* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download View more Animal Life videos
Hologenome theory of evolution wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
List of types of proteins wikipedia , lookup
Cell theory wikipedia , lookup
Human embryogenesis wikipedia , lookup
Evolutionary history of life wikipedia , lookup
Developmental biology wikipedia , lookup
Invertebrate wikipedia , lookup
View more Animal Life videos
McGraw-Hill Science & Technology Encyclopedia:
Coelom
Top
Home > Library > Science > Sci-Tech Encyclopedia
The mesodermally lined body cavity of most animals above the flatworms and nonsegmented
roundworms. Its manner of origin provides one basis for classifying the major higher groups.
Annelids, arthropods, and mollusks have a coelom which develops from solid mesodermal bands. Within
the trochophore larva of annelids, a single pole cell proliferates two strips of mesoblast lying on either
side of the ventral midline. These bands subdivide transversely into bilateral solid blocks, the somites.
Each somite then splits internally to form a hollow vesicle, the cavity of which is the coelom. The
mollusks also form bands of mesoderm from a single pole cell, but these bands do not segment. They
split internally to form single right and left coelomic sacs, but the cavities are soon reduced and the
surrounding mesoblast disperses as separate cells, many of which become muscle. The only remnants of
the coelom in the adult are the pericardial cavity and the cavities of the gonads and their ducts. In
arthropods paired bands of mesoblast may proliferate from a posterior growth center or may separate
inward from a blastoderm, a superficial layer of cells, on the ventral surface of the egg. These bands
divide into linear series of somites which then hollow out. Their cavities represent the coelom.
Echinoderms and chordates constitute a second major group, characterized by the origin of the coelom
from outpocketings of the primitive gut wall. In echinoderms one pair of bilateral pouches evaginates
and separates from the archenteron or primitive digestive cavity. Each pouch constricts into three
portions, not homologous to the metameres of other animals.
The protochordates of the groups Hemichordata and Cephalochordata have three coelomic pouches
formed by separate evaginations of the archenteral roof. In hemichordates the head cavity remains
single as the cavity of the proboscis and has a pore to the exterior on each side. The second pouches
form cavities within the collar and also acquire external pores. The third pair is contained within the
trunk and forms the major perivisceral cavity.
In cephalochordates the head cavity divides into lateral halves. The left side communicates, by a pore, to
an ectodermal pit called the wheel organ. The second pair of pouches forms the pair of mesoblastic
somites, and the third pouches subdivide transversely to give rise to the remainder of the linear series of
somites. The upper or myotomic portion of each somite remains metameric and forms the segmental
muscles. As it enlarges, the coelomic space is displaced ventrally and expands above and below the gut
to form the perivisceral cavities and mesenteries, as described for annelids.
In vertebrates the mesoderm arises as a solid sheet from surface cells that have been involuted through
the blastopore. Lateral to the notochord, beginning at about the level of the ear, the mesoderm
subdivides into three parts: (1) the somites; (2) the nephrotomic cord, temporarily segmented in lower
vertebrates, which will form excretory organs and ducts; and (3) the unsegmented lateral plate. The
coelom arises as a split within the lateral plate. See also Animal kingdom; Gastrulation
Read more: http://www.answers.com/topic/body-cavity#ixzz1nzNGISn8
Fax: 1- 425- 458- 9358 | Toll free: 1- 877- 252 - 7763
. Forgot Password? Click HereRegister | Account
.HomeOnline TutoringHomework HelpSolution LibraryCareersMore
Pricing
About Us
Contact Us
Test Preparation
Content Development Services
Blog
Testimonials
Homework Answers > Zoology> Coelom of vertebrates Need Zoology Homework Help?
Coelom of Vertebrates
Structure of Coelom:
The lower part of Mesoderm is called Hypomere or Lateral plate. This part is non-segmented and is
continuous with a cavity called Coelom or Splanchnocoel. The puter wall of the cavity forms the lining of
the body wall and is called Parietal Peritoneum. The inner wall forms the outer covering of the
alimentary canal and viscera and so called Visceral Peritoneum. Thus the cavity enclosed within the
Parietal and Visceral Peritoneum is the Coelomic cavity. It contains a clear fluid called Coelomic fluid.
The Parietal and Visceral Peritoneum are connected to each other through a double layered Mesentry.
The ventral Mesentry connects the alimentary canal to an organ and is then called Omentum.
Partitions of Coelom:
The anterior and posterior Coelomic cavity is partitioned by a transverse septum. The anterior part
contains heart and hence called Pericardial cavity which contains Pericardial fluid. The lining of the
cavity is called Pericardial membrane. The posterior cavity contains other visceral organs and so called
Peritoneal or Abdominal cavity and the fluid called as Peritoneal fluid. This cavity is lined by Parietal or
Visceral peritoneum.
Differentiation of Coelom in vertebrates:
In anurans and reptiles the Pericardial cavity lies ventral to the anterior Peritoneal cavity. The Peritoneal
cavity has lungs in its anterior region and other visceral organs in its posterior region and is thus called
as Pleuroperitoneal cavity.
In crocodiles, birds and mammals the transverse section extends to the dorsal body wall to form a new
partition behind the lungs. This is membranous in birds and is called Oblique septum. In mammals it is
the highly muscular Diaphragm. This divides the Pleuroperitoneal cavity into Thoracic and Abdominal
cavities.
The Thoracic region has two Pleural cavities each enclosing a lung. The Pericardial cavity lies in between
the ventral portion of the Pleural cavities. The posterior region of the Diaphragm is called the peritoneal
or Abdominal cavity.
The Coelomic fluid cushions and offer protection to the visceral organs present in the Coelomic cavity.
Share these flashcards
Share on Facebook
Share on Twitter
Link or embed
About these flashcards
Created by:
blondygal007 on November 12, 2011
Log in to favorite or report as inappropriate.
Pop outDiscuss
No MessagesYou must log in to discuss this set.
Flashcards: Zoology CHAPTER 29 DEUTEROSTOMES: THE CHORDATES
Term First
Both Sides
dorsal tubular nerve cord
forms the central nervous system
Click to flip
1/27
Study: SpellerLearnTest
Play Games: ScatterSpace Race
All 27 terms
Print new! Export CombineTerms Definitions
dorsal tubular nerve cord forms the central nervous system
notochord dorsal hollow rod that extends the length of the body and serves as a firm but flexible axis
tunicates sea squirts;sessile filter feeders as adults; larvae are free swimming
tunic an enveloping or covering membrane or layer of body tissue
paedogenesis known as juvenification; the retention of juvenile characteristics in an adult
skull/cranium Bone that protects the brain
kidneys organs that filter nitrogen wastes from blood to make urine
Chrondrichthyes predatory fishes such as sharks, rays, and skates that have jaws, paired fins, skeletons
made of cartilage, and skin covered by a unique kind of scale
Amphibia Name the Class of Vertebrata: bone, tetrapod, external fertilization, anamniotic egg, lungs as
adults, gills as young, three chambered heart, ectothermic, lay eggs in water but live on land, skin is
water proof, toxin producing glands, gas exchange through skin
chordates an animal phylum that has a notochord, a dorsal hollow nerve cord, pharyngeal pouches,
and gill slits at some time in its life cycle
pharyngeal pouches openings in the lining of the upper respiratory tract (from gills in fish and
amphibian larvae)
sea squirts = tunicates; P. Chordata, Subphylum Urochordata. sessile adults w/ 2 tubes. siphons moving
h2o into & out of body. hard outer shell; "tunic". toxic secondary compounds. vibrant colors. tadpole
larvae w/ notochord in body and tail (not head)
lancelet small translucent lancet-shaped burrowing marine animal
vertebrate animals having a bony or cartilaginous skeleton with a segmented spinal column and a large
brain enclosed in a skull or cranium
somites Paired blocks of mesoderm just lateral to the notochord of a vertebrate embryo.
