* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Study Manual
Survey
Document related concepts
Transcript
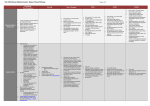
Final revision . September 25, 2014 A randomised clinical trial of intravitreal ranibizumab (Lucentis) vs. intravitreal aflibercept (Eylea) for the treatment of age-related macular degeneration (AMD). Study Manual COORDINATING CENTRE Academic unit of Ophthalmology Australian National University Canberra Hospital Yamba Dve. Garran ACT 2505 Australia CHIEF INVESTIGATOR Professor Ted Maddess Tel +61 2 6125 4099 [email protected] INVESTIGATORS Dr Rohan Essex; (m) 0417 504 965 [email protected] Dr Randev Mendis Prof JM Provis Dr CF Carle (PhD) Dr F Sabeti (PhD) ASSOCIATE INVESTIAGTORS Prof Robyn Guymer Prof Tien Wong Page No. 1 of 17 Final revision . September 25, 2014 1. Research Plan 1.1 Background Age-related Macular Degeneration (AMD) is a very common problem in the elderly. “Wet” AMD is the aggressive form of the disease characterised by choroidal neovascularisation, sub macular fibrosis, and rapid progression to blindness when left untreated. On average there are approximately 3 new patients with wet AMD diagnosed per week in the ACT. Additionally, the fellow eyes of patients with known unilateral wet macular degeneration are at high risk of developing wet macular degeneration (43% at 5 years).1 The mainstay of treatment for Macular degeneration is intraocular injections with either Lucentis or Eylea, both very similar drugs, with clinically proven equivalent efficacy. 2 Initial studies demonstrated the efficacy of monthly treatment, however it has been shown that equivalent results can be achieved with less than monthly dosing.2,3,4 Less than monthly dosing is often given on a pro re nata (PRN) basis, with monthly review, and treatment only when there is clinical evidence of disease activity. There is presently debate as to which of the two agents is the better treatment for Macular degeneration. One study suggests that the frequency of Eylea therapy can be reduced to second monthly with similar results to monthly Lucentis, however postmarketing clinical experience does not support a duration of action twice that of Lucentis.2 Additionally the study design was such that less than monthly Lucentis was not evaluated. Multifocal pupillographic perimetry (mfPOP) is a novel objective measure of visual function developed by the Chief investigator and co-investigator 1. With Coinvestigators 2 and 3, it has been shown to be a sensitive measure of retinal function in AMD, and also to measure improvement in macular function with treatment. References 1. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001 Oct;119(10):1417-36. 2. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012;119:2537-2548. 3. CATT_Research_Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. New England J Med 2011;364:1897-1908. 4. Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, Reeves BC; IVAN study investigators. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013 Oct 12;382(9900):1258-67. 5. Sabeti F, Maddess T, Essex RW, James AC. Multifocal pupillography identifies ranibizumab induced changes in retinal function for exudative age-related macular degeneration. Invest Ophthalmol Vis Sci 2012;53:253-260. 1.2 Objective The investigators plan to randomise 60 patients with new onset wet AMD to either Lucentis or Eylea therapy and to manage them according to standard, accepted clinical practice (prn with monthly review, following a 3 month induction period). They will be Page No. 2 of 17 Final revision . September 25, 2014 followed using both the standard testing and also mfPOP to see if mfPOP is an effective tool at monitoring disease progress on therapy, and also to see if mfPOP can detect any subtle differences between the two medications. The specific aims of the study are to test the following hypotheses: Aim 1: To determine whether the clinical changes in disease activity, particularly early disease recurrence while on treatment, can be detected using mfPOP. Aim 2: To determine the temporal relationship between the clinical changes in disease activity and mfPOP results (i.e. to determine if mfPOP results precede clinical changes, change in parallel, or lag behind clinical changes). Aim 3: To observe whether mfPOP can detect any differences between the two agents. Aim 4: To observe whether there are any differences between the two agents using conventional clinical endpoints (visual acuity, frequency of injections, macular thickness as measured by OCT) Safety outcomes will be: Incidence and severity of ocular adverse events including severe (>15 letter) loss of vision and increase in geographic atrophy (on fundus autofluorescence). Incidence and severity of non ocular adverse events. Page No. 3 of 17 Final revision . September 25, 2014 2. Design Outline The study will be a double blind, randomised controlled trial, comparing ranibizumab and aflibercept for the treatment of new onset wet AMD. Following confirmation of eligibility, patients will be randomised to one of the treatment groups. The treatment regimen used will be a “prn” (pro re nata) regimen, with the first three injections mandated. The patient and the clinicians assessing the patient will be masked to treatment allocation. A separate, un-masked clinician will perform the injections when required. The study will run for 2 years. 