated from eight internodal stem segments of a flowering opium poppy plant at anthesis. Lanes 10 contain total RNA isolated from secondary roots ~2 to 3 mm in diameter. Blots were exposed fourfold longer than in Figure 2 to improve detection of TYDC transcripts in stem samples. Localization of Alkaloid Biosynthetic Genes periderm of well-differentiated mature poppy roots (Figures 5A to 5D). No hybridization was detected when the CTYDC1 (Figure 5D) and cTYDC2 (data not shown) sense probes were used. In situ hybridization of cross-sections of stems from the internode below the first leaf near the shoot apex revealed that rVDCMike transcripts are localized to both the abaxial and adaxial sides of the vascular cambium in all vascular bundles, whereas 7VDC2-like transcripts are more abundant in the phloem than in any other tissue (Figures 5E to 5H). Although the spatial patterns of expression using cTYDCI or CTYDC2 antisense RNA probes were similar, the development time Carpel Developmental Stage 1815 required to visualize the insoluble blue-purple reaction products of the alkaline phosphatase-conjugated anti-digoxigenin antibody was considerably longer when the cTYDCI antisense RNA probe was used (Figure 5F). No hybridization of either antisense RNA probe was apparent in parenchymatous cortical cells adaxial to the vascular bundles or in the sclerenchyma peripheral to the vasculature. Closer examination of vascular bundles revealed that both TYDC1- and r/DC2-like transcripts (Figures 5F and 5G) are associated predominantly with the metaphloem and to a lesser extent with the protoxylem; however, the transcripts were not detected in cells of the cambial layer and not clearly detected in cells associated with the protophloem and metaxylem. No hybridization was apparent when the cTYDCI (data not shown) and CTYDC2 (Figure 5H) sense probes were used. TYDC transcripts were not detected in total RNA extracted from young leaf blade tissue, as shown in Figure 2B. However, in situ hybridization analysis of cross-sections through the midrib of a young leaf and through young axillary buds demonstrated that both cTYDCI and CTYDC2 antisense RNA probes hybridized to mRNAs present in cells in the vascular bundles as well as in cortical and epidermal cells of younger tissues (data not shown). These results suggest that the tissue specificity of TYDC gene expression is associated with the developmental stage of the plant. In emerging axillary buds and in young leaves, TYDC transcripts appeared to be most abundant in the develop- ing vascular bundles but were also found at lower levels in other cell types, whereas in mature roots (Figures 5B and 5C) and stems (Figures 5F and 5G), TYDC gene expression is clearly localized to the vasculature. B 1 2 3 4 5 6 TYDC1 TYDC2 Figure 4. Expression of TYDC Genes during Carpel Development. (A) Opium poppy reproductive organ development from floral bud (stage 1) through anthesis (stage 3) to maturation of the seed capsule just before desiccation and seed ripening (stage 6). (B) TYDC mRNA levels in carpel tissue at six stages (lanes 1 to 6) of development. Ovules were removed from the carpels before RNA extraction. Each lane contains 15 ng of total RNA fractionated on formaldehyde-agarose gels, transferred to nitrocellulose membranes, and hybridized with 32P-labeled cTYDCI or cTYDC2 probes at high stringency. Gels were stained with ethidium bromide before blotting to ensure equal loading of samples. Roots and Aerial Organs Accumulate Different Major Isoquinoline Alkaloids Opium poppy accumulates three major structural types of wellcharacterized tetrahydroisoquinoline alkaloids. In aerial organs, the alkaloids are contained within the vesicle-rich cytoplasm of mature laticifers (Fairbairn and Djote, 1970; Fairbairn et al., 1974; Roberts