Superclass Agnatha superclass of eel-shaped chordates lacking jaws and pelvic fins: lampreys
Class Osteichthyes a class of fish having a skeleton composed of bone in addition to cartilage
Reptilia first completly successful group of animals to move on land; snakes, crocodilians, turtles, dry
skin/claws/amniote egg that can be layed on land
postanal tail tail that continues past the end of the digestive tract (anus)
Subphylum Urochordata "tail cord" sea squirts (tunicates). possess all chordate characteristics as larvar,
but adults only have the pharyngeal gill slits (pouches). sessile filter feeders. 2 siphons, though tunic
siphon a tube running from the liquid in a vessel to a lower level outside the vessel so that atmospheric
pressure forces the liquid through the tube
Subphylum Cephalochordata "head cord" lancelots, amphioxus (genus name). marine filter feeds,
resembles a fish. burrow into sand with head exposed. retain all chordate characteristics as adults.
segmented muscles in tail. develop from SOMITES
vertebral column the series of vertebrae forming the axis of the skeleton and protecting the spinal cord
endoskeleton internal skeleton or supporting framework in an animal
Placodermi extinct group of bony-plated fishes with primitive jaws
tetrapods vertebrate animals having 4 feet, legs, or leglike appendages
Mammalia Class: Endotherms with hair or fur. mammary glands produce milk. glandular skin with hair
or fur. external ear present. teeth are different types. diaphragm between thorax/abdomen
Home > Library > Science > Sci-Tech Encyclopedia
A class of the phylum Coelenterata which includes the fresh-water hydras, the marine hydroids, many of
the smaller jellyfish, a few special corals, and the Portuguese man-of-war. The Hydrozoa may be divided
into six orders: the Hydroida, Milleporina, Stylasterina, Trachylina, Siphonophora, and Spongiomorphida.
See separate article on each order.
The form of the body varies greatly among the hydrozoans. This diversity is due in part to the existence
of two body types, the polyp and the medusa. A specimen may be a polyp, a medusa, a colony of polyps,
or even a composite of the first two. Polyps are somewhat cylindrical, attached at one end, and have a
mouth surrounded by tentacles at the free end. Medusae are free-swimming jellyfish with tentacles
around the margin of the discoidal body.
In a representative life cycle, the fertilized egg develops into a swimming larva which soon attaches itself
and transforms into a polyp. The polyp develops stolons (which fasten to substrates), stems, and other
polyps to make up a colony of interconnected polyps. Medusae are produced by budding and liberated
to feed, grow, and produce eggs and sperm.
Most hydrozoans are carnivorous and capture animals which come in contact with their tentacles. The
prey is immobilized by poison injected by stinging capsules, the nematocysts. Most animals of
appropriate size can be captured, but small crustaceans are probably the most common food. See also
Coelenterata.
WoRMS taxon details
Anthoathecata
AphiaID: 13551
Classification: Biota > Animalia (Kingdom) > Cnidaria (Phylum) > Hydrozoa (Class) > Hydroidolina
(Subclass)
Authority
Status
Cornelius, 1992
accepted
Record
status
Checked by Taxonomic Editor
Rank
Order
Parent
Hydroidolina
Synonymised
taxa
Anthomedusae (synonym)
Athecata (synonym)
Gymnoblastea (synonym.)
Laingiomedusae (synonym)
Sources
original description: Cornelius, P.F.S., 1992. Medusa loss in leptolid Hydrozoan (Cnidaria)
hydroid rafting, and abbreviated life-cycles among their remote-island faunae: an interim review. In: J.
Bouillon, F. Boero, F. Cicogna, J.M. Gili & R.G. Hughes, eds., Aspects of hydrozoan biology. Scientia
Marina 56 2-3: 245-261.
page(s): 245 [details]
basis of record: Bouillon, J.; Boero, F. (2000). Synopsis of the families and genera of the Hydromedusae
of the world, with a list of the worldwide species. Thalassia Salent. 24: 47-296 (look up in IMIS) [details]
from synonym: Howson, C.M.; Picton, B.E. (Ed.) (1997). The species directory of the marine fauna and
flora of the British Isles and surrounding seas. Ulster Museum Publication, 276. The Ulster Museum:
Belfast, UK. ISBN 0-948150-06-8. vi, 508 (+ cd-rom) pp. (look up in IMIS) [details] [view taxon]
from synonym: Brusca, R.C.; Brusca, G.J. (1990). Invertebrates. Sinauer Associates: Sunderland, MA
(USA). ISBN 0-87893-098-1. 922 pp. (look up in IMIS) [details] [view taxon]
from synonym: Hayward, P.J.; Ryland, J.S. (Ed.) (1990). The marine fauna of the British Isles and NorthWest Europe: 1. Introduction and protozoans to arthropods. Clarendon Press: Oxford, UK. ISBN 0-19857356-1. 627 pp. (look up in IMIS) [details] [view taxon]
Direct child
taxa
Suborder Anthoathecata incertae sedis
Suborder Capitata
Suborder Filifera
Environment
marine, brackish, fresh, terrestrial
Fossil range
recent + fossil
Feedingtypes
omnivore [details]
predator [details]
scavenger [details]
Links
To GenBank
To ITIS
Notes
Diagnosis: Hydrozoans that always have a polyp stage. Hydranths either solitary or
colonial, body not covered by firm perisarc. Medusae not colonial, without statocysts, with gonads on
manubrium, with radial canals, with tentacles arising from bell-margin. Cnidome normally includes
desmonemes (not Eudendriidae and Laingiidae). [details]
Taxonomic Remark: The original spelling in Cornelius (1992) was Anthoathecata. Cornelius (1995a)
changed the spelling to Anthoathecatae, which seems unnecessary. [details]
Taxonomic status: The naming of this taxon is disputed, some prefer Anthomedusae, some Athecata
(oldest name). Molecular investigations show that this group is likely not monophyletic, it will thus most
probably
WoRMS taxon details
Anthoathecata
AphiaID: 13551
Classification: Biota > Animalia (Kingdom) > Cnidaria (Phylum) > Hydrozoa (Class) > Hydroidolina
(Subclass)
Authority
Status
Cornelius, 1992
accepted
Record
status
Checked by Taxonomic Editor
Rank
Order
Parent
Hydroidolina
Synonymised
taxa
Anthomedusae (synonym)
Athecata (synonym)
Gymnoblastea (synonym.)
Laingiomedusae (synonym)
Sources
original description: Cornelius, P.F.S., 1992. Medusa loss in leptolid Hydrozoan (Cnidaria)
hydroid rafting, and abbreviated life-cycles among their remote-island faunae: an interim review. In: J.
Bouillon, F. Boero, F. Cicogna, J.M. Gili & R.G. Hughes, eds., Aspects of hydrozoan biology. Scientia
Marina 56 2-3: 245-261.
page(s): 245 [details]
basis of record: Bouillon, J.; Boero, F. (2000). Synopsis of the families and genera of the Hydromedusae
of the world, with a list of the worldwide species. Thalassia Salent. 24: 47-296 (look up in IMIS) [details]
from synonym: Howson, C.M.; Picton, B.E. (Ed.) (1997). The species directory of the marine fauna and
flora of the British Isles and surrounding seas. Ulster Museum Publication, 276. The Ulster Museum:
Belfast, UK. ISBN 0-948150-06-8. vi, 508 (+ cd-rom) pp. (look up in IMIS) [details] [view taxon]
from synonym: Brusca, R.C.; Brusca, G.J. (1990). Invertebrates. Sinauer Associates: Sunderland, MA
(USA). ISBN 0-87893-098-1. 922 pp. (look up in IMIS) [details] [view taxon]
from synonym: Hayward, P.J.; Ryland, J.S. (Ed.) (1990). The marine fauna of the British Isles and NorthWest Europe: 1. Introduction and protozoans to arthropods. Clarendon Press: Oxford, UK. ISBN 0-19857356-1. 627 pp. (look up in IMIS) [details] [view taxon]
Direct child
taxa
Suborder Anthoathecata incertae sedis
Suborder Capitata
Suborder Filifera
Environment
marine, brackish, fresh, terrestrial
Fossil range
recent + fossil
Feedingtypes
omnivore [details]
predator [details]
scavenger [details]
Links
To GenBank
To ITIS
Notes
Diagnosis: Hydrozoans that always have a polyp stage. Hydranths either solitary or
colonial, body not covered by firm perisarc. Medusae not colonial, without statocysts, with gonads on
manubrium, with radial canals, with tentacles arising from bell-margin. Cnidome normally includes
desmonemes (not Eudendriidae and Laingiidae). [details]
Taxonomic Remark: The original spelling in Cornelius (1992) was Anthoathecata. Cornelius (1995a)
changed the spelling to Anthoathecatae, which seems unnecessary. [details]
Taxonomic status: The naming of this taxon is disputed, some prefer Anthomedusae, some Athecata
(oldest name). Molecular investigations show that this group is likely not monophyletic, it will thus most
probably
javascript:popup_window_1();
javascript:popup_window_1();
Scientific
Heteractis malu
Name
Comments A sea anemone (Anthozoa)
Reference
Creator
From D. G. Fautin and G. R. Allen. 1992. Field Guide to Anemonefishes and their
Host Sea Anemones. Western Australia Museum.
