* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Women and Drugs - National Drug Strategy
Pharmacogenomics wikipedia , lookup
Adherence (medicine) wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Prescription costs wikipedia , lookup
Environmental impact of pharmaceuticals and personal care products wikipedia , lookup
National Institute for Health and Care Excellence wikipedia , lookup
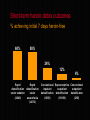
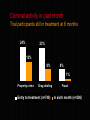
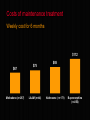
National Evaluation of Pharmacotherapies for Opioid Dependence (NEPOD) Main findings and recommendations Richard P. Mattick, Erol Digiusto, Chris Doran, Susannah O’Brien, Marian Shanahan, Jo Kimber, Nicky Henderson, Courtney Breen, James Shearer, Jenny Gates, Anthony Shakeshaft and NEPOD Trial Investigators*. This presentation was funded by the Australian Government Department of Health and Ageing under the National Illicit Drug Strategy. STRUCTURE OF PRESENTATION o Introduction o Methodology o Results o Recommendations INTRODUCTION Opioid dependence is a chronic, relapsing condition, requiring longterm care strategies Treatment works - reducing heroin use, mortality rates and crime NEPOD background and aims o Commissioned by MCDS, funded by DOH&A o NEPOD was a three year national evaluation of the: o effectiveness o safety o cost and cost-effectiveness of pharmacotherapies for opioid dependence o Trials funded by State/Territory and NH&MRC o Historical context of NEPOD Trial chief investigators ACT: Dr Gabriele Bammer, A/Prof Nick Glasgow Vic: Dr Nick Lintzeris, Dr Alison Ritter SA: Dr Robert Ali, Prof Jason White WA: Dr Allan Quigley Qld: Dr Lynn Hawken, Prof John B. Saunders NSW: Dr James Bell, Prof Richard P. Mattick Pharmacotherapies investigated A range of opioid detoxification procedures and maintenance treatments were evaluated involving: Methadone, Buprenorphine, Naltrexone, and LAAM Methadone Methadone (methadone hydrochloride) is o An opioid receptor agonist with similar pharmacological actions to morphine o Oral syrup – strength 5mgs/mL in Australia o Used to treat opioid dependence in Australia since 1969. Up until 2000, the only maintenance treatment option available Buprenorphine Buprenorphine (buprenorphine hydrochloride) is o An opioid receptor partial agonist, giving an opioid effect, yet reducing the effects of additional opioid use o Sublingual tablet – 0.4mgs, 2mgs, 8mgs o Registered in Australia (as Subutex®) in October 2000 for the treatment of opioid dependence, including detoxification and maintenance Naltrexone Naltrexone (naltrexone hydrochloride) is o A pure opioid receptor antagonist, displacing opioids if present and blocking the effects of subsequent use. It produces no opioid agonist effects o Oral tablet – 50mg o Registered in Australia (as ReVia®) for use as an adjunctive therapy to maintain abstinence following detoxification from opioids. Not currently registered for use in detoxification LAAM LAAM (levo-alpha-acetyl methadol) is o An opioid receptor agonist, chemically related to methadone, with pharmacological actions qualitatively similar to morphine and methadone o Oral syrup o Not currently registered for use in Australia. Registered in the USA since 1993 (ORLAAM®) but no longer being sold or distributed due to safety concerns (as at 2003) Scale of the project o 13 treatment trials o 1,425 participants (355 already in MMT) o More than 250 clinical & research staff o Approximately $7 million funding (mostly spent on providing treatment) METHODOLOGY o Trials collected a ‘core data set’ o Quasi-experimental nature of NEPOD o advantages and disadvantages o Generalisability of NEPOD results o treatment under trial conditions not ‘real-life’ (e.g. explicit selection criteria, close monitoring, cooperative participants) o participants may not have received preferred treatment RESULTS The 1,070 heroin dependent participants o first used heroin at 20 years o ‘habit’ first developed at 23 o At time of entering treatment: o 30 years old, 66% male and 71% not employed o high levels of depression and other mental health problems o In month prior to entering treatment: o only 3 of 28 days ‘heroin-free’ Short-term heroin detox outcomes % achieving initial 7 days heroin-free 60% 58% 24% 12% Rapid Rapid detoxification detoxification under sedation under (24/40) anaesthesia (44/76) 4% Conventional Buprenorphine Conventional inpatient outpatient outpatient