et al., 1983; Nessler et al., 1985; Rush et al., 1985). Latex collected from unripe seed capsules (Figure 4, stage 6), stems, or young leaves exhibited a profile of extractable alkaloids that was both quantitatively and qualitatively similar, as shown in Figure 6. In contrast, the alkaloid profile of the root consisting mainly of sanguinarine and morphine was different from that of the latex of carpels, stems, and leaves in which no sanguinarine was detected. The identities of morphine and sanguinarine were compared with authentic standards and verified by mass spectrometry (see Methods). No sanguinarine was detected in latex samples by HPLC (data not shown). The levels of morphine and sanguinarine in capsule, stem, and leaf latex as well as in roots were approximated by comparison with authentic standards using laser densitometry of thin-layer chromatograms. The levels 1816 The Plant Cell Figure 5. Localization of TYDC Transcripts in the Root and Stem of Opium Poppy by in Situ RNA Hybridization. Localization of Alkaloid Biosynthetic Genes of morphine in capsules, stems, and leaves varied between 43 and 51 ug/mL, whereas roots contained 9.7 and 9.3 mg/g dry weight, respectively, of morphine and sanguinarine. The specific localization of sanguinarine accumulation in roots and the more complex alkaloid pattern found in the latex of aerial organs are representative of the dichotomy of alkaloid accumulation in opium poppy. 1817 Front DISCUSSION In this study, we examined the differential and tissue-specific expression of TYDC genes in opium poppy and compared the localization of TYDC transcripts with the accumulation of different alkaloids produced by the plant. The alkaloids synthesized by opium poppy and related species are of the tetrahydroisoquinoline type. In the Papaveraceae, the major alkaloids of aerial organs are sequestered to vesicles found in the cytoplasm of articulated and anastomized internal secretory cells known as laticifers (Fairbairn et al., 1974; Messier and Mahlberg, 1977,1978,1979). Although laticifers are associated with vascular tissue in all organs, the principal alkaloids that accumulate in roots of various species are often different from those of aerial organs (Phillipson, 1983; Preininger, 1985). In this study, we found that among several major latex alkaloids found in all aerial organs of opium poppy, only morphine also occurred as a major root alkaloid, whereas significant levels of sanguinarine occurred only in poppy roots. All of these compounds represent end products of specific tetrahydroisoquinoline alkaloid branch pathways that originate from common intermediates. TYDC represents the first of these common biosynthetic steps that channel the aromatic amino acids L-tyrosine and L-dopa into the alkaloid biosynthetic pathways (Rueffer and Zenk, 1986; Stadler et al., 1987, 1989). TYDC in opium poppy is encoded by a family of 10 to 14 genes, of which we have cloned four members (Facchini and De Luca, 1994). Within the TYDC gene family, two subgroups based on sequence identity are evident. Each subgroup consists of approximately six members, all of which share ^90% identity at the nucleotide and amino acid levels. In contrast, comparison of subgroup members (represented by TYDC1 and TYDC2) reveals sequence identities of <75°/o. Although the catalytic properties of the different TYDC isoforms are similar Origin Latex Figure 6. Thin-Layer Chromatographic Analysis of Alkaloids Found in Latex and in Root Extracts of Mature Opium Poppy Plants. Alkaloids were extracted in methanol from an equal volume of latex collected from mature seed capsules, leaves, and stems. Roots were extracted in methanol from 100 mg of frozen powdered tissue. Samples were applied to silica gel 60 F254 thin-layer chromatography plates for separation