photographed by Art Reed
Specimen
Live Specimen
Condition
Copyright © 1992 Western Australia Museum
javascript:popup_window_2();
javascript:popup_window_2();
Scientific
Aglantha digitale
Name
Comment A direct-developing holoplanktonic hydromedusa (Hydrozoa) that has no polyp. The
gonads are visible through the transparent bell.
s
Copyright © 1998 Claudia E. Mills
GLOSSARY
N
nares Nostrils; the openings in the nose through which air enters.
nastic movement A plant's response to a stimulus in which the direction of the
response is independent of the direction of the stimulus. Non-directional plant
movements.
natural selection The process of differential survival and reproduction of Þtter
genotypes; can be stabilizing, directional, or disruptive. Better adapted individuals are
more likely to survive to reproductive age and thus leave more offspring and make a
larger contribution to the gene pool than do less Þt individuals. The differential
survival and reproductive successes of individuals in a variable population that
powers the evolutionary process. When all individuals survive and reproduce (except
for chance occurrences) natural selection works at a lower rate, if at all. PICTURE
nectaries Nectar-secreting organs in þowering plants that serve as insect feeding
stations and thus attract insects, which then assist in the transfer of pollen.
negative feedback The stopping of the synthesis of an enzyme by the accumulation
of the products of the enzyme-mediated reaction.
negative feedback control Occurs when information produced by the feedback
reverses the direction of the response; regulates the secretion of most hormones.
negative feedback loop A biochemical pathway where the products of the reaction
inhibit production of the enzyme that controlled their formation.
nektonic organisms "Swimmers"; one of the two main types of organisms in the
pelagic zone of the marine biome.
nephridium The excretory organ in þatworms and other invertebrates; a blind-ended
tubule that expels waste through an excretory pore.
nephron A tubular structure that is the Þltering unit of the kidney; consists of a
glomerulus and renal tubule. PICTURE
nerve cord A dorsal tubular cord of nervous tissue above the notochord of a
chordate.
nerve net An interconnected mesh of neurons that sends signals in all directions;
found in radially symmetrical marine invertebrates, such as jellyÞsh and sea
anemones, that have no head region or brain. PICTURE
nerves Bundles of neuronal processes enclosed in connective tissue that carry signals
to and from the central nervous system. PICTURE
nervous system One of eleven major body organ systems in animals; coordinates and
controls actions of internal organs and body systems, receives and processes sensory
information from the external environment, and coordinates short-term reactions to
these stimuli. PICTURE 1 | PICTURE 2 | PICTURE 3
net primary productivity (NPP) The rate at which producer (usually plants)
biomass is created in a community.
net secondary productivity (NSP) The rate at which consumer and decomposer
biomass is produced in a community.
neural tube A tube of ectoderm in the embryo that will form the spinal cord.
neuromuscular junction The point where a motor neuron attaches to a muscle cell.
neurons Highly specialized cells that generate and transmit bioelectric impulses from
one part of the body to another; the functional unit of the nervous system. A cell of the
nerve tissue having a cell body input zone of dendrites and an output zone of an axon
(of varying length). The electrochemical nerve impulse/message is transmitted by
neurons. PICTURE 1 | PICTURE 2
neurotoxin Chemical that paralyzes nerves. Neurotoxins are produced by a variety of
organisms, most notably some of the heterotrophic dinoflagellates.
neurotransmitters Chemicals released from the tip of an axon into the synaptic cleft
when a nerve impulse arrives; may stimulate or inhibit the next neuron. The chemical
that crosses the synaptic cleft and causes the transmission of the nerve message in an
adjacent neuron or the stimulation of an effector cell (muscle or gland). PICTURE
neutron An uncharged subatomic particle in the nucleus of an atom. The large (mass
approximately equal to 1 atomic mass unit), electrically neutral particle that may
occur in the atomic nucleus.
niche The biological role played by a species.
niche overlap The extent to which two species require similar resources; speciÞes
the strength of the competition between the two species.
nicotine adenine dinucleotide phosphate (NADP+) A substance to which electrons
are transferred from photosystem I during photosynthesis; the addition of the electrons
reduces NADP, which acquires a hydrogen ion to form NADPH, which is a storage
form of energy that can be transferred to the Calvin Cycle for the production of
carbohydrate.
node The stem region of a plant where one or more leaves attach. Where leaves are
attached to stems.
node of Ranvier A gap between two of the Schwann cells that make up an axon's
myelin sheath; serves as a point for generating a nerve impulse.
nondisjunction The failure of chromosomes to separate properly during cell division.
The unequal segregation of chromosomes during meiosis. This forms cells with either
too many (possibly one or more single or sets of chromosomes too many) or too few
chromosomes. Thought to be a common cause for Down Syndrome, where sufferers
often have an extra copy of chromosome 21.
nonvascular plants Plants lacking lignified vascular tissue (xylem), vascularized
leaves, and having a free-living, photosynthetic gametophyte stage that dominates the
life cycle. Common examples are the mosses and liverworts.
norepinephrine A hormone produced in the adrenal medulla and secreted under
stress; contributes to the "Þght or þight" response.
notochord In chordates, a cellular rod that runs the length of the body and provides
dorsal support. Also, a structure of mesoderm in the embryo that will become the
vertebrae of the spinal column. The stiff rod-like structure that all members of the
Phylum Chordata develop at some stage during their life.
nuclear area In prokaryotic cells, a region containing the cell's genetic information.
Unlike the nucleus in eukaryotic cells, it is not surrounded by a membrane.
nuclear pores Openings in the membrane of a cell's nuclear envelope that allow the
exchange of materials between the nucleus and the cytoplasm. PICTURE
nucleic acids Polymers composed of nucleotides; e.g., DNA and RNA.
nucleoid The area of the prokaryotic cytoplasm where the chromatin is localized.
nucleolus A round or oval body in the nucleus of a eukaryotic cell; consists of DNA
and RNA and produces ribosomal RNA (pl.: nucleoli). PICTURE
nucleosomes Spherical bodies formed by coils of chromatin. The nucleosomes in
turn are coiled to form the Þbers that make up the chromosomes.
nucleotide sequences The genetic code encrypted in the sequence of bases along a
nucleic acid.
nucleotides The subunits of nucleic acids; composed of a phosphate, a sugar, and a
nitrogen-containing base. The fundamental structural unit of the nucleic acid group of
organic macromolecules. Some nucleotides are involved in information storage (as
nucleotides in DNA), protein synthesis (as nucleotides in RNA), and energy transfers
(as single nucleotide ATP, GTP, and double nucleotide NADH and NADPH).
nucleus (atom) An atom's core; contains protons and one or more neutrons (except
hydrogen, which has no neutrons).
nucleus (cell) The largest, most prominent organelle in eukaryotic cells; a round or
oval body that is surrounded by the nuclear envelope and contains the genetic
information necessary for control of cell structure and function. PICTURE
nyctinasty A nastic movement in a plant that is caused by light and dark.
Text ©1992, 1994, 1995, 1997, 1998, 2000, M.J. Farabee, all rights reserved.
Back to Table of Contents | Back to Main Glossary Page
Email: mj.farabee
WoRMS taxon details
Anthoathecata
AphiaID: 13551
Classification: Biota > Animalia (Kingdom) > Cnidaria (Phylum) > Hydrozoa (Class) > Hydroidolina
(Subclass)
Rank
Order
Parent
Hydroidolina
Synonymised
taxa
Anthomedusae (synonym)
Athecata (synonym)
Gymnoblastea (synonym.)
Laingiomedusae (synonym)
Sources
original description: Cornelius, P.F.S., 1992. Medusa loss in leptolid Hydrozoan (Cnidaria)
hydroid rafting, and abbreviated life-cycles among their remote-island faunae: an interim review. In: J.
