* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Crystalline phase responsible for the permanent second
Survey
Document related concepts
Transcript
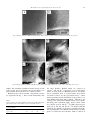
Optical Materials 30 (2007) 338–345 www.elsevier.com/locate/optmat Crystalline phase responsible for the permanent second-harmonic generation in chalcogenide glass-ceramics Marie Guignard a, Virginie Nazabal a,*, Xiang-Hua Zhang a, Frédéric Smektala a, Alain Moréac b, Stanislas Pechev c, Hassina Zeghlache d, Alexandre Kudlinski d, Gilbert Martinelli d, Yves Quiquempois d a c Equipe Verres et Céramiques, Sciences Chimiques de Rennes, UMR-CNRS 6226, Université Rennes 1, 35 042 Rennes Cedex, France b Groupe Matière Condensée et Matériaux, UMR 6626, Université Rennes 1, 35 042 Rennes Cedex, France Institut de Chimie de la Matière Condensée de Bordeaux (ICMCB), CNRS UPR 9048 87, av. du Dr A. Schweitzer, 33608 Pessac Cedex, France d Laboratoire de Physique des Lasers Atomes et Molécules, UMR CNRS 8523, Centre d’Etudes et de Recherches sur les Lasers et Applications, Université Lille 1, 59 655 Villeneuve d’Asq Cedex, France Received 26 January 2006; accepted 26 July 2006 Available online 23 January 2007 Abstract Permanent second-harmonic generation (SHG) has been demonstrated in chalcogenide based glass-ceramics containing non-linear micro-crystals with a size of a few micrometers. A glass composition from the Ge–Sb–S system was chosen as the reference glass for its stability against crystallization and atmospheric corrosion. Metallic cadmium was introduced in this matrix to promote crystallite formation resulting in infrared transparent glass-ceramics. A volume crystallization of b-GeS2 phase was obtained within the glass media by heating the glass samples at 370 °C for different durations. The glass-ceramics were then investigated by Raman spectroscopy, X-ray diffraction and NanoSIMS. The second-order non-linear signals were recorded by using Maker fringes experiment and were studied as a function of the crystallized volume fraction. The results indicated a non-linearity in chalcogenide glass-ceramics about one hundred times lower than a-quartz for a 1 mm thick sample heat treated 144 h. Ó 2006 Published by Elsevier B.V. Keywords: Chalcogenide glasses; Infrared glass-ceramics; Second- harmonic generation; NanoSIMS, Raman spectroscopy 1. Introduction The generation of second-order non-linear optical effects in chalcogenide glasses is still of great interest due to their large infrared transparency (10–20 lm) which allows envisaging of electrooptic modulators in the IR spectral region. In the electric dipolar approximation, second-harmonic generation (SHG) is allowed neither in isotropic media nor in centro-symmetrical media. Nevertheless, SHG has been evidenced in several isotropic glass systems poled by an applied electric field [1–3]. Recently, second-harmonic (SH) signal has been recorded in Ge–(Ga)–Sb–S chalcogen* Corresponding author. Tel.: +33 2 23 23 57 48; fax: +33 2 23 23 56 11. E-mail address: [email protected] (V. Nazabal). 0925-3467/$ - see front matter Ó 2006 Published by Elsevier B.V. doi:10.1016/j.optmat.2006.07.021 ide glasses after a thermal poling treatment leading to a high second-order non-linear susceptibility v(2) up to 8 pm/V depending on the composition [4,5]. However, the induced v(2) is not stable and decreases with time in these low energy band-gap glasses. Another route for generating an optical SH signal is the presence of optical non-linear crystals within a glass media. A macroscopic anisotropy can be introduced into the glasses when the precipitating crystallites are non centrosymmetric. Intrinsic second-order non-linear optical properties have already been induced in several surface crystallized transparent glass-ceramics [6–8]. Recently, Takahashi et al. reported SHG in a 40BaO–20TiO2–40SiO2 nano-crystallized glass where particles were dispersed and randomly oriented within the whole volume of the glass media M. Guignard et al. / Optical Materials 30 (2007) 338–345 leading to a SH intensity about 0.01 times that of a-quartz for a 1 mm thickness sample [9]. At the same time, they reported that efficiency of the SH conversion could be enhanced by highly orienting the crystallization under the surface of the glass-ceramic sample, leading to a secondorder non-linear coefficient v(2) equal to 26 pm/V [10]. We have previously demonstrated for the first time SHG in transparent chalcogenide glass-ceramics [11]. The purpose of the present paper is mainly to present the elaboration of glass-ceramics in Cd-doped Ge–Sb–S system and to characterize the crystalline phase responsible for the second-order non-linear properties in these materials. 