and identification of alkaloids. Authentic standards of sanguinarine (S), noscapine, papaverine, codeine, morphine (M), and opium were also run. Alkaloids were detected by their absorbance or fluorescence after illumination at 365 nm. (Facchini and De Luca, 1994,1995), the TYDC gene family exhibits differential and organ-specific expression (Facchini and De Luca, 1994). In this study, we determined that the tissuespecific expression of TYDC genes is developmentally regulated, because the expression of TYDC genes is strictly associated with vascular tissues in mature roots and stems Figure 5. (continued). Tissues were fixed, dehydrated, embedded in paraffin, cut into 10-nm cross-sections, and hybridized at high stringency with digoxigenin-labeled cTYDd or cTYDC2 RNA probes. Hybridization was detected with anti-digoxigenin antibodies conjugated to alkaline phosphatase and visualized after conversion of enzyme substrates to the insoluble blue-purple product. (A) Root cross-section stained with aniline safranme and astra blue. (B) to (D) Root cross-sections hybridized with cTYDCt (B) and CTYDC2 (C) antisense probes and the cTYDC! (D) sense probe. (E) Stem cross-section stained with aniline safranme and astra blue. (F) to (H) Stem cross-sections hybridized with cTYDCt (F) and CTYDC2 (G) antisense probes and the cTYDC2 (H) sense probe. Bar in (H) = 0.5 mm. pd. penderm; ph. phloem: vc, vascular cambrium; xy, xylem. 1818 The Plant Cell but is also expressed in cortical and epidermal cells of tissues from young leaves and axillary buds at earlier stages of development. In roots, TYDC!-like genes are preferentially expressed, whereas in stems and other aerial organs, TYDCPlike genes exhibit higher expression levels. In mature aerial organs, TYDC gene expression appears to predominate in metaphloem and to a lesser extent in protoxylem of vascular bundles. Laticifers of opium poppy are a highly specialized cell type that has been shown to possess alkaloid biosynthetic enzymes, including TYDC (Fairbairn and Wassel, 1964b; Fairbairn et al., 1968; Roberts et al., 1983). Thus, localizationof abundant TYDC transcripts in the phloem is consistent with fhe expected developmental origins of laticifers. Although not distinguishable in the tissue sections shown in Figure 5, the location of laticifers in mature roots, stems, and leaves is always associated with the phloem, and they are often positioned adjacent to sieve tube members (Nessler and Mahlberg, 1977, 1978, 1979). Our data support the role of vascular tissue as the primary site of isoquinoline alkaloid biosynthesis. In situ detection of TYDC transcripts in developing phloem, together with previous reports on the presence of TYDC and other alkaloid biosynthetic enzymes in the cytoplasm of phloem-derived laticifers (Roberts, 1971, 1974; Roberts and Antoun, 1978; Roberts et al., 1983), suggests that isoquinoline alkaloid biosynthesis occurs in these tissues in stems and roots. We have found that the developing carpel lacks abundant TYDC transcripts and has relatively low enzyme activity compared with other tissues (Facchini and De Luca, 1994). Salutaridine synthase (SS) activity, which catalyzes the sixth to last step in the biosynthesis of morphine, is also low in the developing carpel (Gerardy and Zenk, 1993), suggesting that alkaloids accumulate, but are not synthesized, in the latex of the seed capsule. The articulated laticifers of opium poppy contain perforated transverse walls between adjoining cells that would allow for bulk flow of latex (Nessler and Mahlberg, 1977,1978,1979) and tissue-specific alkaloid accumulation. To account for the high levels of alkaloids found in poppy capsules, it has been suggested that stem latex, where the alkaloids are synthesized, is then translocated into the growing