Bouillon, F. Boero, F. Cicogna, J.M. Gili & R.G. Hughes, eds., Aspects of hydrozoan biology. Scientia
Marina 56 2-3: 245-261.
page(s): 245 [details]
basis of record: Bouillon, J.; Boero, F. (2000). Synopsis of the families and genera of the Hydromedusae
of the world, with a list of the worldwide species. Thalassia Salent. 24: 47-296 (look up in IMIS) [details]
from synonym: Howson, C.M.; Picton, B.E. (Ed.) (1997). The species directory of the marine fauna and
flora of the British Isles and surrounding seas. Ulster Museum Publication, 276. The Ulster Museum:
Belfast, UK. ISBN 0-948150-06-8. vi, 508 (+ cd-rom) pp. (look up in IMIS) [details] [view taxon]
from synonym: Brusca, R.C.; Brusca, G.J. (1990). Invertebrates. Sinauer Associates: Sunderland, MA
(USA). ISBN 0-87893-098-1. 922 pp. (look up in IMIS) [details] [view taxon]
from synonym: Hayward, P.J.; Ryland, J.S. (Ed.) (1990). The marine fauna of the British Isles and NorthWest Europe: 1. Introduction and protozoans to arthropods. Clarendon Press: Oxford, UK. ISBN 0-19857356-1. 627 pp. (look up in IMIS) [details] [view taxon]
Links
To GenBank
To ITIS
Notes
Diagnosis: Hydrozoans that always have a polyp stage. Hydranths either solitary or
colonial, body not covered by firm perisarc. Medusae not colonial, without statocysts, with gonads on
manubrium, with radial canals, with tentacles arising from bell-margin. Cnidome normally includes
desmonemes (not Eudendriidae and Laingiidae). [details]
Taxonomic Remark: The original spelling in Cornelius (1992) was Anthoathecata. Cornelius (1995a)
changed the spelling to Anthoathecatae, which seems unnecessary. [details]
Taxonomic status: The naming of this taxon is disputed, some prefer Anthomedusae, some Athecata
(oldest name). Molecular investigations show that this group is likely not monophyletic, it will thus most
probably
Siphonophorae
Top
Home > Library > Miscellaneous > Wikipedia
Siphonophores
http://en.wikipedia.org/wiki/File:Portuguese_Man-O-War_(Physalia_physalis).jpg
Siphonophorae or Siphonophora, the siphonophores, are an order of the Hydrozoa, a class of marine
invertebrates belonging to the phylum Cnidaria. They are colonial, but the colonies can superficially
resemble jellyfish; although they appear to be a single organism, each specimen is actually a colony of
Siphonophora. The best known species is the dangerous Portuguese Man o' War (Physalia physalis).
With a body length of 40–50 m, another species of siphonophore, Praya dubia, is one of the longest
animals in the world.[1]
Contents
1 Description
2 Systematics
3 Footnotes
4 References
5 External links
Description
Siphonophores are especially scientifically interesting because they are composed of medusoid and
polypoid zooids that are morphologically and functionally specialized. Each zooid is an individual, but
their integration with each other is so strong that the colony attains the character of one large organism.
Indeed, most of the zooids are so specialized that they lack the ability to survive on their own.
Siphonophorae thus exist at the boundary between colonial and complex multicellular organisms. Also,
because multicellular organisms have cells which, like zooids, are specialized and interdependent,
siphonophores may provide clues regarding their evolution.[1]
Like other hydrozoans, certain siphonophores can emit light. A siphonophore of the genus Erenna has
been discovered at a depth of around 1,600 meters off the coast of Monterey, California. The individuals
from these colonies are strung together like a feather boa. They prey on small animals using stinging
cells. Among the stinging cells are stalks with red glowing ends. The tips twitch back and forth creating a
twinkling effect. It is theorized that twinkling red light attracts small fish that have been found eaten by
these siphonophores. While many sea animals produce blue and green bioluminescence, this
siphonophore was only the second lifeform found to produce a red light (the first being the scaleless
dragonfish Chirostomias pliopterus).[2]
Systematics
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpghttp://en.wikipedia.org/wiki/File:Haec
kel_Siphonophorae_37.jpg
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpghttp://en.wikipedia.org/wiki/File:Haec
kel_Siphonophorae_37.jpgAspects of Physophora hydrostatica (Physonectae: Physophoridae).
Plate 37 in Kunstformen der Natur by Ernst Haeckel (1904). See also below.
Due to their highly specialized colonies, siphonophores have long misled scientists. They were for a long
time believed to be a highly distinct group, but now are known to have evolved from simpler colonial
hydrozoans similar to Anthomedusae or Leptomedusae. Consequently, they are now united with these
in a subclass Leptolinae.
The Siphonophorae have long fascinated scientists and layfolk alike, due to their dramatic appearance as
well as the large size and dangerous sting of several species. Compared to their relatives, their
systematics are relatively straightforward:[3]
Suborder Calycophorae
Family Abylidae
Family Clausophyidae
Family Diphyidae
Family Hippopodiidae
Family Prayidae
Family Sphaeronectidae
Suborder Cystonectae
Family Physaliidae
Family Rhizophysidae
Suborder Physonectae
Family Agalmatidae
Family Apolemiidae
Family Athorybiidae
Family Erennidae
Family Forskaliidae
Family Physophoridae
Family Pyrostephidae
Family Rhodaliidae
The genus Stepanyantsia is of unclear affiliations; it might belong in the Agalmatidae.
-23.
Top
Home > Library > Miscellaneous > Wikipedia
Siphonophores
http://en.wikipedia.org/wiki/File:Portuguese_Man-O-War_(Physalia_physalis).jpg
Siphonophorae or Siphonophora, the siphonophores, are an order of the Hydrozoa, a class of marine
invertebrates belonging to the phylum Cnidaria. They are colonial, but the colonies can superficially
resemble jellyfish; although they appear to be a single organism, each specimen is actually a colony of
Siphonophora. The best known species is the dangerous Portuguese Man o' War (Physalia physalis).
With a body length of 40–50 m, another species of siphonophore, Praya dubia, is one of the longest
animals in the world.[1]
Contents
1 Description
2 Systematics
3 Footnotes
4 References
5 External links
Description
Siphonophores are especially scientifically interesting because they are composed of medusoid and
polypoid zooids that are morphologically and functionally specialized. Each zooid is an individual, but
their integration with each other is so strong that the colony attains the character of one large organism.
Indeed, most of the zooids are so specialized that they lack the ability to survive on their own.
Siphonophorae thus exist at the boundary between colonial and complex multicellular organisms. Also,
because multicellular organisms have cells which, like zooids, are specialized and interdependent,
siphonophores may provide clues regarding their evolution.[1]
Like other hydrozoans, certain siphonophores can emit light. A siphonophore of the genus Erenna has
been discovered at a depth of around 1,600 meters off the coast of Monterey, California. The individuals
from these colonies are strung together like a feather boa. They prey on small animals using stinging
cells. Among the stinging cells are stalks with red glowing ends. The tips twitch back and forth creating a
twinkling effect. It is theorized that twinkling red light attracts small fish that have been found eaten by
these siphonophores. While many sea animals produce blue and green bioluminescence, this
siphonophore was only the second lifeform found to produce a red light (the first being the scaleless
dragonfish Chirostomias pliopterus).[2]
Systematics
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpghttp://en.wikipedia.org/wiki/File:Haec
kel_Siphonophorae_37.jpg
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpghttp://en.wikipedia.org/wiki/File:Haec
kel_Siphonophorae_37.jpgAspects of Physophora hydrostatica (Physonectae: Physophoridae).
Plate 37 in Kunstformen der Natur by Ernst Haeckel (1904). See also below.
Due to their highly specialized colonies, siphonophores have long misled scientists. They were for a long
time believed to be a highly distinct group, but now are known to have evolved from simpler colonial
hydrozoans similar to Anthomedusae or Leptomedusae. Consequently, they are now united with these
in a subclass Leptolinae.