2. Experimental 2.1. Materials synthesis Glass-ceramic samples were obtained by heating the reference glass, whose chemical composition is Ge23Sb11S65Cd1, at 370 °C for different durations as reported previously [11]. In order to be used as references, both a- and b-GeS2 polycrystalline compounds were prepared by direct synthesis from high purity (5N) germanium and sulfur elements in a silica tube sealed under vacuum. The ampoule containing the melt was heated and homogenized 12 h at 1000 °C. a-GeS2 was obtained by cooling this silica tube from 1000 °C to 20 °C at a rate of 2 °C/min. b-GeS2 was obtained by crystallization of glassy GeS2 by heating it 168 h at 480 °C. Note that vitreous GeS2 was prepared by quenching the stoichiometric germanium–sulfur mixture from 1000 °C to 20 °C at a rate of 50 °C/s. A dc electric field was applied during the ceramization process to generate an oriented crystallization in the glass-ceramics [12] and thus to enhance the SH efficiency. For that purpose, a glass sample was placed between two silicon electrodes and a dc electric field was applied through the sample during the heat treatment (410 °C). The temperature of the heat treatment was increased in this experiment compared to the simple heat treatment to decrease its duration. A sample was simply heat treated in the same conditions to be used as a reference. In this experimental set-up, the cathode is grounded and the field intensity cannot exceed 1 kV. 2.2. Chemical and physical characterizations Thermal analyses were carried out on small pieces of glass, about 50 mg, in sealed aluminum pans in the temperature range of 25–500 °C. The measurements were performed at a heating rate of 10 °C/min by means of a differential scanning calorimeter (DSC) with an accuracy of about ±2 °C. X-ray diffraction (XRD) data were collected while heating the reference glass at 380 °C using diffractometer operating with Co Ka radiation (k = 1.7905 Å) (voltage: 35 kV, current: 30 mA) with a step width of 0.017° and a counting time of 4.7 s/step. This temperature close to the crystallization process temperature (370 °C) 339 was selected after a preliminary study in temperature in the range of (25 °C–460 °C), to allow an easier characterization of the crystallized phases by decreasing the duration of the experiment. Ten acquisitions were realized under nitrogen gas, each one lasting about 5 h. Micro-particles crystallizing in the reference glass were observed using optical and scanning electronic microscopes (SEM). SEM observations were performed on a freshly broken surface leading to a better contrast. Energy Dispersive X-Ray Spectroscopy (EDS) was then used to identify the chemical composition of both glassy and crystalline phases of the studied samples. Raman spectra were recorded at room temperature with a micro-spectrometer. A Ti:Sapphire laser was used as a source emitting a beam at a wavelength of 775 nm (300/ 400 mW). Note that the spatial resolution of the focused laser beam is about 1 lm in width and 5 lm in depth. aand b-GeS2 Raman spectra were collected on respective polycrystalline powders. Glass media and particles Raman spectra were collected on glass-ceramics heated 72, 96, 110 and 144 h at 370 °C. Observation of the samples with an optical microscope while analyzing permitted us to record glass media and then particle spectra by focusing the laser beam in different parts of the samples. NanoSIMS analyses were realized at the CAMECA Company (Courbevoie, France) on a glass-ceramic heated 144 h at 370 °C by using a NanoSIMS 50 spectrometer. A cesium primary ionic source was used for the measurement of 16O , 32S , 74Ge , 121Sb . The Cs+ ions were accelerated by a high voltage of 16 kV and an electron gun was used to compensate the surface charging. The surface of the samples was coated with gold thin film to avoid any electrical charging phenomena while it is subjected to the bombardment of the Cs+ ionic beam. Images were obtained by collecting simultaneously the four different ions. 