* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture notes 10
Survey
Document related concepts
Transcript
Explaining puzzles of the H-R diagram:
The interiors of stars
Slide
The mass-luminosity relation for 192 stars in
double-lined spectroscopic binary systems.
L ~ M3.5 much stronger than inferred from L ~ R2 ~ M2/3
Slide
Specific segments of the main sequence are occupied
by stars of a specific mass
Majority of stars are here
Slide
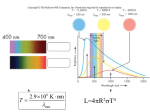
HR diagram
Absolute Magnitude (M)
(Luminosity)
Brighter
Spectral
Type
Hotter
Temperature (Color, B-V)
Age of the cluster from
turnoff point
Turnoff point: stars
of that mass are
going to die and
move away from
the main sequence
Slide
Stars spent most of their lives on the Main Sequence
At the end of its life the star moves away from the
Main Sequence
More massive and more luminous stars die faster
Hypothesis: Stars on the Main Sequence live
due to nuclear fusion of hydrogen!
A. Eddington (1920), G. Gamow (1928), H. Bethe (1939)
• Stars stay on the main sequence until all hydrogen in
the core is consumed
• Then something should happen
Slide
Life of stars:
Mass/gravity is everything
• Stars are born due to gravitational collapse of gas
clouds
• Star’s life is a battle between thermal pressure
generated by nuclear reactions and gravity
• Eventually, a star loses this battle, and gravity
overwhelms
Slide
Stars are gravitating spheres: they are held together by their own
gravity. The gravity force acting on each volume element of a star
is exactly balanced by gas pressure (Hydrostatic equilibrium)
This balance is steady
No gravity: the Sun will disperse in 1 day
gas pressure
gravity
No gas pressure: the Sun will
collapse in 20 minutes
Central pressure ~ 1010 atmospheres
Slide
Hydrostatic equilibrium
Temperature in the center of a star
A =1 m2
=
Slide
Internal structure
Central temperature for the sun Tc ≈ 1.5×107 K
Surface temperature of the sun Ts ≈ 5800 K
Heat transfer from the center to the surface!
Heat transfer determines both the internal composition
and the luminosity of the stars
Slide
Internal source of energy
The Sun’s luminosity is L = 4x1026 Watt. Where does this
energy come from?
• Gravitational energy?
• Chemical energy?
• Nuclear reactions?
Slide
Chemical energy?
This is the energy associated with breaking chemical bonds in molecules
1. Typical energy released per proton is ~ 1-10 eV
2. There are M/mp ~ 1057 protons in the Sun
Total available energy is Echem ~ 10x1057 = 1058 eV ~ 2x1039 J
Chemical energy will be radiated away during the time
But the Sun’s age is at least 4.6 billion years!
Also, there is too hot for molecules in the sun
Slide
Note:
If E is total energy stored in the sun (in J);
L is luminosity, or the rate with which this energy is spent
(in J/sec);
Then the time it takes to spend all energy is T = E/L sec
Slide
Gravitational energy?
As the Sun radiates its thermal energy to outer space,
it shrinks, and the central temperature is increased (!)
The energy source is the
gravitational energy of a star
If the energy is radiated away with luminosity L = 4x1026 J/s,
The Sun would radiate all its energy during the time
But the Sun’s age is at least 4.6 billion years!
Slide
Stellar Energy Sources
Gravitational Energy?
Recall that Gravitational Potential Energy is U = -G Mm/r. This says that as particles
move toward each other, the potential energy is more negative.
For two particles may eventually end up in a bound orbit. The Virial Theorem says
that the total energy in the bound system is E=(1/2)U. So, 50% of the energy is
available to be radiated away.
Mr
r
dmi
Stellar Energy Sources
Gravitational Energy
Now, instead of a point mass, dmi, consider a shell of thickness dr with mass
dm = 4πr2 ρ dr
Differential potential energy is
ρ
Mr
Integrating gives Potential Energy
R
dr
Approximate density
as constant over
volume
ρ ≈ ρ = M/(4/3 πR3)
Mr ≈ 4/3 πr3 ρ
Stellar Energy Sources
Gravitational Energy
Inserting these into the Potential
Energy equation gives:
Solving gives:
Applying the Virial Theorem,
E=U/2:
This is the amount of energy “Lost” in the gravitational collapse
of system that ends up in a bound state.
Stellar Energy Sources
Gravitational Energy
Example, assume the Sun started as a spherical cloud of Hydrogen with a very,
very large Radius, Ri >> R⊙. The energy radiated away is
ΔEg = - (Ef - Ei) ≈ -Ef ≈ (3/10) GM⊙2 / R⊙ = 1.1 x 1041 J.