The Siphonophorae have long fascinated scientists and layfolk alike, due to their dramatic appearance as
well as the large size and dangerous sting of several species. Compared to their relatives, their
systematics are relatively straightforward:[3]
Suborder Calycophorae
Family Abylidae
Family Clausophyidae
Family Diphyidae
Family Hippopodiidae
Family Prayidae
Family Sphaeronectidae
Suborder Cystonectae
Family Physaliidae
Family Rhizophysidae
Suborder Physonectae
Family Agalmatidae
Family Apolemiidae
Family Athorybiidae
Family Erennidae
Family Forskaliidae
Family Physophoridae
Family Pyrostephidae
Family Rhodaliidae
The genus Stepanyantsia is of unclear affiliations; it might belong in the Agalmatidae.
ZOO2010 Laboratory Study Guide
Chapter 9, The Radiate Animals
Type your email address in the space provided.
Type your full name in the space provided.
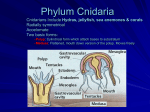
Terms to Know in This Chapter:
germ layers
gastrovascular cavity [gas trow VASS cue lar]
hydrostatic skeleton [high drow STAT ik]
polymorphism [pol eh MORE fiz um]
colloblast (sing.) [COL oh blast]
polyp (sing.) [POL up]
medusa (sing.) [meh DUE sah]
medusae (pl.) [meh DUE see]
jellyfish
sea anemone [ah NEM oh knee]
radially symmetrical
coral
tentacle (sing.) [TEN tah kul]
hypostome [HIGH po stome]
mouth
gonad (sing.) [GO nad]
basal disc
bud
cnidocyte (sing.) [NIDE oh sight]
nematocyst (sing.) [neh MAD oh sist]
cnidocil (sing.) [NIDE oh sill]
symbiont [SIM bee ont]
glutathione [glut ah THIGH on]
extracellular
intracellular
Bouin's fluid [BOW ins]
barb
hypnotoxin [hip no TOX in]
epidermis (sing.) [ep eh DERM iss]
mesoglea [mez oh GLEE ah]
diploblastic [dip low BLAST ik]
epitheliomuscular cell [ep eh THEL ee oh MUSS cue lar]
nerve net
interstitial cell [in tur STEH shul]
nutritive-muscular cell [NEW trah tive]
sensory cell
asexual
budding
monoecious [moe KNEE shuss]
dioecious [die EE shuss]
testis (sing.) [TEST tiss]
testes (pl.) [TEST tees]
egg
spermatozoan (sing.) [spur mat oh ZOE ah]
spermatozoa (pl.) [spur mat oh ZOE an]
zygote [ZIE goat]
planula (sing.) [PLAN you lah]
planulae (pl.) [PLAN you lee]
hydranth (sing.) [HIGH dranth]
gonangium (sing.) [go NAN gee um]
gonangia (pl.) [go NAN gee ah]
hydrorhiza (sing.) [high drow RISE ah]
hydrorhizae (pl.) [high drow RISE ee]
hydrocaulus (sing.) [high drow CALL us]
hydrocauli [high drow CALL eye]
perisarc [PERRY sark]
coenosarc [SIN oh sark]
hydrothecum (sing.) [high drow THEE cum]
hydrotheca (pl.) [high drow THEE kah]
blastostyle [BLAST oh style]
gonothecum (sing.) [go no THEE cum]
gonotheca (pl.) [go no THEE kah]
exumbrella [ex UM brah lah]
subumbrella [sub UM brah lah]
tentacular bulb [ten TACK you lar]
statocyst (sing.) [STAT oh sist]
manubrium (sing.) [mah NUBE ree um]
manubria (pl.) [mah NUBE ree ah]
stomach
radial canal
ring canal
tetramerous [tet rah MEER us]
rhopalium (sing.) [row FALE ee um]
rhophalia (pl.) [row FALE ee ah]
lappet (sing.) [LAP it]
oral arm
gastric pouch
scyphistoma (sing.) [sky FIST oh mah]
scyphistomae (pl.) [sky FIST oh mee]
strobilum (sing.) [STROW bee lum]
strobila (pl.) [STROW bee lah]
ephyra (sing.) [EE frah]
ephyrae (pl.) [EE free]
acontium (sing.) [ah CON tee um]
acontia (pl.) [ah CON ee ah]
columm
siphonoglyph [seh FON oh glif]
peristome (sing.) [PEAR eh stome]
pharynx (sing.) [FAIR inks]
pharynges (pl.) [fair IN geez]
septum (sing.) [SEP tum]
septa (pl.) [SEP tah]
thecum (sing.) [THEE cum]
theca (pl.) [THEE kah]
Portugeuse man-of-war
ocellus (sing.) [oh CELL us]
ocelli (pl.) [oh CELL ee]
larva (sing.) [LAR vah]
larvae (pl.) [LAR vee]
vellum (sing.) [VEL um]
vella (pl.) [VEL ah]
Genera You Need to Know:
Hydra [HIGH drah]
Daphnia [DAFF knee ah]
Obelia [oh BEEL yah]
Gonionemus [go knee oh NEE mus]
Physalia [feh SAIL ee ah]
Aurelia [or REEL yah]
Metridium [meh TRID ee um]
Classification You Need to Know:
Kingdom Animalia [an eh MALE yah]
Class Hydrozoa [high drow ZOE ah]
Class Scyphozoa [sky foe ZOE ah]
Class Anthozoa [an thow ZOE ah]
What You Need to Know:
You should be able to:
name the three classes of Cnidaria, give the characteristics of each and recognize each, on sight, in lab,
identify living Hydra and under the microscope, be able to identify tentacles, hypostome, mouth,
gonads, nematocysts, and the two layers of cells,
name the basic cell types in the epidermis and gastrodermis of Hydra,
distinguish between Hydra budding and with testes and ovaries,
identify both preserved specimens and prepared slides of Obelia and identify the major parts of the
colony,
identify preserved specimens and prepared slides of Gonionemus and recognize the major structures,
distinguish among statocysts, tentacular bulbs, rhophalia, and ocelli,
explain what is meant by polymorphism and dimorphism,
explain how you can tell most jellyfish of the class Scyphozoa from jellyfish of the class Hydrozoa,
identify from preserved specimens Aurelia and recognize the major structures associated with it,
provide life cycles for Hydra, Obelia, Aurelia and the larval types of each,
identify the major structures associated with Metridium from preserved specimens, and,
recognize several stony and soft corals provided by the instructor.
Exercises: Fill in the Blank.
The genus Obelia belongs to the class while Metridium belongs to the class .
Which class of Cnidarians has no medusae?
Which class of Cnidarians typically has a velum?
How many tentacles does a Hydra typically have? .
The stinging cell of Cnidarians is called the while the trigger is called the and the "stinger" itself is called
the .
Which cell type in Cnidarians may give rise to other types of cells?
Balance structures in the Cnidarians are called .
The middle layer of tissue in Cnidarians is called the while the middle layer in sponges is called .
The larval type in sponges is the larva and the larval type in Cnidarians is called the larva.
The gonads of the class Scyphozoa and Anthozoa are found in the .
The name of the medusae produced by Aurelia is and the polyps are called .
This is a tiny opening on either side of the mouth in Metridium used to circulate water while keeping
prey inside the pharynx.
Stinging cells in Metridium are found on tiny threads called
The walls of the cup of stoney corals is called the
Reproductive polyps in Obelia are called .
Exercises: Multiple Choice. Select the Best Answer.
Which genus has no medusae and only polyps?
(1) Hydra
(2) Obelia
(3) Metridium
(4) both 1 and 3
(5) All of the above have no medusae.
Which class has polyps?
(1) Hydrozoa
(2) Scyphozoa
(3) Anthozoa
(4) both 1 and 2
(5) all of the above
Which of the following is a colonial polyp form that floats in the ocean and is carried by winds and
waves?
(1) Obelia
(2) Aurelia
(3) Physalia
(4) Metridium
(5) Gonionemus
Which cell type is found in the gastrodermis of Hydra?
(1) interstitial cell
(2) nutritive-muscle cell
(3) epitheliomuscle cell
(4) cnidocyte
(5) sensory cell
Which terms are correctly matched?
(1) Hydra-medusa
(2) Velum-Aureila
(3) acontium-Obelia
(4) ephyra-Aurelia
(5) Physalia-scyphistoma
View more Animal Life videos
McGraw-Hill Science & Technology Encyclopedia:
Coelom
Top
Home > Library > Science > Sci-Tech Encyclopedia
The mesodermally lined body cavity of most animals above the flatworms and nonsegmented
roundworms. Its manner of origin provides one basis for classifying the major higher groups.