capsule during its rapid expansion after peta1 fall (Fairbairn et al., 1974). These results establish provocativecorrelations betweenthe differential expression of TYDC genes, the tissue-specific localization of TYDC transcripts in mature organs, and the accumulation of specific alkaloids in opium poppy. For example, TYDC7-like genes are expressed predominantly in tissue corresponding to the phloem in roots, where sanguinarine and morphine are the principal alkaloids. The lack of detectable quantities of sanguinarine in latex and the low levels of TYDC7like transcripts suggest that the expression of TYDCI-like genes may be coordinately regulated with that of other genes specifically involved in benzophenanthridine alkaloid biosynthesis. The abundance of morphine in latex and its presence in root extracts, together with the high levels of expression and localization of TYDCP-like transcripts in the phloem of mature stems and roots, suggest a possible association between the regulation of TYDCP-like genes and others specific to the biosynthesis of phenanthrene alkaloids. However, the reguiation of other alkaloid biosynthetic enzymes in opium poppy and related species is poorly understood. Only the spatial pattern of SS activity has been determined (Gerardy and Zenk, 1993), and it resembles that of TYDC (Facchini and De Luca, 1994). A key step in sanguinarine biosynthesis, the berberine bridge enzyme (BBE), has been cloned and characterized from Eschscholzia californica (Dittrich and Kutchan, 1991). ExpresSion of BBE has been shown to be induced by various elicitors in E. californica cell cultures; however, the tissue-specific expression of BBE has not been investigated. The availability of genes, such as SS and BBE, from specific branch pathways is essential for further study of the coordinated regulation of specific genes and the developmental, temporal, and spatial patterns of isoquinoline alkaloid biosynthesis. Because the functions in plants of most alkaloids are not known, interpretations of the occurrence of specific alkaloids in specific organs are highly speculative. Most isoquinoline alkaloids, including morphine and noscapine, induce potent pharmacological effects, and their accumulation in aerial organs could serve as a deterrent to herbivores. Sanguinarine has been shown to be an active fungitoxin at concentrations as low as 10 mM, although its role as a phytoalexin in plants has not been verified unequivocally (Cline and Coscia, 1988). The occurrence of sanguinarine in the root of opium poppy and other Papaveraceae may confer protection against soilborne pathogens. It is also likely that the protective potency and adaptability of the chemical arsenal of plants increase as a function of the structural diversity of the products that they accumulate. The complex development-, organ-, and tissuespecific regulation oí alkaloid biosynthesis illustrated in this and other reports suggests that the roles of plant secondary metabolites may be more significant than previously suspected. METHODS Plant Material Plants (Papaversomniferum cv Marianne)were grown under standard greenhouse conditions at a daylnight temperature regime of 20/18"C. Tissue samples were harvested from mature flowering plants 1 day after anthesis or at specific stages of carpel development as indicated. Latex was isolated from capsules, stems, and leaves by cutting the organ from the plant and collecting the white exudate with a glass capillary. Tyrosine/Dopa Decarboxylase Probes Previously,we describedthe isolation of full- and partial-lengthcDNA and genomic clones for tyrosine/dopadecarboxylasefrom ?f somniferum. They are designated cTYDC1, TYDC1, cTYDC2, cTYDC3, and TYDC4 (Facchini and De Luca, 1994).AI1 inserts were cloned using Localization of Alkaloid Biosynthetic Genes