We know the Sun has a Luminosity of 3.826 x 1026 W (J/s). Dividing ΔEg by the
luminosity gives an estimate for how long it would take the Sun to radiate away
its gravitational potential energy.
t = ΔEg / L⊙ ≈ 107 yr.
Called the “Kelvin-Helmholtz” timescale (people who first worked it out). Does
this make sense ?
Nuclear reactions?
Slide
Nuclear reactions?
• Fission: decay of heavy nuclei into lighter fragments
•Fusion: synthesis of light nuclei into a heavier nucleus
Energy released per proton is ~10-20 MeV!!
Slide
Energy is released in fusion reaction if the sum of masses of initial
nuclei is larger that the mass of the final nucleus
hydrogen
mp + m p
hydrogen
Positron (antielectron)
Deuterium
MD + m e < 2 m p
ΔM = 2 mp- MD - me
Energy released E = ΔM c2
neutrino
Famous Einstein’s relation: E = mc2
Deuterium has larger binding energy than protons (more tightly bound)
Slide
What is binding energy?
It exists due to attractive forces between parts of a
compound system: protons and neutrons in a nucleus,
electrons and ion in an atom, Earth and moon, etc.
Binding energy is negative!: Ub = -|Ub|
Total energy of a system is the sum of energies of
its parts plus binding energy:
E = E1 + E2 + Ub = E1 + E2 - |Ub|
Slide
When is the energy released in fission reactions?
Energy is released in fission reaction if the mass of an initial nucleus is
larger that the sum of masses of all final fragments
MU > MRb + MCs + 3 mn
Rubidium and Cesium are more
tightly bound, or have larger
binding energy than Uranium.
It is energetically favorable for
Uranium to split.
ΔM = MU – (MRb + MCs + 3 mn)
Energy released E = ΔM c2
Famous Einstein’s relation: E = mc2
Slide
|Ub|
There are no heavy elements
on the stars
Slide
Energy Production
Energy generation in the sun
(and all other stars):
Nuclear Fusion
= fusing together 2 or more
lighter nuclei to produce
heavier ones.
Nuclear fusion can
produce energy up to
the production of iron;
For elements heavier than
iron, energy is gained by
nuclear fission.
Slide
Binding energy
due to strong
force = on short
range, strongest
of the 4 known
forces:
electromagnetic,
weak, strong,
gravitational
Slide
Hydrogen Fusion
Proton-proton cycle: four hydrogen nuclei fuse
to form one helium nucleus
Slide
Einstein’s relation: E = mc2
Energy released in one cycle:
(Binding energy)
0.007, or 0.7% of the rest energy of
protons (4mpc2) is released
Hans Bethe 1939
Slide
This is 107 times more efficient than
chemical reactions!
Does nuclear fusion provide
enough energy to power the Sun?
Assume 1056 protons in the core:
There is more than enough nuclear fuel
for 1010 years!
Slide
How much hydrogen should be fused per second to provide the Sun’s luminosity?
Nuclear fusion efficiency:
0.7% of the hydrogen mass is converted into radiation in the p-p cycle
600 million tons of hydrogen are fused every second on the Sun!
Matter-antimatter annihilation has even higher efficiency: 100% !!
Slide
Proton-proton cycle
Slide
Proton-proton cycle
Step 1
Step 2
Step 3
All positrons annihilate with electrons creating gamma-quanta
Slide
Step 1:
1H
+ 1H --> 2H + positron + neutrino
Nuclear Fusion is governed by strong nuclear force. But, to fuse two hydrogen
atoms, they must overcome the Coulomb barrier and come at ~ 1 fm from each other.
Recall that the electric (repulsive!) force between two protons is (1/4πε0) q2/r2.
Protons should be hot!
Slide
How hot??
Make assumption that the energy to overcome the Coulomb barrier is the thermal
energy of the gas. Refer to relative velocity of two nuclei (protons) using their reduced
mass, μm.
The point where the total energy is zero is where the two nuclei get to their closest
approach and then are repelled by the Coulomb force:
(1/2)μmv2 = (3/2)kT = (1/4πε0) (Z1Z2e2)/r
T = (Z1Z2e2) / 6πε0 kr
For two hydrogen atoms, Z1=Z2=1 and r≈1 fm = 10-15 m. This then gives T≈1010 K.
But, the center of the Sun is only ~1.6 x 107 K.
How does nuclear fusion take place ?
George Gamow 1930s
Answer is Quantum Mechanics. Recall that we learned that the Heisenberg
Uncertainty principle states that the uncertainties in the position and momentum of a
particle are related by
ΔxΔp > h/2
Quantum Mechanics allows
that the location can not be
known precisely. Allows
particle to “exist” past
Coulomb barrier, and eventually
fuse !