2.3. Optical characterizations For optical characterization, glass-ceramic samples were polished to get 1 mm thick discs with plane and parallel sides. For SH intensity measurement, b-GeS2 compound was crushed into powder and sieved into six different size ranges: less than 20, 20–40, 40–50, 50–63, 63–80, and 80– 100 lm. These powders were then placed between two microscope plates to obtain 0.5 mm thick samples. Visible–IR spectra were recorded at room temperature between 500 and 2000 nm using a CARY spectrophotometer (Varian). In the mid- and far-infrared region, optical transmission was measured using a Fourier transform spectrophotometer (Bruker). SHG measurements were realized using a process based on a Maker Fringes set-up [13]. This experiment utilized a pump beam produced by an optical parametric oscillator (type I, BBO crystal in a resonator), itself pumped by a Nd:YAG pulsed laser operating at 532 nm with a 10 Hz repetition rate. The BBO crystal produced two beams: one signal at 727 nm and the idler at 340 M. Guignard et al. / Optical Materials 30 (2007) 338–345 around 2 lm. Appropriate filters allowed only the idler beam to reach the sample creating SH signal. The glassceramic samples were fixed on a rotating table whose vertical rotation axis crossed the pump. The Maker fringes setup was configured and calibrated using an a-quartz crystal 3. Results In the Ge–Sb–S glass forming system, the Ge25Sb10S65 glass composition was chosen for its stability against crystallization. Different amounts of metallic cadmium were then introduced in this glass in order to obtain non-centrosymmetric CdS micro-crystals. Indeed, these crystals exhibit a large second-order non-linear susceptibility [14]. A maximum of 1 mol% could be added with higher contents leading to uncontrollable crystallization. A systematic study of the thermal stability of the obtained glasses indicates that the Ge23Sb11S65Cd1 composition is the best one to fabricate reproducible glass-ceramics. Thermal analysis has shown that this reference glass has a glass transition temperature Tg of about 305 °C and a small crystallization peak beginning at 380 °C for a heating rate of 10 °C/min. The XRD patterns of the reference glass powder heated at 380 °C are represented on Fig. 1. After 5 h of heat treatment at 380 °C, the diffraction diagram shows the formation of a crystalline form superimposed over the glassy matrix and indicates that the main crystalline phase in the glass-ceramic is at this time Cd4GeS6 (Cc space group, monoclinic symmetry [15]). For longer durations of the heat treatment, characteristic peaks of the b-GeS2 phase (Fdd2 space group, orthorhombic symmetry [16]) appear and become predominant after 10 h at 380 °C indicating that this phase preferentially crystallizes in the reference Fig. 1. XRD pattern for Ge23Sb11S65Cd1 glass powder heat treated at 380 °C. a, b and c represent characteristic peaks in a-GeS2, b-GeS2 and Cd4GeS6 diagrams, respectively. glass. Few other peaks cannot be interpreted and a-GeS2 allotropic phase might also be present in the glass-ceramic. Both Cd4GeS6 and b-GeS2 are non centro-symmetric crystals and consequently, can present second-order non-linear optical properties. Then we fixed the heat treatment temperature at 370 °C which gave the best compromise between the particle size and the duration of the heat treatment. Several samples were heat treated at 370 °C for 48 to 144 h to study the evolution of chemical and physical properties. The crystallization process was monitored by following the transmission in the visible region. The glass-ceramic samples become less and less transparent on account of the scattering losses induced by the precipitation of particles within the whole glass volume. No significant change was observed on the transmission spectrum for the 48 h heated sample compared to the reference glass (Fig. 2). The maximum of the transmission is almost constant in the mid-infrared range for the 72 and 96 h heat treated samples. Whereas it decreases to about 54% and 39% for the 110 and 144 h heat treated samples, respectively at the wavelength of 10.6 lm. The observation of the glass-ceramic samples under SEM indicates that two different kinds of crystals are generated after heating the reference glass. Nano-crystals with a size of about 10–20 nm are observed in large quantity even before the