StratagenepBluescript SK+ (genomicclones)or SK- (cDNAs). Probes for DNA and RNA gel blot hybridizationswere synthesizedfrom cDNA inserts isolated by digestingplasmidswith EcoRl and Xhol. The partiallength cTYDCl and cTYDC3 inserts were 1554 and 927 bp, respectively, whereas the full-length cTYDC2 insert was 1876 bp. A 1.9-kb genomic fragment containing the 3’ translated region of TYDC4 was subcloned and subsequently isolated using Hindlll. Probesfor in situ RNA hybridizationanalysiswere synthesizedfrom plasmids that were linearizedwith either Sacl (antisense) or Kpnl (sense) and treated with 2 mg mL-’ proteinase K for 30 min at 37%. Nucleic Acid lsolation and Analysis lsolated DNA inserts were separated on 1.0% agarose gels and immobilized on nylon membranes. Total RNA for gel blot analysis was isolated according to Logemann et al. (1987), and 15 pg was fractionated on 1.O% formaldehyde-agarosegels before being transferred to nitrocellulose membranes(Sambrooket al., 1989). DNA and RNAgel blots were hybridized with random primed 3ZP-labeled-DNAinserts (Feinbergand Vogelstein, 1984) at 65OC in 0.25 M sodium phosphate buffer, pH 8.0, 7% SDS, 1% BSA, 1 mM EDTA. Blots were washed at 55OC twice with 2 x SSC (1 x SSC is 0.15 M NaCI, 0.015 M sodium citrate, pH 7.0), 0.1% SDS, and twice with 0.2 x SSC, 0.1% SDS (Sambrook et al., 1989). Blots were autoradiographed with an intensifying screen on RXlOO x-ray film (Fuji, Montreal, Canada) for 24 hr or longer. In Situ Hybridization In situ RNA hybridizations were performed essentially as described by Cox and Goldberg (1988), with modifications used by Wang et al. (1992). Tissue samples were fixed in FAA (50% ethanol, 5% acetic acid, 10% formalin) at room temperature for 1 to 2 days, depending on the size of the material. After dehydration with tert-butanol and embedding in paraffin, samples were cut into 10-pm sections and mountedon replicateslides coated with poly-L-lysine. Tissue sections were deparaffinized, hydrated, and subjected to the following series of treatments: 0.2 N HCI for 20 min at room temperature, 2 pg mL-l proteinase K for 30 min at 37% and 0.25% acetic anhydride for 10 min at room temperature. Sense and antisensetranscriptsfor cTYDCl and cTYDC2 were synthesized and labeled in vitro with digoxigenin11-UTP(Boehringer Mannheim)usingT7 or T3 RNA polymerase. Slides were prehybridized for 1 hr at 5OoC in 50% formamide, 30 mM NaCI, 10 mM Tris-HCI, pH 6.8, 10 mM sodium phosphate, pH 6.8,5 mM EDTA, 10% dextran sulfate, 10 mM DTT, 150 mg mL-l yeast tRNA, and 500 mg mL-’ polyadenylic acid. Tissue sections were hybridized in the same solution containing 0.2 mg mL-’ digoxigenin-labeled probe for 15 hr at 50OC. After hybridization, slides were treated with 50 pg mL-’ RNaseA for 30 min at 37OC to digest nonhybridized probe and subsequently washed in 2 x SSC for 2 hr at room temperature, 1 x SSC for 2 hr at room temperature, and 0.1 x SSC for 1 hr at 65%. Hybridized probe was detectedwith anti-digoxigenin antibodies conjugated to alkaline phosphatase(Boehringer Mannheim)and visualized by color developmentusing5-bromo-4-chlorc-3-indolylphosphateand nitro blue tetrazolium as substrates. Sections were photographed with a Leitz Ortholux II microscope(Leica Canada Ltd., Toronto, Canada)on Kodak RoyalGold 100 film. 1819 Alkaloid Extraction and Thin-Layer Chromatography Alkaloids were extracted from fresh latex (50 pL) collected from mature poppy seed capsules, stems, or leaves with 0.2 mL of methanol for 10 min at 100OC. Roots (100 mg) were ground to a fine powder under liquid nitrogen and extracted with 0.5 mL of methanol for 10 min at 100OC.Debrisfrom all sampleswas remwed by centrifugation.Samples (10 pL) of plant extracts were applied to silica gel 60 FZs4 thin-layer chromatography plates (EM