Annelids, arthropods, and mollusks have a coelom which develops from solid mesodermal bands. Within
the trochophore larva of annelids, a single pole cell proliferates two strips of mesoblast lying on either
side of the ventral midline. These bands subdivide transversely into bilateral solid blocks, the somites.
Each somite then splits internally to form a hollow vesicle, the cavity of which is the coelom. The
mollusks also form bands of mesoderm from a single pole cell, but these bands do not segment. They
split internally to form single right and left coelomic sacs, but the cavities are soon reduced and the
surrounding mesoblast disperses as separate cells, many of which become muscle. The only remnants of
the coelom in the adult are the pericardial cavity and the cavities of the gonads and their ducts. In
arthropods paired bands of mesoblast may proliferate from a posterior growth center or may separate
inward from a blastoderm, a superficial layer of cells, on the ventral surface of the egg. These bands
divide into linear series of somites which then hollow out. Their cavities represent the coelom.
Echinoderms and chordates constitute a second major group, characterized by the origin of the coelom
from outpocketings of the primitive gut wall. In echinoderms one pair of bilateral pouches evaginates
and separates from the archenteron or primitive digestive cavity. Each pouch constricts into three
portions, not homologous to the metameres of other animals.
The protochordates of the groups Hemichordata and Cephalochordata have three coelomic pouches
formed by separate evaginations of the archenteral roof. In hemichordates the head cavity remains
single as the cavity of the proboscis and has a pore to the exterior on each side. The second pouches
form cavities within the collar and also acquire external pores. The third pair is contained within the
trunk and forms the major perivisceral cavity.
In cephalochordates the head cavity divides into lateral halves. The left side communicates, by a pore, to
an ectodermal pit called the wheel organ. The second pair of pouches forms the pair of mesoblastic
somites, and the third pouches subdivide transversely to give rise to the remainder of the linear series of
somites. The upper or myotomic portion of each somite remains metameric and forms the segmental
muscles. As it enlarges, the coelomic space is displaced ventrally and expands above and below the gut
to form the perivisceral cavities and mesenteries, as described for annelids.
In vertebrates the mesoderm arises as a solid sheet from surface cells that have been involuted through
the blastopore. Lateral to the notochord, beginning at about the level of the ear, the mesoderm
subdivides into three parts: (1) the somites; (2) the nephrotomic cord, temporarily segmented in lower
vertebrates, which will form excretory organs and ducts; and (3) the unsegmented lateral plate. The
coelom arises as a split within the lateral plate. See also Animal kingdom; Gastrulation
Read more: http://www.answers.com/topic/body-cavity#ixzz1nzNGISn8
Fax: 1- 425- 458- 9358 | Toll free: 1- 877- 252 - 7763
. Forgot Password? Click HereRegister | Account
.HomeOnline TutoringHomework HelpSolution LibraryCareersMore
Pricing
About Us
Contact Us
Test Preparation
Content Development Services
Blog
Testimonials
Homework Answers > Zoology> Coelom of vertebrates Need Zoology Homework Help?
Coelom of Vertebrates
Structure of Coelom:
The lower part of Mesoderm is called Hypomere or Lateral plate. This part is non-segmented and is
continuous with a cavity called Coelom or Splanchnocoel. The puter wall of the cavity forms the lining of
the body wall and is called Parietal Peritoneum. The inner wall forms the outer covering of the
alimentary canal and viscera and so called Visceral Peritoneum. Thus the cavity enclosed within the
Parietal and Visceral Peritoneum is the Coelomic cavity. It contains a clear fluid called Coelomic fluid.
The Parietal and Visceral Peritoneum are connected to each other through a double layered Mesentry.
The ventral Mesentry connects the alimentary canal to an organ and is then called Omentum.
Partitions of Coelom:
The anterior and posterior Coelomic cavity is partitioned by a transverse septum. The anterior part
contains heart and hence called Pericardial cavity which contains Pericardial fluid. The lining of the
cavity is called Pericardial membrane. The posterior cavity contains other visceral organs and so called
Peritoneal or Abdominal cavity and the fluid called as Peritoneal fluid. This cavity is lined by Parietal or
Visceral peritoneum.
Differentiation of Coelom in vertebrates:
In anurans and reptiles the Pericardial cavity lies ventral to the anterior Peritoneal cavity. The Peritoneal
cavity has lungs in its anterior region and other visceral organs in its posterior region and is thus called
as Pleuroperitoneal cavity.
In crocodiles, birds and mammals the transverse section extends to the dorsal body wall to form a new
partition behind the lungs. This is membranous in birds and is called Oblique septum. In mammals it is
the highly muscular Diaphragm. This divides the Pleuroperitoneal cavity into Thoracic and Abdominal
cavities.
The Thoracic region has two Pleural cavities each enclosing a lung. The Pericardial cavity lies in between
the ventral portion of the Pleural cavities. The posterior region of the Diaphragm is called the peritoneal
or Abdominal cavity.
The Coelomic fluid cushions and offer protection to the visceral organs present in the Coelomic cavity.
Share these flashcards
Share on Facebook
Share on Twitter
Link or embed
About these flashcards
Created by:
blondygal007 on November 12, 2011
Log in to favorite or report as inappropriate.
Pop outDiscuss
No MessagesYou must log in to discuss this set.
Flashcards: Zoology CHAPTER 29 DEUTEROSTOMES: THE CHORDATES
Term First
Both Sides
dorsal tubular nerve cord
forms the central nervous system
Click to flip
1/27
Study: SpellerLearnTest
Play Games: ScatterSpace Race
All 27 terms
Print new! Export CombineTerms Definitions
dorsal tubular nerve cord forms the central nervous system
notochord dorsal hollow rod that extends the length of the body and serves as a firm but flexible axis
tunicates sea squirts;sessile filter feeders as adults; larvae are free swimming
tunic an enveloping or covering membrane or layer of body tissue
paedogenesis known as juvenification; the retention of juvenile characteristics in an adult
skull/cranium Bone that protects the brain
kidneys organs that filter nitrogen wastes from blood to make urine
Chrondrichthyes predatory fishes such as sharks, rays, and skates that have jaws, paired fins, skeletons
made of cartilage, and skin covered by a unique kind of scale
Amphibia Name the Class of Vertebrata: bone, tetrapod, external fertilization, anamniotic egg, lungs as
adults, gills as young, three chambered heart, ectothermic, lay eggs in water but live on land, skin is
water proof, toxin producing glands, gas exchange through skin
chordates an animal phylum that has a notochord, a dorsal hollow nerve cord, pharyngeal pouches,
and gill slits at some time in its life cycle
pharyngeal pouches openings in the lining of the upper respiratory tract (from gills in fish and
amphibian larvae)
sea squirts = tunicates; P. Chordata, Subphylum Urochordata. sessile adults w/ 2 tubes. siphons moving
h2o into & out of body. hard outer shell; "tunic". toxic secondary compounds. vibrant colors. tadpole
larvae w/ notochord in body and tail (not head)
lancelet small translucent lancet-shaped burrowing marine animal
vertebrate animals having a bony or cartilaginous skeleton with a segmented spinal column and a large
brain enclosed in a skull or cranium
somites Paired blocks of mesoderm just lateral to the notochord of a vertebrate embryo.
Superclass Agnatha superclass of eel-shaped chordates lacking jaws and pelvic fins: lampreys
Class Osteichthyes a class of fish having a skeleton composed of bone in addition to cartilage
Reptilia first completly successful group of animals to move on land; snakes, crocodilians, turtles, dry
skin/claws/amniote egg that can be layed on land
postanal tail tail that continues past the end of the digestive tract (anus)
Subphylum Urochordata "tail cord" sea squirts (tunicates). possess all chordate characteristics as larvar,
but adults only have the pharyngeal gill slits (pouches). sessile filter feeders. 2 siphons, though tunic
siphon a tube running from the liquid in a vessel to a lower level outside the vessel so that atmospheric
pressure forces the liquid through the tube
Subphylum Cephalochordata "head cord" lancelots, amphioxus (genus name). marine filter feeds,
resembles a fish. burrow into sand with head exposed. retain all chordate characteristics as adults.
segmented muscles in tail. develop from SOMITES
vertebral column the series of vertebrae forming the axis of the skeleton and protecting the spinal cord
endoskeleton internal skeleton or supporting framework in an animal
Placodermi extinct group of bony-plated fishes with primitive jaws
tetrapods vertebrate animals having 4 feet, legs, or leglike appendages
Mammalia Class: Endotherms with hair or fur. mammary glands produce milk. glandular skin with hair
or fur. external ear present. teeth are different types. diaphragm between thorax/abdomen
Home > Library > Science > Sci-Tech Encyclopedia
A class of the phylum Coelenterata which includes the fresh-water hydras, the marine hydroids, many of
the smaller jellyfish, a few special corals, and the Portuguese man-of-war. The Hydrozoa may be divided
into six orders: the Hydroida, Milleporina, Stylasterina, Trachylina, Siphonophora, and Spongiomorphida.