Stellar Energy Sources
Nuclear Energy
Estimate quantum mechanical effect on temperature. Use that the “wavelength” of a
particle is λ=h/p, which means we can rewrite the Kinetic energy as:
(1/2)μmv2 = p2/2μm = (h/λ)2 / 2μm
Then we can set the distance of closest approach equal to one wavelength (where
the height of the potential barrier is equal to the Kinetic Energy).
(1/4πε0) Z1Z2e2 / λ = (h/λ)2 / 2μm
Solving for λ and substituting r=λ into
T = (Z1Z2e2) / 6πε0 kr
we have
Tquantum = Z1Z2e4 μm / (12π2ε02h2k)
For two protons, μm = mp/2 and Z1=Z2=1 which gives Tquantum ≈ 107 K. That’s
better, and quantum mechanics matters !
Step 2
Takes 6 seconds to occur
Slide
Step 3
Takes 1 million years to occur
Slide
More on stellar nucleosynthesis
Common notations
An Element is specified by a # of protons, Z.
How do protons stay together in nuclei ?
Turns out, then need neutrons to glue the nuclei together or the +e charge of the
protons would break the nuclei apart.
An Isotope of an element is identified by the # of neutrons, N, in a nucleus.
The number of nucleons (protons + neutrons) is A = Z + N.
A is refereed to as the mass number. Masses of atomic particles are:
mp = 1.67262158 x 10-27 kg = 1.00727646688 u
mn = 1.67492716 x 10-27 kg = 1.00866491578 u
me = 9.10938188 x 10-31 kg = 0.0005485799110 u
1 u is the mass of a Carbon-12 nucleus ÷ 12 (definition).
But, a carbon 12 nucleus might have a mass of 6mp + 6mn = 12.096 u > 12 u.
What is “missing” is the binding energy of C
Stellar Energy Sources
Nuclear Energy
Nuclear Fusion releases energy. It converts mass into energy. Recall Relativity, E=mc2.
1 u = 931.494013 MeV/c2.
Note that the mass of hydrogen in the ground state, mH = 1.00782503214 u. This says
that mH < mp + me = 1.00783. The difference is actually -13.6 eV.
The Sun is fusing He from H. A He-4 nucleus has a mass of 4.0026 u.
4 Hydrogen atoms have a mass of 4.0313 u.
Δm = 0.028697 u, or 0.7% of the total energy.
This is an energy of E=Δmc2 = 26.731 MeV. This is the binding energy of a He-4
nucleus. To break apart a He-4 nucleus takes this much energy.
Stellar Nucleosynthesis
Conservation laws
There certain conservation laws in nature, that must be obeyed. Some are
Conservation of electric charge : all products have same net charge as reactants.
Conservation of Lepton number : Leptons are “light things”, electrons, positrons,
muons, neutrinos. Must have same lepton # before and after a reaction.
Example: e- + e+ → 2γ
On LHS an electron and positron annihilate.
Initial total charge is qi = -1 + 1 = 0.
Total lepton # is Li = 1 (for e-) + -1 (for e+) = 0.
Final charge qf = 0 and final lepton # Lf = 0.
Two photons are required to conserve momentum (another conservation law).
Stellar Nucleosynthesis
Fusion Reactions in Stars
There are several paths that Stars can use to fuse He from H.
The Proton-Proton (PP) chain
4 1 1H → 24He + 2e+ + 2νe + 2γ
The intermediate steps involve the intermediate products Deuterium (2H) and
1
3
helium-3 ( He)
2
1
1H
1
2
+ 11H→ 21H + e+ + νe
1H
3He
+ 12H→ 32He + γ
+23He → 42He + 2 1H
1
Each reaction has its own rate because each has its own Coulomb
Barrier to overcome.
Stellar Nucleosynthesis
Fusion Reactions in Stars
A variant is the PP II chain (starting with step 3 in the PP)
2
+ 24He → 74Be + γ
3He
4
7Be
+ e- → 73Li + νe
7Li
+ 11H → 2 42He
3
Yet another variant is the PP III chain (starting with Step 2 in PPII)
4
7Be
8
5 B
+ 11H → 85B + γ
→ 48Be + e+ + νe
8
4 Be
→ 2 42He
There are other possibilities. You can imagine others, then you work out what the timescales
are (how long does the reaction take). This determines if it matters.