Separations, Darmstadt, Germany) and developed in a solvent system consistingof acetone/toluene/ethanol/concentratedammonia (45:45:7:3 [v/v]) (Wagner et al., 1984). Standards for sanguinarine, noscapine, and papaverine were purchasedfrom Sigma. Morphine, codeine, and opium standards were a kind gift from U. Eilert (Technische Universitat, Braunschweig, Germany).Plateswere dried at room temperatureand photographedunder ultraviolet illumination at 365 nm on Kodak RoyalGold 100 film. Extracts were separated by HPLC (600E HPLC and 991 photodiodearray detector; Waters, Montreal, Canada) on a Waters Nova Pak C18 reverse phase (3.9 x 300 mm) column using an isocratic gradient of methanol/water (6:4) containing 0.1% triethanolamine (Kraml and DiCosmo, 1993). The retention time for sanguinarine in root extracts was 16.1min and was identical with that obtained for the corresponding standard. Furthermore, an absorption spectrum identical to that of sanguinarine standard was obtainedfrom the root-derivedalkaloids eluting at this retention time. ldentification of Morphine and Sanguinarine by Mass Spectrometry Morphine and sanguinarine standards as well as morphins from latex, morphine from roots, and sanguinarine from roots were purified by thin-layer chromatography.Spots corresponding to morphine and sanguinarinestandards were removed, and alkaloids were redissolved in methanol. lnsoluble silica was removed by centrifugation, and the supernatants were subjected to mass spectrometry. Low-resolution mass spectra (direct probe mass spectrometry with a VG 7070F gas chromatography/massspectrometer)(V.G. Analytical Ltd., Manchester, UK) obtained for morphine (mlz: 285 [IOO], 268 [l8]. 215 127,174 [19], 162 [27], and 124 [26]) in latex were similar to those obtained for morphine in roots, and these were identical to those obtained for the morphine standard(mlz: 285 [lOO], 268 [13], 215 [%I, 174 [IA, 162 [34], and 124 [lg]). The low-resolutionmassspectraobtained for sanguinarine ( m h : 332 [IOO], 317 [34], 194 [26], and 149 [IOO]) in roots were identical to those obtainedfor sanguinarine standards ( m h : 332 [lOO], 317 [30], 194 [38], and 149 [32]). When a sample is placed in a mass spectrometer, it is bombarded with a beam of energetic electrons that causes the molecules to ionize and break down into many fragments. Reported above are the masses of the major ions obtained from the morphine standard, with 285 being the mass of the largest ion produced and 100 being the relativevalue for the largest peak. Subsequent masses(268,215, 174, 162, and 124) representother major ions produced that are representative of morphine. The numbers in bracketsrepresentthe intensities of each ion relative to the largest peak, which is 100. ACKNOWLEDGMENTS We are grateful to Drs. Udo Eilert for the generous gifts of alkaloid standards, Joachim Vieth (Institut de Rechercheen Biologie VkgBtale) 1820 The Plant Cell for his invaluable assistancewith the interpretation of anatomicaldata, James Berry (State University of New York at Buffalo) for providing us with the in situ hybridization protocol used in his laboratory, Edward YeUng (University of Calgary) for assistance with photomicrography, and Gijs van Rooijen for assistancewith the laser densitization of thinlayer chromatography results. We also thank Sylvain Lebeurier for maintaining plants in the greenhouse, Julie Poupartfor technical assistance with alkaloid extractions, Christiane Morisset for advice on the preparation of tissue sections, Jean-Luc Verville for photographic work, and Drs. Normand Brisson and Benoit St.