2.1 Eligibility and consent Patients who are thought to be eligible for the study will be referred to the study centre (by an ophthalmologist) where they will be seen within 2 weeks. Baseline assessments will be performed, and fluorescein angiography will be performed at this visit (or a copy of the angiogram will be reviewed if it has been performed elsewhere). Study inclusion/exclusion criteria will be checked. If the patient is found to be eligible for the study then the study will be explained, and an information sheet will be provided to the patient. Provided the patient understands the study and has no questions, informed consent will be obtained. Randomisation then occurs, and injection 1 will be scheduled within 1 week of this visit (max 2 weeks), and the study visit schedule will be mapped. If the patient is not found to be eligible for the study they will be referred back to their ophthalmologist for prompt treatment. 2.1.1 Inclusion Criteria Age >= 50 years New diagnosis of wet AMD requiring treatment with anti-VEGF agents in the opinion of the investigator. Bilateral new disease is acceptable, as is unilateral new disease and end-stage disease in the fellow eye. Best corrected visual acuity of >=20 letters (6/120) 2.1.2 Exclusion Criteria Wet AMD in the fellow eye already receiving anti-VEGF therapy, or other disease in the fellow eye requiring anti-vegf therapy. (end stage wet amd in the fellow eye is acceptable, as are drusen of any extent) Anti-VEGF therapy in the fellow eye within the last 6 months, or systemically within 6 months. Previous treatment with anti-VEGF agents in the study eye ever. (previous laser greater than 6 months ago is acceptable, but not within 6 months) Haemorrhage greater than 50% of the lesion area. Glaumomatous optic neuropathy in the study eye Presence of disease known to affect pupil movement in response to efferent stimuli, eg posterior synechiae, third nerve lesion, etc. Cataract surgery within the last 6 months. Cataract that is likely to require surgery within 2 years Maculopathy due to other causes Page No. 4 of 17 Final revision . September 25, 2014 o diabetic macular oedema, myopic macular degeneration, retinal vein occlusion). o Myopic refraction <=-6 diopters (spherical equivalent). In patients who have had refractive procedures (cataract surgery or refractive surgery), the refraction prior to the procedure is used. If not available, then the presence of fundus features consistent with high myopia (significant periparillary atrophy, posterior staphyloma) will be used as exclusion criteria. o Choroidal neovascularisation due to causes other than AMD (eg pathologic myopia, multifocal choroiditis, angioid streaks, sorsby’s macular dystrophy, previous macular laser, Central serous retinopathy ) An ocular condition that would prevent visual acuity improvement despite resolution of oedema (such as foveal atrophy, sub-foveal fibrosis or optic atrophy) Any condition which would affect follow-up or photographic documentation (e.g. geographical, psycho-social) Patient has a condition or is in a situation that in the investigator’s opinion may put the patient at significant risk, may confound the study results, or may interfere significantly with the patient’s participation in the study. Known allergy to ranibizumab or aflibercept. 2.2 Randomisation The method of randomisation will be block randomisation, with variable block size (2 to 4). There will be stratification by study site if more than one is participating. Randomisation allocations will be placed in sequentially numbered opaque envelopes, and the number on the envelope will be the study number allocated to the patient. Randomisation will occur at visit #-1 (see study schedule). 2.2.1 Second eye involvement Patients (not eyes) will be randomised. Only one eye of a patient will be included in the study. If both eyes of a patient are eligible for the study at baseline then the right eye will be included. If the fellow eye subsequently develops wet AMD requiring anti-VEGF therapy then it will receive the same medication as the study eye. Fellow eyes requiring anti-VEGF therapy during the study will be managed using a prn protocol with monthly review. These reviews will occur at the usual study visits, and treatment will be given by the un-masked “injecting” investigator. 