Stellar Nucleosynthesis
Fusion Reactions in Stars
Stellar Nucleosynthesis
Fusion Reactions in Stars
The Carbon-Nitrogen-Oxygen (CNO) Cycle
This cycle uses C, N, and O as a catalyst for the fusion of He. Proposed by Hans Bethe in
1938. There are several variants.
Starting with the last line of
the Primary CNO cycle
12
6 C
+ 11H→ 137 N + γ
13
7 N
→ 13C + e+ + νe
13
6 C
+ 11H → 147N + γ
14
7 N
+ 11H
15O
15N
7
→
→
15N
7
15O
8
+
+γ
e+ +
νe
+ 11H → 126C + 4He
2
Primary CNO Cycle
+ 11H → 168O + γ
15
7 N
16
8 O
17
9 F
17O
8
+ 11H → 179 F + γ
+
→ 17
8 O + e + νe
+ 11H → 147N + 42He
Variant CNO Cycle (occurs
0.04% of the time)
Stellar Nucleosynthesis
Fusion Reactions in Stars
Note that as H → He the mean molecular weight increases. The ideal gas law predicts that the
central pressure, PC, will then decrease. The star will no longer be in equilibrium and will
collapse, raising the temperature. At some point He begins to “burn” (fuse to C).
Triple Alpha Process
Fusion of Three 4He nuclei to form 12C. Called “alpha”
because an alpha particle is a 4He nucleus.
4
2 He
4
8Be
+ 24He⇌ 84Be
+ 24He → 126C + γ
Stellar Nucleosynthesis
Fusion Reactions in Stars
Carbon and Oxygen Burning
After sufficient Carbon has been produced, further fusion occurs. Generally
this is done by adding 4He nuclei. These
elements are called “α” elements
2
(created by α-particle capture).
12
6 C
+ 24He → 168O + γ
16
8 O
+ 24He → 20
10Ne + γ
At higher temperatures still:
{
16O
8
12C
6
+ 126C→
+ 2 4He
***
2
20
10 Ne
+ 42He
23
11 Na
+ 11H
23Mg
12
24Mg
12
+ n ***
+γ
{
24
12 Mg
16O
8
+ 168O→
+ 2 4He
***
2
28
16 Si
+ 42He
31
15 P
+ 11H
31S
16
+n
32S
16
+γ
*** reactions are endothermic - they absorb energy rather than release it. These are rare.
|Ub|
There are no heavy elements
on the stars
Slide
Most Abundant Elements in the
Solar Photosphere.
Most abundant cosmic elements are H, He,
O, C, Ne, N, Mg, Si, Fe. True for cosmos and
the Sun.
Note: These are all α elements. Stars are
very efficient at making α elements !
Element
Atomic #
Log Relative Abundance
H
1
12.00
He
2
10.93 ± 0.004
O
8
8.83 ± 0.06
C
6
8.52 ± 0.06
Ne
10
8.08 ± 0.06
N
7
7.92 ± 0.06
Mg
12
7.58 ± 0.05
Si
14
7.55 ± 0.05
Fe
26
7.50 ± 0.05
S
16
7.33 ± 0.11
Al
13
6.47 ± 0.07
Ar
18
6.40 ± 0.06
Ca
20
6.36 ± 0.02
Ng
11
6.33 ± 0.03
Ni
28
6.25 ± 0.04
Stellar Nucleosynthesis
Fusion Reactions in Stars
Where do elements with Z > 26 come from ?
s-Process Nucleosynthesis
One way is by s-process, s- for “slow”. Free neutrons do not feel Coulomb Barrier and
collide with nuclei. Occasionally they stick, making larger nuclei. If neutron flux is not too
great, these heavier nuclei decay before more neutron captures.
Technetium (Tc) has no stable isotopes (all decay). But it is found in the atmospheres of
giant stars. Most abundant isotope, 99Tc has a half-life of
200,000 yrs, much less than
43
lifetime of star. Must be forged in the star and dredged up.
However, there are occasions when the neutron flux is much, much higher... especially when
nucleosynthesis stops in stars, causing the cores to collapse, which increases the neutron
density.
The solar neutrino problem
Slide
Neutrino have zero or very small mass and almost do not interact with matter
10,000 years
Slide
Neutrino image of the Sun
Slide
The Davis experiment
400,000 liters of perchlorethylene
buried 1 mile deep in a gold mine
About 1 Chlorine atom per day is
converted into Argon as a result of
interaction with solar neutrino
There are 1032 Cl atoms in a tank!
Much more difficult than finding
a needle in a haystack!!
Slide
Sudbury neutrino observatory: 1000 tons of heavy water D2O
Slide
32,000 ton of ultra-pure water
13,000 detectors
Slide
Slide
Slide
Observed neutrino flux is 2 times lower than the theoretical prediction!
Slide
Slide
The problem has been finally solved just recently:
Neutrinos “oscillate”!
They are converted into other flavors: mu and tau neutrinos
Neutrinos should have mass
Particle physics models should include this
effect
Slide
Slide