-Pierre for critical reading of the manuscript and helpful discussions. This work was funded by Natural Sciences and Engineering ResearchCouncil of Canadaoperating and strategic grants to V.D.L. P.J.F. was supported in part by a Natural Sciences and Engineering Research Council of Canada Postdoctoral FellowshiD. Fairbairn,J.W., and Wassel, G. (1964b). The alkaloids of fapaversomniferum L. (I. Biosynthesis in isolated latex. Phytochemistry 3, 583-585. Fairbairn, J.W., Djote, M., and Paterson, A. (1968).The alkaloids of fapaver somniferum L. VIL Biosynthetic activity of the isolated latex. Phytochemistry 7, 2111-2116. Fairbairn, J.W., Hakim, F., and El Kheir, Y. (1974).Alkaloidal storage, metabolism, and translocation in the vesicles of fapaver somniferum latex. Phytochemistry 13, 1133-1139. Feinberg, A.P., and Vogelstein, B. (1984).A techniquefor radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 137, 266-269. Gerardy, R., and Zenk, M.H. (1993). Formation of salutaridine from (R)-reticulineby a membrane-boundcytochrome P-450 enzyme from fapaver somniferum. Phytochemistry 32, 79-86. Hahlbrock, K., and Scheel, D. (1989).Physiology and molecular bi- Received August 11, 1995; accepted August 30, 1995. REFERENCES ology of phenylpropanoid metabolism. Annu. Rev. Plant Physiol. Plant MOI.Biol. 40, 347-369. Kawalleck, P., Keller, H., Hahlbrock, K., Scheel, D., and Somssich, I.E. (1993). A pathogen-responsive gene of parsley encodes tyrosine decarboxylase. J. Biol. Chem. 268, 2189-2194. Kraml, M.M., and DiCosmo, F. (1993).A rapid high performance liq- Antoun, M.D., and Roberts, M.F. (1975).Some enzymes of general metabolism in the latex of fapaversomniferum. Phytochemistry 14, 909-914. Brochmann-Hanssen,E. (1985). Biosynthesisof morphinan alkaloids. In The Chemistry and Biology of lsoquinoline Alkaloids, J.D. Phillipson, M.F. Roberts, and M.H. Zenk, eds (Berlin: SpringerVerlag), pp. 229-239. Brownstein, M.J. (1993). A brief history of opiates, opioid peptides, and opioid receptors. Proc. Natl. Acad. Sci. USA 90, 5391-5393. Cline, S.D., and Coscia, C.J. (1988). Stimulation of sanguinarine production by combined funga1 elicitation and hormonal deprivation in cell suspensioncultures of fapaverbracteatum. Plant Physiol. 86, 161-165. Cox, K.H., and Goldberg, R.B. (1988). Analysis of plant gene expression. In Plant Molecular Biology: A Practical Approach, C.H. Shaw, ed (Oxford: IRL Press), pp. 1-35. Dittrich, H., and Kutchan, T.M. (1991).Molecular cloning, expression, and induction of berberine bridge enzyme, an enzyme essential to the formation of benzophenanthridine alkaloids in the response of plants to pathogenic attack. Proc. Natl. Acad. Sci. USA 88, 9969-9973. Facchini, P.J., and De Luca, V. (1994).Differential and tissue-specific expressionof a gene family for tyrosine/dopa decarboxylase in opium poppy. J. Biol. Chem. 269, 26684-26690. Facchini, P.J., and De Luca, V. (1995).Expression in Escherichia coli and partia1 characterization of two tyrosine/dopa decarboxylases from opium poppy. Phytochemistry 38, 1119-1126. Fairbairn, J.W., and Djote, M. (1970). Alkaloid biosynthesis and metabolism in an organelle fraction of fapaver somniferum. Phytochemistry 9, 739-742. Fairbairn, J.W., and Kapoor, L. (1960). The laticiferous vessels of fapaver somniferum L. Planta Med. 13, 1133-1139. Fairbairn, J.W., and Wassel, G. (1964a).The alkaloidsof fapaversomniferum L. I. Evidence for a rapid turnover of the major alkaloids. Phytochemistry 3, 253-258. uid chromatography method for the separation of the alkaloid precursor L-tyrosine and six tetrahydroisoquinoline alkaloids of fapaver somniferum. Phytochem. Anal. 4, 103-104. Kutchan, T.M., and Zenk, M.H. (1993). Enzymology and molecular biology of benzophenanthridine alkaloid biosynthesis.J. Plant Res. 3, 165-173. Logemann, J., Schell, J., and Willmitzer, L. (1987). lmprovedmethod for the isolationof RNAfrom plant tissues. Anal. Biochem.163, 16-20. Marques, I.A., and Brodelius, P. (1988). Elicitor-induced L-tyrosine decarboxylase from plant cell suspension cultures. 