* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electromagnetic spectrum - Purdue Physics
Bremsstrahlung wikipedia , lookup
Microplasma wikipedia , lookup
Van Allen radiation belt wikipedia , lookup
Health threat from cosmic rays wikipedia , lookup
Cosmic microwave background wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Astrophysical X-ray source wikipedia , lookup
Circular dichroism wikipedia , lookup
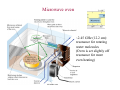
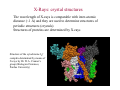
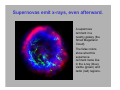
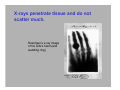
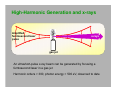
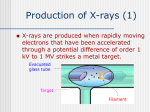
Electromagnetic spectrum Radio waves > 30 cm Used primarily for communication AC current in wires: 60 Hz, emits constant EM wave background (=5000 km) Energies of radio wave ‘photons’ are very low and quantized nature of EM radiation at these frequencies is not noticeable. Microwaves ~ 300 m - 30 cm Radars Cell phones, Satellite TV Outer space: neutral hydrogen emits 21 cm microwaves Molecular state transitions - rotational and vibrational Cesium clock: two closely spaced levels, ~ 9.2 GHz Microwave oven Microwave oven ~2.45 GHz (12.2 cm): resonance for rotating water molecules (Oven is set slightly off resonance for more even heating) Big bang and microwaves 1960s: Arno Penzias and Robert Wilson discovered that the whole universe is full of microwave radiation, that was similar to radiation of a body at 2.7 K (about -270°C). This cosmic background radiation is a leftover of the ‘Big Bang’ - the origin of the universe (Nobel Prize 1978) Infrared (IR) light =780 nm - 1 mm Warm matter emits broad band of infrared light - at higher temperatures band shifts to shorter wavelength. Can use for IR imaging. IR image IR movie www.footagehouse.com CO2 laser (~20m) cutting metal Visible light =400 nm - 700 mm Sun spectrum: Sources of light: Sun, light bulb, fire Most objects do not emit light but reflect or scatter light emitted by other objects Mixture of all wavelengths is percepted by people as ‘white’ light. Ultraviolet (UV) =100 nm - 350 mm Photon energy is high and it is readily absorbed by electronic transitions in atoms and molecules, and could be enough to ionize molecules - remove an electron from a molecule (photoelectric effect). UV is damaging to living tissue X-Rays: discovery =0.1 Å - 10 nm X-rays - discovered by Röntgen in 1895 The first X-Ray by Röntgen: the hand a famous anatomist Kölliker photographic plate X-Rays can penetrate through matter Late 1895, John Winthrop Wright, MD, used X-Ray of a patient’s swollen hand to locate 22-caliber bullet induction coil Crookes tube X-Rays: crystal structures The wavelength of X-rays is comparable with inter-atomic distance (~1 Å) and they are used to determine structures of periodic structures (crystals) Structures of proteins are determined by X-rays Structure of the cytochrome b6f complex determined by means of X-rays by Dr. W.A. Cramer’s group (Biological Sciences, Purdue University) Gamma-Rays << 1 Å Due to extremely short wavelength wave-properties of gamma rays are hard to observe. Gamma rays are emitted by nuclear transitions (nuclear bomb) The Electromagnetic Spectrum: More Fun Things • Sources of light: gases, liquids, and solids • • • Boltzmann's Law Blackbody radiation The electromagnetic spectrum • Long-wavelength sources • and applications • Visible light and the eye Courtesy Prof. Rick Trebino, Georgia Tech • Short-wavelength sources and applications The electromagnetic spectrum gamma-ray microwave 1 106 visible radio infrared 0 105 41 10 3 10 UV 2 10 wavelength (nm) The transition wavelengths are a bit arbitrary… X-ray 1 10 0 10 -1 10 60-Hz radiation from power lines Yes, this very-low-frequency current emits 60-Hz electromagnetic waves. No, it is not harmful. A flawed epidemiological study in 1979 claimed otherwise, but no other study has ever found such results. Also, electrical power generation has increased exponentially since 1900; cancer incidence has remained essentially constant. Also, the 60-Hz electrical fields reaching the body are small; they’re greatly reduced inside the body because it’s conducting; and the body’s own electrical fields (nerve impulses) are much greater. 