2.3 Masking The investigators will be masked to the randomisation allocation. This includes all investigators involved in patient assessment, including those performing acuity assessments, and the doctors(s) who determine disease activity. They must not perform nor witness the injections, nor be involved in generating the prescriptions for the patient. No record of the study medication will be made in the clinical chart other than in the “sealed section” (see below). Page No. 5 of 17 Final revision . September 25, 2014 The patient will be masked to treatment allocation. They cannot handle their medication at any stage (e.g. pick it up from the pharmacy). The un-masked injecting investigator (and their assistant if present) will not mention the name of the study medication within earshot of the patient. Each study site must have at least one un-masked investigator. The clinician(s) performing the injections (the “injecting investigator”) and the clinician who writes the prescription for the anti-VEGF medication will not be masked. This will probably (but not necessarily) be the same person. The clinical chart of each study participant will have a sealed section containing the randomisation allocation and the injection details. Prior to any injections the un-masked injecting clinician will double check the sealed chart against the open chart to ensure the correct medication will be given. Any inadvertent or deliberate unmasking of investigator or patient, will be documented and reported. Page No. 6 of 17 Final revision . September 25, 2014 2.4 Visit schedule and Testing Regardless of the specific anti-VEGF the patient is randomised to, the injection schedule and indications for treatment will be the same. Patients will be reviewed every 4 weeks. On enrolment the visit schedule will be planned for the two years of the study. A visit may be brought forward or delayed by no more than 2 weeks, however subsequent visits will still occur at the originally planned time. If a visit cannot be performed within this time-period due to unavoidable circumstances is will be regarded as missed, and the next scheduled visit will be attended. Re-mapping the visit schedule will be permitted during the study if necessary, but there will still be 28 study visits. The visit schedule is presented in appendix 1. The enrolment/screening visit will occur one week prior to the first injection. Randomisation will occur at this screening visit. On the day of the first injection the investigators will ensure that a significant new haemorrhage has not occurred since the previous enrolment visit. If there is a significant new haemorrhage present (>50% of the lesion area) the eye will be withdrawn from the study. This will be reported, but the eye will not contribute to the results. Injections will be given at baseline, visit 1 and visit 2. At all subsequent visits injections will be given as required, based on lesion activity. The lesion activity and the indication for injection must be documented on the visit form. If necessary for patient care, appointments may be made for any patient to be seen between scheduled visits. Such visits do not require documentation unless a complication is detected or treatment is performed. Patients will be asked at every visit whether they have been hospitalised, or suffered a stroke or a heart attack since the last visit. Any patient missing an appointment will be contacted within 1 week to establish their status and another appointment will be made. All admissions to hospital for any reason will be treated as severe adverse events and reported. 2.4.1 Visual acuity testing and refraction Refraction and visual acuity measurements will be performed for all patients by a trained vision examiner. Subjective refraction will be conducted prior to visual acuity testing to obtain bestcorrected vision at screening visit, week 12, week 52 and week 104. Refraction equipment required includes: 1. Snellen projection distance visual acuity chart or retroilluminated LOGMAR chart. 2. Phoroptor with minus cylinder lenses, or trial lens set. 3. Jackson cross-cylinders of 0.25 and/or 0.5. Page No. 7 of 17 Final revision . September 25, 2014 Best corrected visual acuity will be measured on a retro-illuminated logMAR chart mounted on a stand, starting with the chart at 4m. The lens correction from the subjective refraction will be placed in the trial frame worn by the patient. The patient’s non-tested eye will be occluded. The patient may fixate eccentrically or turn or shake his/her head to improve visual acuity. With the chart at 4m, the examiner records the total letters read. If a patient can read all the letters on a line it is acceptable to assume they can read all larger letters. Provided the patient can read all the letters on the top line at 4m, the letter score will be thirty plus the number of letters read If the patient cannot read all of the top line on the chart at 4.0 metres, the total number of letters read at 4.0m is recorded. The patient is then moved to 1.0 meter from the chart [the spherical correction in the trial frame should be changed by adding +0.75 diopters to correct for the closer test distance]. The number of letters read at 1.0 m on the top 6 lines of the chart only is then recorded. In this setting the letter score is the number of letters read at 1m, plus the number read at 4m. NOTE – visual acuity must be 20 letters or more at baseline for study eligibility. If the patient’s visual acuity is so poor that he/she cannot read any chart letters when tested at one meter then the patient’s ability to count fingers, detect hand motion, or have light perception should be evaluated. 