11. Partia1characterization. Plant Physiol. 88, 52-55. Nessler, C.L., and Mahlberg, P.G. (1977). Cell wall perforations in laticifers of fapaver somniferum L. Bot. Gaz. 138, 402-408. Nessler, C.L., and Mahlberg, P.G. (1978).Ontogeny and cytochemistry of alkaloid vesicles in laticifers of fapaver somniferum L. Am. J. Bot. 64, 541-551. Nessler, C.L., and Mahlberg, P.G. (1979).Ultrastructure of laticifers in redifferentiated organs on callus from fapaver somniferum (Papaveraceae).Can. J. Bot. 57, 675-685. Nessler, C.L., Allen, R.D., and Galewsky, S. (1985). ldentification and characterization of latex-specific proteins in opium poppy. Plant Physiol. 79, 499-504. Phillipson, J.D. (1983).lntraspecific variation and alkaloids of fapaver species. Planta Med. 48, 187-192. Preininger, V. (1985).Chemotaxonomyof the Papaveraceaealkaloids. In The Chemistry and Biology of lsoquinoline Alkaloids, J.D. Phillipson, M.F. Roberts, and M.H. Zenk, eds (Berlin: SpringerVerlag), pp. 23-37. Roberts, M.F. (1971). Polyphenolasesin the 1OOOgfractions of fapaver somniferumlatex. Phytochemistry 10, 3021-3027. Roberts, M.F. (1974). Oxidation of tyrosine by fapaver somniferum latex. Phytochemistry 13, 119-123. Roberts, M.F. (1988). lsoquinolines (fapaver alkaloids). In Cell Cul- ture and Somatic Cell Genetics of Plants, Vol. 5, F. Constabel and I.K. Vasil, eds (New York: Academic Press), pp. 315-334. Localization of Alkaloid Biosynthetic Genes Roberts, M.F., and Antoun, M.D. (1978). The relationship between L-dopadecarboxylase in the latex of Papaversomniferum and alkaloid formation. Phytochemistry 17, 1083-1087. Roberts, M.F., McCarthy, D., Kutchan, T.M., and Coscia, C.J. (1983). Localization of enzymes and alkaloidal metabolites in Papaver latex. Arch. Biochem. Biophys. 222, 599-609. Rueffer, M., and Zenk, M.H. (1986). Distant precursors of benzylisoquinoline alkaloids and their enzymatic formation. 2. Naturforsch. Sect. C Biosci. 42, 319-332. Rush, M.D., Kutchan, T.M., and Coscia, C.J. (1985). Correlation of the appearance of morphinan alkaloids and laticifer cells in germinating Papaver bracteatum seedlings. Plant Cell Rep. 4, 237-240. Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory). 1821 Stadler, R., Kutchan, T.M., Loeffler, S.,Nagakura, N., Cassels, B., and Zenk, M.H. (1987). Revision of the early steps of reticuline biosynthesis. Tetrahedron Lett. 28, 1251-1254. Stadler, R., Kutchan, T.M., and Zenk, M.H. (1989).(S)-Norcoclaurine is the central intermediate in benzylisoquinolinealkaloidbiosynthesis. Phytochemistry 28, 1083-1086. Wagner, H., Bladt, S.,and Zgainski, E.M. (1984). Plant Drug Analysis. (Berlin: Springer-Verlag), pp. 78-79. Wang, J.-L., Klessig, D.F., and Berry, J.O. (1992). Regulation of C4 gene expression tn developing Amaranth leaves. Plant Cell 4, 173-184. Zenk, M.H. (1985).Enzymology of benzylisoquinoline alkaloid formation. In The Chemistry and Biology of lsoquinoline Alkaloids, J.D. Phillipson, M.F. Roberts, and M.H. Zenk, eds (Berlin: SpringerVerlag), pp. 240-256. Phloem-Specific Expression of Tyrosine/Dopa Decarboxylase Genes and the Biosynthesis of Isoquinoline Alkaloids in Opium Poppy. P. J. Facchini and V. De Luca Plant Cell 1995;7;1811-1821 DOI 10.1105/tpc.7.11.1811 This information is current as of June 15, 2017 Permissions https://www.copyright.com/ccc/openurl.do?sid=pd_hw1532298X&issn=1532298X&WT.mc_id=pd_hw1532298X eTOCs Sign up for eTOCs at: http://www.plantcell.org/cgi/alerts/ctmain CiteTrack Alerts Sign up for CiteTrack Alerts at: http://www.plantcell.org/cgi/alerts/ctmain Subscription Information Subscription Information for The Plant Cell and Plant Physiology is available at: http://www.aspb.org/publications/subscriptions.cfm © American Society of Plant Biologists ADVANCING THE SCIENCE OF PLANT BIOLOGY