60-Hz magnetic fields inside the body are < 0.002 Gauss; the earth’s magnetic field is ~ 0.4 G. The longwavelength electromagnetic spectrum Arecibo radio telescope Radio & microwave regions (3 kHz – 300 GHz) Global positioning system (GPS) It consists of 24 orbiting satellites in “half-synchronous orbits” (two revolutions per day). Four satellites per orbit, equally spaced, inclined at 55 degrees to equator. Operates at 1.575 GHz (1.228 GHz is a reference to compensate for atmospheric water effects) 4 signals are required; one for time, three for position. 2-m accuracy (100 m for us). Microwave ovens Microwave ovens operate at 2.45 GHz, where water absorbs very well. Percy LeBaron Spencer, Inventor of the microwave oven Geosynchronous communications satellites 22,300 miles above the earth’s surface 6 GHz uplink, 4 GHz downlink Each satellite is actually two (one is a spare) Cosmic microwave background Peak frequency is ~ 150 GHz The 3° cosmic microwave background is blackbody radiation left over from the Big Bang! Wavenumber (cm-1) Microwave background vs. angle. Note the variations. Interestingly, blackbody radiation retains a blackbody spectrum despite the expansion the universe. It does get colder, however. TeraHertz light (a region of microwaves) TeraHertz light is light with a frequency of ~1 THz, that is, with a wavelength of ~300 m. THz light is heavily absorbed by water, but clothes are transparent in this wavelength range. IR is useful for measuring the temperature of objects. Hotter and hence brighter in the IR Old Faithful Such studies help to confirm that Old Faithful is in fact faithful and whether human existence is interfering with it. IR Liedetection I don’t really buy this, but I thought you’d enjoy it… He’s really sweating now… The military uses IR to see objects it considers relevant. IR light penetrates fog and smoke better than visible light. Jet engines emit infrared light from 3 to 5.5 µm Energy (m) This light is easily distinguished from the ambient infrared, which peaks near 10 m and is relatively weak in this range The infrared space observatory Stars that are just forming emit light mainly in the IR. Using mid-IR laser light to shoot down missiles Wavelength = 3.6 to 4.2 m The Tactical High Energy Laser uses a high-energy, deuterium fluoride chemical laser to shoot down short range unguided (ballistic flying) rockets. Laser welding Near-IR wavelengths are commonly used. Atmospheric penetration depth (from space) vs. wavelength Visible light Wavelengths and frequencies of visible light Auroras Solar wind particles spiral around the earth’s magnetic field lines and collide with atmospheric molecules, electronically exciting them. Auroras are due to fluorescence from molecules excited by these charged particles. Different colors are from different atoms and molecules. O: 558, 630, 636 nm N2+: 391, 428 nm H: 486, 656 nm Dye lasers cover the entire visible spectrum. Fluorescent lights “Incandescent” lights (normal light bulbs) lack the emission lines. The human retina Rods Cones The retina is a mosaic of two basic types of photoreceptors, rods, and cones. Cones are highly concentrated in a region near the center of the retina called the fovea. The maximum concentration of cones is roughly 180,000 per mm2 there and the density decreases rapidly outside of the fovea to less than 5,000 per mm2. Note the blind spot caused by the optic nerve, which is void of any photoreceptors. The eye’s response to light and color The eye’s cones have three receptors, one for red, another for green, and a third for blue. The eye is poor at distinguishing spectra. Because the eye perceives intermediate colors, such as orange and yellow, by comparing relative responses of two or more different receptors, the eye cannot distinguish between many spectra. The various yellow spectra below appear the same (yellow), and the combination of red and green also looks yellow! How film and digital