See separate article on each order.
The form of the body varies greatly among the hydrozoans. This diversity is due in part to the existence
of two body types, the polyp and the medusa. A specimen may be a polyp, a medusa, a colony of polyps,
or even a composite of the first two. Polyps are somewhat cylindrical, attached at one end, and have a
mouth surrounded by tentacles at the free end. Medusae are free-swimming jellyfish with tentacles
around the margin of the discoidal body.
In a representative life cycle, the fertilized egg develops into a swimming larva which soon attaches itself
and transforms into a polyp. The polyp develops stolons (which fasten to substrates), stems, and other
polyps to make up a colony of interconnected polyps. Medusae are produced by budding and liberated
to feed, grow, and produce eggs and sperm.
Most hydrozoans are carnivorous and capture animals which come in contact with their tentacles. The
prey is immobilized by poison injected by stinging capsules, the nematocysts. Most animals of
appropriate size can be captured, but small crustaceans are probably the most common food. See also
Coelenterata.
WoRMS taxon details
Anthoathecata
AphiaID: 13551
Classification: Biota > Animalia (Kingdom) > Cnidaria (Phylum) > Hydrozoa (Class) > Hydroidolina
(Subclass)
Authority
Status
Cornelius, 1992
accepted
Record
status
Checked by Taxonomic Editor
Rank
Order
Parent
Hydroidolina
Synonymised
taxa
Anthomedusae (synonym)
Athecata (synonym)
Gymnoblastea (synonym.)
Laingiomedusae (synonym)
Sources
original description: Cornelius, P.F.S., 1992. Medusa loss in leptolid Hydrozoan (Cnidaria)
hydroid rafting, and abbreviated life-cycles among their remote-island faunae: an interim review. In: J.
Bouillon, F. Boero, F. Cicogna, J.M. Gili & R.G. Hughes, eds., Aspects of hydrozoan biology. Scientia
Marina 56 2-3: 245-261.
page(s): 245 [details]
basis of record: Bouillon, J.; Boero, F. (2000). Synopsis of the families and genera of the Hydromedusae
of the world, with a list of the worldwide species. Thalassia Salent. 24: 47-296 (look up in IMIS) [details]
from synonym: Howson, C.M.; Picton, B.E. (Ed.) (1997). The species directory of the marine fauna and
flora of the British Isles and surrounding seas. Ulster Museum Publication, 276. The Ulster Museum:
Belfast, UK. ISBN 0-948150-06-8. vi, 508 (+ cd-rom) pp. (look up in IMIS) [details] [view taxon]
from synonym: Brusca, R.C.; Brusca, G.J. (1990). Invertebrates. Sinauer Associates: Sunderland, MA
(USA). ISBN 0-87893-098-1. 922 pp. (look up in IMIS) [details] [view taxon]
from synonym: Hayward, P.J.; Ryland, J.S. (Ed.) (1990). The marine fauna of the British Isles and NorthWest Europe: 1. Introduction and protozoans to arthropods. Clarendon Press: Oxford, UK. ISBN 0-19857356-1. 627 pp. (look up in IMIS) [details] [view taxon]
Direct child
taxa
Suborder Anthoathecata incertae sedis
Suborder Capitata
Suborder Filifera
Environment
marine, brackish, fresh, terrestrial
Fossil range
recent + fossil
Feedingtypes
omnivore [details]
predator [details]
scavenger [details]
Links
To GenBank
To ITIS
Notes
Diagnosis: Hydrozoans that always have a polyp stage. Hydranths either solitary or
colonial, body not covered by firm perisarc. Medusae not colonial, without statocysts, with gonads on
manubrium, with radial canals, with tentacles arising from bell-margin. Cnidome normally includes
desmonemes (not Eudendriidae and Laingiidae). [details]
Taxonomic Remark: The original spelling in Cornelius (1992) was Anthoathecata. Cornelius (1995a)
changed the spelling to Anthoathecatae, which seems unnecessary. [details]
Taxonomic status: The naming of this taxon is disputed, some prefer Anthomedusae, some Athecata
(oldest name). Molecular investigations show that this group is likely not monophyletic, it will thus most
probably
WoRMS taxon details
Anthoathecata
AphiaID: 13551
Classification: Biota > Animalia (Kingdom) > Cnidaria (Phylum) > Hydrozoa (Class) > Hydroidolina
(Subclass)
Authority
Status
Cornelius, 1992
accepted
Record
status
Checked by Taxonomic Editor
Rank
Order
Parent
Hydroidolina
Synonymised
taxa
Anthomedusae (synonym)
Athecata (synonym)
Gymnoblastea (synonym.)
Laingiomedusae (synonym)
Sources
original description: Cornelius, P.F.S., 1992. Medusa loss in leptolid Hydrozoan (Cnidaria)
hydroid rafting, and abbreviated life-cycles among their remote-island faunae: an interim review. In: J.
Bouillon, F. Boero, F. Cicogna, J.M. Gili & R.G. Hughes, eds., Aspects of hydrozoan biology. Scientia
Marina 56 2-3: 245-261.
page(s): 245 [details]
basis of record: Bouillon, J.; Boero, F. (2000). Synopsis of the families and genera of the Hydromedusae
of the world, with a list of the worldwide species. Thalassia Salent. 24: 47-296 (look up in IMIS) [details]
from synonym: Howson, C.M.; Picton, B.E. (Ed.) (1997). The species directory of the marine fauna and
flora of the British Isles and surrounding seas. Ulster Museum Publication, 276. The Ulster Museum:
Belfast, UK. ISBN 0-948150-06-8. vi, 508 (+ cd-rom) pp. (look up in IMIS) [details] [view taxon]
from synonym: Brusca, R.C.; Brusca, G.J. (1990). Invertebrates. Sinauer Associates: Sunderland, MA
(USA). ISBN 0-87893-098-1. 922 pp. (look up in IMIS) [details] [view taxon]
from synonym: Hayward, P.J.; Ryland, J.S. (Ed.) (1990). The marine fauna of the British Isles and NorthWest Europe: 1. Introduction and protozoans to arthropods. Clarendon Press: Oxford, UK. ISBN 0-19857356-1. 627 pp. (look up in IMIS) [details] [view taxon]
Direct child
taxa
Suborder Anthoathecata incertae sedis
Suborder Capitata
Suborder Filifera
Environment
marine, brackish, fresh, terrestrial
Fossil range
recent + fossil
Feedingtypes
omnivore [details]
predator [details]
scavenger [details]
Links
To GenBank
To ITIS
Notes
Diagnosis: Hydrozoans that always have a polyp stage. Hydranths either solitary or
colonial, body not covered by firm perisarc. Medusae not colonial, without statocysts, with gonads on
manubrium, with radial canals, with tentacles arising from bell-margin. Cnidome normally includes
desmonemes (not Eudendriidae and Laingiidae). [details]
Taxonomic Remark: The original spelling in Cornelius (1992) was Anthoathecata. Cornelius (1995a)
changed the spelling to Anthoathecatae, which seems unnecessary. [details]
Taxonomic status: The naming of this taxon is disputed, some prefer Anthomedusae, some Athecata
(oldest name). Molecular investigations show that this group is likely not monophyletic, it will thus most
probably
Complete
Containing Groups
Animals
Eukaryotes
Life on Earth
/Animals
Other Animals
Bilateria
Myxozoa
Cnidaria
Ctenophora
Placozoa
Porifera
/Myxozoa
/Ctenophora
Subgroups
Scyphozoa
Hydrozoa
Anthozoa
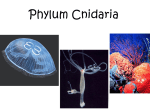
Cnidaria
Sea anemones, corals, jellyfish, sea pens, hydra
Daphne G. Fautin and Sandra L. Romano
Click on an image to view larger version & data in a new window javascript:popup_window();
javascript:popup_window();javascript:popup_window_0();
javascript:popup_window_0();
]Hydrozoa" coords=84,104,131,90]Anthozoa"
coords=84,32,131,18]Scyphozoa" coords=84,86,139,72<--]Animals" coords=5,41,14,50
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group
and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of
the other groups in the tree. This ancestor diversified over time into several descendent
subgroups, which are represented as internal nodes and terminal taxa to the right.