2.4.2 OCT assessment OCT can be performed through an undilated pupil, however if quality is poor the scan will be performed post dilation. The Heidelberg Spectralis OCT will be used for all scans, using the “Dense” Spectral-Domain OCT protocol. 2.4.3 mfPOP assessment Two mfPOP stimulus variants that test the central 30° (macular) and 60° (wide-field) of the visual fields of both eyes will be utilised. They will use the new clustered volleys mfPOP presentation method and use yellow stimuli presented on a 10 cd/m 2 background. 2.4.4 Fundus autofluorescence Fundus autofluorescence images will be acquired using the Heidelberg Spectralis device. 2.4.5 Fundus photography Colour fundus photography will be performed using the Canon CR-2 digital nonmydriatic camera or equivalent. Mydriasis is permissible if image quality is poor. A single photo, centred on the fovea will be used. Page No. 8 of 17 Final revision . September 25, 2014 2.5 Activity assessment, and indications for treatment Indications for treatment at visits 3 thru 26 At each visit from visit 3 thru visit 26, treatment is indicated if the lesion is active. Treatment is withheld if the lesion is inactive or borderline. If an injection (either mandatory or as required) is contraindicated on the day of assessment (eg due to concurrent conjunctivitis), it must be performed within 2 weeks of this visit. If this cannot be done it will be given at the next study visit, and the reason for delay will be documented and reported. Definitions of lesion activity Inactive A lesion will be defined as inactive if there is no intra- or sub- retinal fluid attributable to leak from choroidal neovascularisation*, no new macular haemorrhage, and no increase in volume of pigment epithelial detachment. *The following examples are not regarded as fluid due to leak from choroidal neovascularisation small, stable pockets of intra-retinal fluid over areas of geographic atrophy, outer retinal tubulation subretinal fluid due to retinal deformation at the margins of tall drusen or tall PED. Stable serous pigment epithelial detachment (PED). See below for definition of stable. Active Intra- or sub- retinal fluid attributable to leak from choroidal neovascularisation, New macular haemorrhage Serous of fibrovascular pigment epithelial detachment which is not stable. This could be o increasing in size since last review, or o decreasing in size in response to previous injection at last review. In this setting injections will be indicated monthly until the volume of the PED stabilises. Borderline If it is unsure whether some changes on an OCT represent fluid due to leak from CNV, but the clinician is not prepared to grade the lesion as active (or inactive), then the lesion may be graded as borderline, provided the visual acuity is not more than 5 letters worse than the previous visit, and the patient does not report any subjective loss of vision. This would also be the case if it is unclear whether a fleck of haemorrhage is present. Treatment is withheld in this setting. Page No. 9 of 17 Final revision . September 25, 2014 2.6. Intravitreal injection Treatment with intravitreal ranibizumab or aflibercept will be performed in a minor procedures area in the clinic under sterile conditions. The injection eye will be prepared with several drops of g. amethocaine 1% The eye is flushed with 5% providone-iodine (or aqueous chorhexidine if the patient has a known allergy or sensitivity to betadine) A small amount of 2% lignocaine may be then administered subconjunctivally with a 30G needle to the site of the injection, if it is necessary for adequate anaesthesia. After inserting an eyelid speculum, sterilized callipers are used to mark the injection site (3mm from the limbus if pseudophakic, 4mm if phakic. 0.05 ml of the study drug will be injected into the vitreous with a 30G needle. No antibiotics are necessary post injection. 2.7 Cataract Surgery Ideally cataract surgery would be deferred until the end of the study (104 weeks). If there is progression of cataract to the extent that withholding surgery would be unethical, then surgery is permissible. If surgery is to take place, the study medicine will be given at the time of surgery (or within 1 week), regardless of disease activity. Page No. 10 of 17 Final revision . September 25, 2014 3 Adverse events At each study visit the following adverse events to be reported are to be documented (since the previous visit): Ocular Infectious Endophthalmitis Retinal Detachment Vitreous Haemorrhage Damage to the Crystalline Lens Cataract Progression to surgery Systemic Admission to hospital Cerebrovascular events (transient ischaemic attack and stroke) Cardiac ischaemic events (unstable angina, new onset angina, acute myocardial infarction) Page No. 11 of 17 Final revision . September 25, 2014 4 Statistical analysis Data will be analysed on the basis of intention to treat. The primary clinical endpoint will be change in BCVA from baseline at 