ceramization process (Fig. 3a). These particles slightly grow during the heat treatment (Fig. 3b). The second type of particles are micro-crystals with a size of about several micrometers, which appear during the heat treatment. As shown on Fig. 4, the number and the size of these micro-particles both increase during the ceramization process indicating that nucleation and crystal growth simultaneously occur in the reference glass while heating at 370 °C. Their crystallized volume fractions are estimated from the calculation of the crystallized surface fraction determined from five SEM images of each glass-ceramic Fig. 2. Optical transmission of Ge23Sb11S65Cd1 non-heat treated glass (nt) and of Ge23Sb11S65Cd1 glass-ceramics heat treated 72 (a), 96 (b), 110 (c) and 144 h (d) at 370 °C (1 mm thick). M. Guignard et al. / Optical Materials 30 (2007) 338–345 341 Fig. 3. Images obtained by SEM of Ge23Sb11S65Cd1 glass-ceramics non-heat treated (a) and heat treated 72 h (b) at 370 °C. Fig. 4. Images obtained by SEM of Ge23Sb11S65Cd1 glass-ceramics heat treated 72 (a), 96 (b), 110 (c) and 144 h (d) at 370 °C. sample. The calculated crystallized volume fraction as well as the average size of the particles are given in Table 1 for glass-ceramics heat treated for 72 to 144 h at 370 °C. Raman spectra collected froma- and b-GeS2 powders are represented in Fig. 5. These results demonstrate that Table 1 Average particles’ size and crystallized volume fraction in Ge23Sb11S65Cd1 glass-ceramics heat treated during 72, 96, 110 and 144 h at 370 °C Duration of the heat treatment at 370 °C (Hours) 72 96 120 144 a (lm) ± 5% q (%) ± 25% 1.5 0.1 2.5 0.8 3.5 2.9 6.0 10.8 the most intensive Raman bands are centered at 361 cm 1 and 343 cm 1, respectively, in good agreement with a previous study [17]. Raman spectra for in a glassy and a crystallized areas of a glass-ceramic heat treated for 144 h at 370 °C, are also represented in Fig. 5. Glass media spectrum is mostly dominated by the presence of a large Raman band from 230 to 460 cm 1 induced by the overlap of the characteristic Raman bands of the pyramidal [SbS3] and tetrahedral [GeS4] entities. These bands are centered at about 302 cm 1 for [SbS3] trigonal pyramids [18], at 343, 369 and 433 cm 1 for [GeS4] tetrahedra vibrations [19]. Finally, for the micro-crystallites spectrum, the superimposing of the Raman bands related to b-GeS2 phase, for which the most intensive band is centered at 342 M. Guignard et al. / Optical Materials 30 (2007) 338–345 Fig. 5. Raman spectra of a-(a) and b-GeS2 (b) polycrystalline powders and crystallized (c) and amorphous (a) areas recorded in a Ge23Sb11S65Cd1 glass-ceramic heat treated 144 h at 370 °C. 343 cm 1, on the Raman spectrum from the glass media is obvious. The images obtained by NanoSIMS analyses from a glass-ceramic heat treated 144 h at 370 °C are given in Fig. 6. Concentration scale is represented as a color scale and indicates that black and red colors correspond to the Fig. 6. Images obtained by NanoSIMS for 16 O, 32 S, 74 Ge, 121 rejection and the saturation of each element, respectively. The intensities of the signals recorded for the four components depend on their respective sensitivity and thus only a qualitative information can be extracted from this scale. In these images, particles seem to crystallize homogeneously into a Ge- and S-rich phase with a parallel epipedal form. At the same time, a depletion in germanium is observed all around the crystals while an accumulation in antimony is clearly shown. Considering that oxygen signal recording cannot be attributed only to a charge effect, the image obtained from oxygen analyses evidences that this element is still present in the glass media even after the purification. However, Fig. 6 shows that oxygen is not present in the micro-crystals and may have been pushed away from the nuclei during the ceramization process. Maker fringe pattern collected from our chalcogenide glass-ceramics has been previously described [11]. The SH signal reaches a maximum for a laser beam angle of incidence on the glass-ceramic disc around 0°. Then the SH signal decreases when the absolute angle of incidence increased indicating that particles are randomly oriented within the glass media. In the present work, it is clearly proved in Fig. 7a that SH signal intensity, recorded for an angle of incidence of 