cameras work Most digital cameras interleave different-color filters Color wheels Hue = wavelength Saturation = spectral width Value = brightness (intensity) The Ultraviolet The UV is usually broken up into three regions, UVA (320-400 nm), UVB (290-320 nm), and UVC (220-290 nm). UVC is almost completely absorbed by the atmosphere. You can get skin cancer even from UVA. Flowers in the UV Since bees see in the UV (they have a receptor peaking at 345 nm), flowers often have UV patterns that are invisible in the visible. Arnica angustifolia Vahl Visible UV (false color) The sun in the UV Image taken through a 171-nm filter by NASA’s SOHO satellite. The very short-wavelength regions Vacuum-ultraviolet (VUV) 180 nm > > 50 nm Soft x-rays 5 nm > > 0.5 nm Absorbed by <<1 mm of air Ionizing to many materials Strongly interacts with core electrons in materials Extreme-ultraviolet (XUV or EUV) 50 nm > > 5 nm Ionizing radiation to all materials Synchrotron Radiation Formerly considered a nuisance to accelerators, it’s now often the desired product! Synchrotron radiation in all directions around the circle Synchrotron radiation only in eight preferred directions EUV Astronomy The solar corona is very hot (30,000,000 degrees K) and so emits light in the EUV region. EUV astronomy requires satellites because the earth’s atmosphere is highly absorbing at these wavelengths. The sun also emits x-rays. The sun seen in the x-ray region. Matter falling into a black hole emits x-rays. Nearby star Black hole A black hole accelerates particles to very high speeds. Supernovas emit x-rays, even afterward. A supernova remnant in a nearby galaxy (the Small Magellanic Cloud). The false colors show what this supernova remnant looks like in the x-ray (blue), visible (green) and radio (red) regions. X-rays are occasionally seen in auroras. On April 7th 1997, a massive solar storm ejected a cloud of energetic particles toward planet Earth. The “plasma cloud” grazed the Earth, and its high energy particles created a massive geomagnetic storm. Atomic structure and x-rays Ionization energy ~ 100 – 1000 e.v. Ionization energy ~ .01 – 1 e.v. Fast electrons impacting a metal generate x-rays. High voltage accelerates electrons to high velocity, which then impact a metal. Electrons displace electrons in the metal, which then emit xrays. The faster the electrons, the higher the x-ray frequency. X-rays penetrate tissue and do not scatter much. Roentgen’s x-ray image of his wife’s hand (and wedding ring) X-rays for photo-lithography You can only focus light to a spot size of the light wavelength. So x-rays are necessary for integratedcircuit applications with structure a small fraction of a micron. 1 keV photons from a synchrotron: 2 micron lines over a base of 0.5 micron lines. High-Harmonic Generation and x-rays Amplified femtosecond laser pulse x-rays gas jet An ultrashort-pulse x-ray beam can be generated by focusing a femtosecond laser in a gas jet Harmonic orders > 300, photon energy > 500 eV, observed to date HHG is a highly nonlinear process resulting from highly nonharmonic motion of an electron in an intense field. The strong field smashes the electron into the nucleus—a highly non-harmonic motion! Ion x-ray electron Wavelengths of a few Angstroms has been created this way. Gamma rays result from matterantimatter annihilation. An electron and positron self-annihilate, creating two gamma rays whose energy is equal to the electron mass energy, mec2. ee+ h = 511 kev More massive particles create even more energetic gamma rays. Gamma rays are also created in nuclear decay, nuclear reactions and explosions, pulsars, black holes, and supernova explosions. Gamma-ray bursts emit massive amounts of gamma rays. A new one appears almost every day, and it persists for ~1 second to ~1 minute. The gamma-ray sky They’re probably supernovas. In 10 seconds, they can emit more energy than our sun will in its entire lifetime. Fortunately, there don’t seem to be any in our galaxy. The universe in different spectral regions… Gamma Ray X-Ray Visible The universe in more spectral regions… IR Microwave