12 months. Other clinical endpoints will be Change in BCVA from baseline at 3, and 24 months Proportion with >0 letter change. At 3, 12 and 24 months Number of injections in the two year study period. Change in autofluorescence, specifically area of georgraphic atrophy, at 24 months. Clinical Endpoints to be compared at each timepoint will be OCT central macular thickness Change in BCVA from baseline Safety endpoints will be those discussed in section 3, compared at 24 months. mfPOP output will be compared at 3, 12 and 24 months, looking for any differences in response between the two medications. Temporal responsiveness of mfPOP to clinical activity will be assessed by presenting plots of mfPOP results, synchronised with observed changes in clinical activity. The mfPOP device generates visual field indices for sensitivity and response delay at 44 locations per visual field, the two eyes being measured concurrently, and by responses measured from the two pupils. Thus, a given mfPOP test generates 176 (=2 pupils, x 2 eyes x 44 locations per visual field) data points. This creates problems of multiple comparisons. This issue will be dealt with in two ways depending on requirements. The first is to generate a single number per eye, such as the mean of the worst Ndeviation from normal within the visual field measured by the pupil providing the best signal to noise ratio. This provides full Bonferroni correction. These summary statistics will be used for methods such as receiver operator characteristic (ROC) plot analysis, and Kaplan-Meier survival (KMS) analysis, and cross-correlation anlaysis. ROC analysis will look at the sensitivity and specificity for 1) predicting progression during prn management or 2) based on the baseline mfPOP predicting which patients are responders after the first three injections. The KMS analysis would look at issue like which patients had outcomes worse that some criterion after N-months of prn management. The cross-correlation analysis will look at the time lag between changes in mfPOP measures and disease becoming active under prn management. These summary measures will also be combined with summary measures from other instruments, such as the OCT or acuity. This would involve created combined scores using method such as Fisher’s linear discriminant analysis. The second method to deal with multiple comparisons will to use linear mixed models where the variables with possible repeated (correlated) measures are entered as Page No. 12 of 17 Final revision . September 25, 2014 random effects. In this way the maximum power can be obtained to investigate subjectwise effects like the differences between treatment with ranibizumab or aflibercept. We will also use these models to attempt a components of variance analysis looking at the proportions variance accounted for by eye, subject, visit and disease stage, and the correltions between each, and their significance. Page No. 13 of 17 Final revision . September 25, 2014 5. Quality assurance Standardized protocols have been developed for mfPOP, assessment of lesion activity, intraocular injection, measurement of best-corrected visual acuity, OCT and intraocular pressure. The major quality assurance features of the study are: Standardised eligibility and exclusion criteria Prospective, random treatment allocation Adherence to treatment protocol and follow-up program Intention to treat statistical analysis Double-blind study design. Page No. 14 of 17 Final revision . September 25, 2014 Appendix 1. Schedule of Visits – Year 1 (schedule is identical regardless of treatment allocation) Screen Wk -1 Week 0 Wk 4 Wk 8 Wk 12 Wk 16 Wk 20 Wk 24 Wk 28 Wk 32 Wk 36 Wk 40 Wk 44 Wk 48 Wk 52 Visit# -1 0 1 2 3 4 5 6 7 8 9 10 11 12 13 Study Consent X X X X X X X X X X X X X X Concomitant Meds & Concurrent Procedures LogMAR BCVA X X X X LogMAR VA using previous refraction Low contrast VA X IOP Matrix 10-2 X X Dilated fundal examination X Cataract grading OCT Fundus autofluorescence mfPOP X X X X Fundus Colour Photography X Fluorescein angiography X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X Treatment with Study medication Y Y Y prn prn prn prn prn prn prn prn prn prn prn Adverse Events X X X X X X X X X X X X X X Page No. 15 of 17 Final revision . September 25, 2014 Appendix 1. (cont) Schedule of visits - Year 2 (schedule is identical regardless of treatment allocation) Visit # Concomitant Meds & Concurrent Procedures Wk 56 14 Wk 60 15 Wk 64 16 Wk 68 17 Wk 72 18 Wk 76 19 Wk 80 20 X X X X X X X Wk 84 21 X Wk 88 22 Wk 92 23 Wk 96 24 Wk 100 25 X X X X X X X X X X X X X X X X X X X X X X X X X X X X X Matrix 10-2 Dilated fundal examination X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X Not counted. X Fundus Colour Photography Treatment with Study medication Adverse Events X X Fundus autofluorescence mfPOP X X Cataract grading OCT X X Low contrast VA IOP 26 X LogMAR BCVA LogMAR VA using previous refraction Wk 104 prn prn prn prn prn prn prn prn prn prn prn prn X X X X X X X X X X X X Page No. 16 of 17 Final revision . September 25, 2014 Page No. 17 of 17