0°, is improved by increasing the crystallized volume fraction during the ceramization process whereas a decrease of the optical transmission is Sb elements from a Ge23Sb11S65Cd1 glass-ceramic heat treated 144 h at 370 °C. M. Guignard et al. / Optical Materials 30 (2007) 338–345 343 Fig. 7. Second-harmonic intensity in Ge23Sb11S65Cd1 glass-ceramics heat treated for 72 to 144 h at 370 °C (1 mm thick) (a) and in b-GeS2 polycrystalline powder (0.5 mm thick) (b) compare to a-quartz. Fig. 9. Maker fringes pattern of Ge23Sb11S65Cd1 glass-ceramics heat treated 8 h at 410 °C (1 mm thick) (continuous line), heat treated 8 h at 410 °C and subjected to a 1 kV dc electric filed (1 mm thick) (dashed line). observed. In parallel, SHG is highlighted here in b-GeS2 polycrystalline powder and an increase of the SH signal intensity is observed in a first approach with an increase of the particles size (Fig. 7b). The influence of a dc electric field on the crystallization process has been studied. When applying a dc electric field (1.0 kV) through a glass sample during the ceramization process, in addition to the classical volume crystallization, a randomly oriented surface crystallization occurs. This phenomenon is only observed below the sample surface in contact with the anodic electrode (Fig. 8). Finally, note that no significant change of the SH intensity was observed in glass-ceramics after applying a dc electric field during the ceramization process compared to glass-ceramics sample obtained for an equivalent simple heat treatment (Fig. 9). On the contrary, a slight decrease of the SH intensity is observed when the sample is subjected to the dc field. duced in Ge–Ga–S glass system, up to 8 mol% [20,21]. The specific thermal melting treatments and the batch weight of about 60 g used in our study can partially explain these observations. Instead of expected CdS crystallites, Cd4GeS6 phase precipitated in Ge23Sb11S65Cd1 glass. The presence of nano-particles with a size of about 10 nm before the ceramization process might represents the precipitation of these crystals during the quenching of the melt. After the ceramization process, the b-GeS2 phase seems to be the most stable crystallized phase. The formation of the germanium disulfide (GeS2) (DfH°298 = 125.4 kJ/mol [22]) is not thermodynamically favored compared to the diantimony trisulfide (Sb2S3) or the cadmium sulfide (CdS), which have standard enthalpies of formation at 298 K of 138.4 kJ/mol [22] and 161.9 [23], respectively. The large quantity of germanium in the glass composition compared to cadmium and antimony may be responsible for the preferential crystallization of GeS2 micro-particles with a size of about a few micrometers. An increase of both the number and the size of these micro-particles is observed when duration of the heat treatment at 370 °C increases. This behavior suggests that both nucleation and growth 4. Discussion Only a slight amount of cadmium can be introduced in our reference glass compared to the large quantities intro- Fig. 8. Images obtained by SEM of the anodic side (a) and the cathodic side (b) of a Ge23Sb11S65Cd1 glass-ceramic heat treated for 8 h at 410 °C. 344 M. Guignard et al. / Optical Materials 30 (2007) 338–345 of the crystals occur in the same time. However, b-GeS2 phase seems to crystallize into mono-crystals with relatively homogeneous spatial distribution and size within the glass matrix. At this temperature, the viscosity may be low enough to allow the particles growth by diffusion of the germanium atoms towards the nuclei and the diffusion of the antimony atoms away from them in a reasonable time as it was shown by NanoSIMS analyses. We assume that the non-linear optical behavior of the obtained glass-ceramics is analogous to that of a polycrystalline powder with randomly oriented non-centro-symmetric particles dispersed within a glass media In this case, the main contribution of the SHG may take place in each separate micro-particles. The glass matrix might play the role of index matching of the micro-crystallites which would then permit the second harmonic not to be significantly scattered due to reflection and refraction at particle interfaces. Consequently, the whole SH flux could be contained in a narrow cone in the forward direction [24]. For non-linear polycrystalline powder, the expected Maker fringe pattern should