* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download NOVERIL 240 MG TABLETS
Survey
Document related concepts
Tablet (pharmacy) wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Environmental impact of pharmaceuticals and personal care products wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Psychopharmacology wikipedia , lookup
Transcript
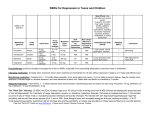
פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר על ידו בנובמבר 05 NOVERIL® 240 MG TABLETS )(dibenzepin Prescribing Information Novartis NPI 1 Page 2 Noveril Name of the medicinal product NOVERIL® 240 mg tablets 2 Qualitative and quantitative composition Dibenzepin* hydrochloride 240 mg * INN rec. 3 Pharmaceutical form Coated tablets 4 Clinical particulars 4.1 Therapeutic indications Adults For the treatment of depression Children and adolescents In children and adolescents, there is not sufficient evidence of safety and efficacy of Noveril® in the treatment of symptoms of depression. The use of Noveril in children and adolescents (0-17 years of age) is therefore not recommended. 4.2 Posology and method of administration Dosage Doses must be adapted to individual requirements. Lower-than-usual doses are recommended for elderly patients. The dosages are the following: Oral administration Age group Average Maximum (mg per day) (mg per day) Adults 480 720 Elderly patients 240 480 The dose should be gradually increased up to an optimum therapeutic level and continued for 4 to 8 weeks. As with all tricyclic antidepressants, 1 to 3 weeks of treatment may be needed until the optimal therapeutic effect is achieved. Following remission, the dose may usually be reduced to one-half to one-third for maintenance therapy. In adult patients, treatment should begin with Noveril 240 mg tablets, the daily dose being given as a single dose or in 2 divided doses. (Note: tablet remnants of the Noveril 240 mg NOV API NOV05 MoH V1 REF17082005 Novartis NPI Page 3 Noveril form may occasionally be passed with the feces. They contain no active substance.) If coated tablets are used, the daily dose must be divided into 3 doses. 4.3 Contraindications Known hypersensitivity to the drug, narrow-angle glaucoma. 4.4 Special warnings and special precautions for use Risk of suicide is inherent to severe depression and may persist until significant remission occurs. Patients with depressive disorders, both adult and paediatric, may experience worsening of depression and/or suicidality or other psychiatric symptoms, whether or not they are taking antidepressant medication. Antidepressants increased the risk of suicidal thinking and behaviour (suicidality) in short-term studies in children and adolescents with depressive disorders and other psychiatric disorders. All patients being treated with Noveril for any indication should be observed closely for clinical worsening, suicidality and other psychiatric symptoms (see section 4.8 Undesirable effects), especially during the initial phase of therapy or at times of dose changes. Modifying the therapeutic regimen, including possibly discontinuing the medication, should be considered in these patients, especially if these changes are severe, abrupt in onset, or were not part of the patient's presenting symptoms. Families and caregivers of both paediatric and adult patients being treated with antidepressants for both psychiatric and nonpsychiatric indications, should be alerted about the need to monitor patients for the emergence of other psychiatric symptoms (see section 4.8 Undesirable effects), as well as the emergence of suicidality, and to report such symptoms immediately to health care providers [2]. Prescriptions for Noveril should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose. Caution should be exercised in patients at risk of paralytic ileus, with prostatic enlargement, heart failure, hyperthyroidism, bronchial asthma and impaired hepatic or renal function. In epileptic patients, Noveril may lower seizure threshold. It should only be used if seizures are adequately controlled under anti-epileptic medication. Regular checks should be made on blood pressure if the patient is receiving antihypertensive agents. If the patient experiences difficulty in falling asleep, the evening dose should either be taken before 4 p.m. or combined with a sedative. Caution is indicated in patients with a history of thrombosis because Noveril may increase the risk of thromboembolism due to sedation and immobilization of the patient. 