detoxification detoxification detoxificaton (12/50) (19/158) (2/56) Post-detoxification treatment o Detoxification is a starting point o Advantage of buprenorphine detoxification in engaging participants in ongoing treatment o When given a choice o 5% chose naltrexone o 60% chose methadone or buprenorphine Retention in treatment methadone, buprenorphine & LAAM vs naltrexone Heroin-free days in past 28 days Trial participants still in treatment at 6 months 22 22 3 3 Methadone (n=282) (n=102) Buprenorphine (n=250) (n=77) Entry to treatment 28 25 4 2 LAAM (n=41) (n=23) Naltrexone (n=283) (n=8) In sixth month Criminal activity in past month Trial participants still in treatment at 6 months 24% 23% 13% 8% 8% 1% Property crime Drug dealing Entry to treatment (n=748) Fraud In sixth month (n=206) Serious Adverse Events (SAEs) Rates per 100 person-years o Overall rate of SAEs during treatment low - 14 per 100 person-years o SAE rates increased after participants left treatment - approximately three times higher o Heroin overdose the most common SAE, with higher rates among participants entering naltrexone than agonist treatment Costs of detoxification $2,879 $489 $605 Buprenorphine outpatient detoxification (n=158) Conventional outpatient detoxification (n=56) $2,056 $2,073 Conventional inpatient detoxification (n=50) Rapid detoxification under sedation (n=25) Rapid detoxification under anaesthesia (n=76) Cost-effectiveness ratios for detoxification The cost of achieving initial 7 days heroin-free $16,945 $3,317 $4,056 $4,645 Rapid detoxification under sedation Buprenorphine outpatient detoxification Rapid detoxification under anaesthesia $5,824 Conventional inpatient detoxification Conventional outpatient detoxification % achieving initial 7 days heroin-free 60% 12% 58% 24% 4% Cost-effectiveness of detoxification The cost of achieving initial 7 days heroin-free o Rapid detoxification under sedation appears the most cost-effective detoxification procedure o Buprenorphine outpatient detoxification appears more cost-effective than conventional procedures o Detoxification through a conventional outpatient setting is the least cost-effective method Costs of maintenance treatment Weekly cost for 6 months $112 $67 Methadone (n=287) $75 LAAM (n=44) $85 Naltrexone (n=171) Buprenorphine (n=250) Cost-effectiveness ratios for treatment Cost per extra heroin-free day in sixth month $318 $353 $195 $89 $118 LAAM (n=32)(n=12) $106 Methadone (n=61) (n=226) G.P. setting $133 Buprenorphine (n=37) (n=213) Naltrexone (n=151) Specialist clinic Cost-effectiveness of treatment The cost of an extra heroin-free day o Methadone appears the most cost-effective treatment currently available o Naltrexone treatment is the least cost-effective o Treatment in G.P. (shared-care) setting appears more cost-effective than in specialist clinics RECOMMENDATIONS Recommendations regarding clinical practice 1. Continue to support methadone o o the most cost-effective treatment available but look for cost-efficiencies with buprenorphine 2. Provide a range of effective treatment options o all treatments provide benefits Recommendations regarding clinical practice Improve retention 3. o o o o range of treatments provide incentives (convenient treatment pathways from clinics to G.P. care to abstinence) eliminate disincentives (e.g., costs to patients) address psychosocial needs Encourage GP shared-care model 4. o o cost-effective accessible Recommendations regarding clinical practice 5. Link detoxification to continuing treatment 6. Use buprenorphine for outpatient detoxification 7. Provide rapid detoxification under sedation o o o for patients who want naltrexone treatment anaesthesia is not necessary naltrexone is not registered for rapid detoxification Recommendations regarding further research o Buprenorphine in pregnancy & ‘medical maintenance’ o Combined buprenorphine / naloxone tablet (Suboxone®) o Transfer from buprenorphine to naltrexone o Naltrexone retention – sustained release preparations? o GP shared-care costs & effects o LAAM o Cost-benefit of treatment – crime o Monitor implementation of buprenorphine Contacts for further information o For further information regarding the NEPOD Project, contact the: National Drug and Alcohol Research Centre (NDARC) on (02) 9385 0333 http://ndarc.med.unsw.edu.au o For further details regarding individual trials, refer to the published articles, or contact the investigators who conducted the research