correspond to an invariable straight line with the incident angle variation of the fundamental beam. In our case, micro-crystallites precipitate in a high refractive index glass matrix (about 2.2). The decrease of the SH signal observed on Maker fringes pattern when the absolute incident angle of the laser beam increases is mostly due to the Fresnel losses at the glass interfaces at high angles. According to Kurtz and Perry [24], the SHG intensity in a polycrystalline powder can be analyzed with regard to the r/lc ratio where r is the particle size and lc is the coherent length of the particle, lc = kx/[4(n2x nx)]. Taking into account of an approximation of the refractive index of both the crystallized phases and the working wavelength kx, we estimate that their coherent length is in the range of 10–40 lm at the fundamental beam of about kx = 2 lm. The SHG intensity of Cd4GeS6 powders (90 lm) measured with a reference of quartz powder has already been reported and presents an attractive I 2x =I 2x quartz value equal to 50 (at x = 1.064 lm) [25]. It has been observed that glass-ceramics containing only nano-crystals of Cd4GeS6 (10 nm) do not show any SHG probably because the crystal size is too small compared to the coherent length in these samples and the crystallized volume fraction is too low. b-GeS2 micro-crystals have a size close to the coherent length in our glass-ceramics and the existence of secondorder non-linear optical properties has clearly been evidenced inb-GeS2 polycrystalline powder. Consequently, b-GeS2 phase is the predominant phase which can generate permanent SH signal in our materials. An increase of the SHG intensity versus the crystallized volume fraction clearly related tob-GeS2 crystallites is qualitatively observed. Thus an increase of the SH intensity with an increase of the crystal size can be expected [24]. However, the increase of the number of the particles may also be responsible for the enhancement of the SH intensity in our glass-ceramics and it is difficult to distinguish and sep- arate these two contributions. Because nano-structured glasses containing only crystals of about 10 nm precipitated during the quenching do not show any SHG, we consider that the contribution of the nano-scopic heterogeneity on the second-order non-linearity in our glass-ceramics is nil or low compared to the SHG induced by the presence of the non-centrosymmetric b-GeS2 micro-crystals. This theory, firstly proposed in potassium niobium silicates by Sigaev et al. [26], suggests that SHG in nano-structured glasses results from the coupling of local electric fields, induced by the abrupt change of permittivity from a phase to another, and the third-order non-linear susceptibility v(3) [27]. SH intensities recorded in these materials remain about 10 times lower than intensities recorded in thermally poled chalcogenide glasses and are about 0.01 to 0.06 times that of a-quartz. We assume that a part of the generated SH signals is scattered out of the optical set-up and thus it is not recorded by the spectrometer. Indeed the experimental setup does not comprise of any spherical mirror or integrating sphere to collect scattered photons in backward direction so these intensities are underestimated. For the same reason, our experiment does not allow us to conclude about the phase-matchable or the non-phase-matchable character of GeS2 compound [24]. We have to highlight that the most promising feature of these glass-ceramics compared to thermal poled chalcogenide glasses is their temporal permanency. In order to optimize our results, the most efficient way to minimize scattering losses while retaining an efficient SH process should be to control the crystal size to be under 100 nm with a relatively high crystallized fraction. Another way should be to decrease the difference between the refractive index of the glass matrix and the micro-crystal. The migration of mobile cations (Na+) under the applied electric field has been reported in a similar composition as the composition of the reference glass [5]. This phenomenon may generate an internal electric field in the opposite direction which partially offset the external field. Thus the presence of the external dc electric field might be limited to the first micrometers under the anodic side of the sample and an increase of the temperature of the glass due to energy Joule resistance heating might occur in this area only as it has been suggested in CdS-doped glasses [28]. Thus a thermal effect may be at the origin of the randomly oriented surface crystallization induced by the dc electric field. Additional scattering losses due to this surface crystallization may be responsible for the decrease of SH intensity in the sample subjected to the dc electric field compare to the one simply heat treated. 