4.5 Interactions with other medicinal products and other forms of interaction Tricyclic antidepressants may enhance: • the response to alcohol especially during the first days of treatment (consumption of alcohol should be avoided during treatment with Noveril) NOV API NOV05 MoH V1 REF17082005 Novartis NPI • • • Page 4 Noveril the effects of CNS depressants and anticholinergics the cardiovascular effects of sympathomimetics including amphetamine (concomitant treatment may result in tachycardia, arrhythmia and hypertension) the effects of oral anticoagulants, possibly by inhibiting enzymatic metabolism of the anticoagulant. Tricyclic antidepressants may decrease: • the antihypertensive effect of methyldopa and guanethidine • the effect of anticonvulsants (see section 4.4 Special warnings and special precautions for use) Effectiveness of tricyclic antidepressants may be reduced by concomitant intake of barbiturates due to hepatic enzyme induction, whereas cimetidine may increase the effectiveness due to inhibition of their metabolism. Concomitant intake of MAO inhibitors may increase the risk of neuroleptic malignant syndrome with hyperpyretic episodes, convulsions and hypertensive crises. 14 days should elapse between the discontinuation of antidepressant and the initiation of MAO-inhibitor therapy. 4.6 Pregnancy and lactation Noveril should be used during pregnancy and lactation only under compelling circumstances. 4.7 Effects on ability to drive and use machines Noveril can impair the patient's reactions, e.g. driving a vehicle and operating machinery. 4.8 Undesirable effects In the initial stage of therapy, Noveril may cause headache, tiredness, drowsiness or agitation and inner restlessness. Anticholinergic effects such as dry mouth, accommodation disturbances, constipation and micturition disorders may also be observed. Although therapeutic doses have little effect on blood pressure or the ECG, the following cardiovascular symptoms may occur: tachycardia, hypotension, dizziness and, rarely, arrhythmia. On rare occasions, skin rashes, agranulocytosis, vasculitis have been reported. 4.9 Overdose Symptoms: accommodation disturbances; dryness of mucosa; vertigo, dizziness, alternation between excitation and apathy; hyperthermia; cramps, grand mal seizures, coma; tachycardia, hypotension; AV-block, arrhythmia; cardiac arrest; respiratory depression. Treatment: elimination of the drug from the gastrointestinal tract by gastric lavage (in case of overdosage with Noveril 240 mg tablets use the stomach tube with the largest possible lumen), administration of activated charcoal. Symptomatic treatment of the cardiovascular and respiratory system. Cardiac arrhythmias may be controlled by alkalinization of blood or, in refractory cases, by phenytoin under ECG monitoring. Administration of physostigmine to control anticholinergic effects and of anticonvulsants to control convulsions. Special attention NOV API NOV05 MoH V1 REF17082005 Novartis NPI Page 5 Noveril should be paid to metabolic acidosis. Close monitoring for not less than 5 days (because of late effects!). 5 Pharmacological properties 5.1 Pharmacodynamic properties Pharmacotherapeutic group: Tricyclic antidepressant, non selective monoamine reuptake inhibitors, ATC code: N06A A08 Noveril is a tricyclic antidepressant of the dibenzo-epine group. It is a selective noradrenaline uptake inhibitor, which exhibits in vitro and in vivo imipramine-like effects. It is also qualitatively comparable to desipramine, nortriptyline and maprotiline. It binds strongly to histamine H1 receptors in the brain and, to a lesser extent, to cholinergic receptors. The pharmacological profile of Noveril corresponds widely to its biochemical properties: histamine antagonism, tetrabenazine antagonism, potentiation of various noradrenergic effects and anticholinergic effects. 5.2 Pharmacokinetic properties After oral administration of the 80 mg tablet, dibenzepin is absorbed with a half-life of 0.5 hours, giving rise to maximal plasma concentrations of about 100 ng/mL. The absolute bioavailability does not exceed 25% due to first-pass metabolism. The volume of distribution is approx. 300 L. Protein binding is 80%. Dibenzepin is mainly eliminated by metabolization, with a terminal half-life of 5 hours. Only 1% of the dose is excreted unchanged in the urine. The main metabolite in plasma arises from demethylation of dibenzepin in the side chain. Its plasma concentration is approximately 1.5 times that of dibenzepin. This metabolite possesses similar pharmacological properties as dibenzepin. Once-a-day long-term dosing of the 240 mg tablet yields similar plasma concentrations as t.i.d. administration of the 80 mg tablet. 6 Pharmaceutical particulars Noveril must be kept out of the reach and sight of children. Manufacturer: Novartis Farma S.p.A., Italy for Novartis Pharma AG, Basel, Switzerland License Holder: PharmaExcel Ltd. 23 Hasivim St., Petach-Tikva NOV API NOV05 MoH V1 REF17082005