5. Conclusion Permanent second-order non-linear optical effect is revealed in these sulfide glass-ceramics where b-GeS2 micro-crystallites mostly precipitate homogenously within the glass media by chemical species migration. The M. Guignard et al. / Optical Materials 30 (2007) 338–345 obtained transparent glass-ceramics generate a secondorder non-linear intensity about 0.01 that a–quartz and existence of second-order non-linear properties was evidenced in b-GeS2 polycrystalline powder. The induced crystal size is between 1 and 6 lm, which is not large enough to reveal the phase-matchable or the non-phasematchable character of the GeS2. Effect of applying an electric field during the ceramization process was studied and it was demonstrated an increase of the crystallization rate probably due to a local warming of the glass sample. Even these glass-ceramics cannot be used to fabricate waveguides because of the scattering losses, they still remain promising materials for frequency doubling in the infrared domain where few laser sources exist at this time. Acknowledgements The authors thank François Horréard and François Hillon from Cameca France (103 Boulevard Saint Denis, BP 6, 92403 Courbevoie Cedex, France) and Fabien Grasset (Sciences Chimiques de Rennes, UMR-CNRS 6226, Université Rennes 1, 35 042 Rennes Cedex, France) for useful discussions and experiments performed on NANOSIMS apparatus. References [1] R.A. Myers, N. Mukherjee, P.St.J. Russel, Opt. Lett. 16 (22) (1991) 1732. [2] K. Tanaka, A. Narazaki, K. Hirao, Opt. Lett. 25 (4) (2000) 251. [3] M. Dussauze, E. Fargin, M. Lahaye, V. Rodriguez, F. Adamietz, Opt. Express 13 (11) (2005) 4064. [4] M. Guignard, V. Nazabal, J. Troles, F. Smektala, H. Zeghlache, Y. Quiquempois, A. Kudlinski, G. Martinelli, Opt. Express 13 (3) (2005) 789. 345 [5] M. Guignard, V. Nazabal, J. Troles, F. Smektala, H. Zeghlache, Y. Quiquempois, A. Kudlinski, G. Martinelli, Proc. SPIE 5949 (2005) 202. [6] Y.H. Kao, Y. Hu, H. Zheng, J.D. Mackenzie, K. Perry, G. Bourhill, J.W. Perry, J. Non-cryst. Solids 167 (1994) 247. [7] K. Shioya, T. Komatsu, H.G. Kim, R. Sato, K. Matusita, J. Noncryst. Solids 189 (1995) 16. [8] Y. Takahashi, Y. Benino, V. Dimitrov, T. Komatsu, J. Non-cryst. Solids 260 (1999) 155. [9] Y. Takahashi, K. Kitamura, S. Inoue, Y. Benino, T. Fujiwara, T. Komatsu, J. Ceram. Soc. Jpn. 113 (6) (2005) 419. [10] Y. Takahashi, Y. Benino, T. Fujiwara, T. Komatsu, J. Appl. Phys. 95 (7) (2004) 3503. [11] M. Guignard, V. Nazabal, H.L. Ma, X.H. Zhang, H. Zeghlache, G. Martinelli, Y. Quiquempois, F. Smektala, Phys. Chem. Glass, in press. [12] J.J. Shyu, Y.H. Chen, J. Mater. Sci. 39 (1) (2004) 159. [13] P.D. Maker, R.W. Terhune, M. Nisenoff, C.M. Savage, Phys. Rev. Lett. 8 (1) (1962) 21. [14] R.C. Miller, D.A. Kleinman, A. Savage, Phys. Rev. Lett. 11 (4) (1963) 146. [15] M. Julien-Pouzol, S. Jaulmes, Acta Cryst. C51 (1995) 1966. [16] W.H. Zachariasen, J. Chem. Phys. 4 (9) (1936) 618. [17] Z. Černošek, E. Černošková, L. Beneš, J. Mol. Struct. 435 (1997) 193. [18] G. Lucovsky, F.L. Galeener, R.C. Keezer, R.H. Geils, H.A. Six, Phys. Rev. B 10 (12) (1974) 5134. [19] L. Koudelka, M. Frumar, M. Pisárčik, J. Non-cryst. Solids 41 (1980) 171. [20] S. Barnier, M. Guittard, C. Julien, Mater. Sci. Eng. B 7 (1990) 209. [21] J. Le Person, V. Nazabal, J.-L. Adam, Solid State Sci. 7 (2005) 303. [22] J.C. Philips, Phys. Rev. B 30 (10) (1984) 6195. [23] Handbook of Chemistry and Physics, CRC Press. [24] S.K. Kurtz, T.T. Perry, J. Appl. Phys. 39 (8) (1968) 3798. [25] A. Bubenzer, R. Nitsche, J. Appl. Cryst. 11 (1978) 152. [26] V.N. Sigaev, S.Yu. Stefanovich, B. Champagnon, I. Gregora, P. Pernice, A. Aronne, R. LeParc, P.D. Sarkisov, C. Dewhurst, J. Noncryst. Solids 306 (2002) 238. [27] R.T. Hart, K.M. Ok, P.S. Halasyamani, J.W. Zwanziger, Appl. Phys. Lett. 85 (6) (2004) 938. [28] Y. Yamamoto, H. Nasu, T. Hashimoto, K. Kamiya, J. Non-cryst. Solids 281 (2001) 198.