You can click on the root to travel down the Tree of Life all the way to the root of all Life, and
you can click on the names of descendent subgroups to travel up the Tree of Life all the way to
individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification.
To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close box
Tree following Werner (1973) and Bridge et al. (1995).
Containing group: Animals
Introduction
The exclusively aquatic phylum Cnidaria is represented by polyps such as sea anemones and
corals, and by medusae such as jellyfish. A polypoid or a medusoid cnidarian is a radially or
biradially symmetrical, uncephalized animal with a single body opening, the mouth. The mouth
is surrounded by tentacles studded with microscopic stinging capsules known as nematocysts
that are the agents of offense and defense. The possession of intrinsic nematocysts is the defining
characteristic of the phylum (Hessinger and Lenhoff 1988); nematocysts are the most diverse and
widespread of three types of cnidae (cnidos = thread) -- hence the preferred name of the phylum.
Cnidarians are diploblastic -- that is, the body and tentacles consist of two cell layers, the
endoderm (sometimes referred to as the gastrodermis) and the ectoderm (the epidermis).
Between the two cell layers is the mesoglea, which ranges from little more than a glue to bind
the layers (for example, in Hydra) to the vast bulk of the animal (for example, in jellyfish of
Class Scyphozoa). The body encompasses a single sac-like body space, the coelenteron (koilos =
cavity; enteron = intestine), which communicates with the surrounding medium through the
mouth. The less preferred name of the phylum, Coelenterata, is based on this attribute. The
coelenteron (also termed the gastrovascular cavity) serves for gas exchange and digestion.
All cnidarians are carnivorous, with cnidae and tentacles active in prey capture. Because polyps
are typically sessile, and only some medusae possess sensory structures (the most sophisticated
occur in the Cubozoa; Pearse and Pearse 1978), cnidarians are generally believed to be passive
predators, feeding on prey items that blunder into their tentacles. Some cnidarians can absorb
dissolved organic matter directly from seawater (e.g. Schlichter 1975), but it is not known how
widespread this ability is. Living within the tissues of anthozoans of many species and
hydrozoans and scyphozoans of a small number of species are unicellular algae from which the
animals derive reduced carbon (Shick 1991). Dinoflagellate symbionts, termed zooxanthellae,
are by far the most common algal symbionts; they are exclusively marine. Green algal
symbionts, termed zoochlorellae, occur in both marine and freshwater cnidarians.
The text-book depiction of the typical cnidarian life cycle is an alternation between a medusa and
a polyp (termed metagenesis), the former the sexually reproductive stage and the latter the
asexual stage. In fact, an attribute of the entire class Anthozoa is the absence of a medusa. At
least some individuals of all anthozoan species form gametes; those of some species may
reproduce vegetatively as well. The other three classes -- Cubozoa, Hydrozoa, and Scyphozoa -are often grouped as the "Medusozoa" because the medusa phase is present in them all. Indeed,
the medusa dominates the life cycle of members of the classes Cubozoa and Scyphozoa
(Cubozoa was formerly considered an order of Scyphozoa, and some specialists still consider it
as such). Life cycles of the Hydrozoa are the most diverse in the phylum: although the polyp is
the more conspicuous and persistent stage in most taxa, some lack the medusa phase, whereas
others lack the polyp phase. Hydra, which is used in many textbooks to illustrate the phylum, is
utterly atypical: a hydrozoan, it lacks a medusa, it has aggregations of gametogenic tissue that
function as gonads, and it is among only a handful of freshwater cnidarian species.
The cnidarian larva is the planula, a pear-shaped, entirely ciliated animal. In the "typical"
cnidarian life cycle, male and female medusae spawn freely into the sea, where fertilization
occurs and a planula develops. At metamorphosis, the planula settles on and attaches to the
substratum, where it metamorphoses into a polyp. The primary polyp produces additional polyps
asexually, by budding, stolonic outgrowth, or some other process, to form a clone or a colony. At
the appropriate time, determined perhaps by size of the colony or environmental conditions,
rather than or in addition to polyps, medusae are produced asexually (in Cubozoa, each polyp
metamorphoses into a medusa). They are released to take up a pelagic existence and the cycle
begins anew.
Click on an image to view larger version & data in a new window javascript: w =
window.open('/onlinecontributors/app?service=external/ViewImageData&sp=2189', '2189',
'resizable,height=500,width=713,scrollbars=yes'); w.focus();
javascript: w =
window.open('/onlinecontributors/app?service=external/ViewImageData&sp=2189', '2189',
'resizable,height=500,width=713,scrollbars=yes'); w.focus();
Idealized lifecycle of the Cnidaria.
Characteristics
The cnida, or nematocyst, which is the sine qua non of the phylum, is secreted by the Golgi
apparatus of a cell termed a cnidoblast (Watson 1988). A cnida therefore is technically not an
organelle, but, rather, the most complex secretory product known. Upon receiving the
appropriate physical and/or chemical stimulus, a cnida fires, everting a tubule many times the
length of the capsule. The tubule may deliver a toxin, may stick to a prey item, or may entangle
an object, depending on the type of cnida. A cnida can fire but once. There are three major types
of cnidae: nematocysts, spirocysts, and ptychocysts. Nematocysts occur in all classes of
Cnidaria, but some of the 30-plus varieties of nematocysts are restricted to members of certain
classes (Fautin and Mariscal 1991). Spirocysts are found only in Anthozoa; they are adhesive in
nature. Ptychocysts are the most taxonomically restricted in distribution, occurring only in the
anthozoan order Ceriantharia; their function is to entangle bits of mud among their robust tubules
to form the feltwork that constitutes the tube of these burrowing animals.
Click on an image to view larger version & data in a new window javascript: w =
window.open('/onlinecontributors/app?service=external/ViewImageData&sp=2447', '2447',
'resizable,height=880,width=658,scrollbars=yes'); w.focus();
javascript: w =
window.open('/onlinecontributors/app?service=external/ViewImageData&sp=2447', '2447',
'resizable,height=880,width=658,scrollbars=yes'); w.focus();javascript: w =
window.open('/onlinecontributors/app?service=external/ViewImageData&sp=3173', '3173',
'resizable,height=500,width=500,scrollbars=yes'); w.focus();
javascript: w =
window.open('/onlinecontributors/app?service=external/ViewImageData&sp=3173', '3173',
'resizable,height=500,width=500,scrollbars=yes'); w.focus();javascript: w =
window.open('/onlinecontributors/app?service=external/ViewImageData&sp=639', '639',
'resizable,height=856,width=739,scrollbars=yes'); w.focus();
javascript: w =
window.open('/onlinecontributors/app?service=external/ViewImageData&sp=639', '639',
'resizable,height=856,width=739,scrollbars=yes'); w.focus();
Left: Fired "basitrich" (basitrichous isorhiza) from a sea anemone. The now empty capsule is in
the lower right of the photo; the spiny basal part of the fired tubule extends to the upper left;
beyond the frame of the photo is the non-spiny, distal part of the tubule, which is many times
longer than the capsule. Middle: "Holotrich" (holotrichous isorhiza) from a corallimorpharian.
Right: Unfired "basitrichs" (basitrichous isorhizas) from a sea anemone. The longitudinal line
inside each capsule is the spiny basal part of the unfired tubule.
Two body forms are characteristic of cnidarians -- the polyp and the medusa. With a few
exceptions, a columnar polyp is sedentary, being attached to or burrowed into the substratum by
the end opposite the mouth. Thus its tentacles are typically considered to point upward and
outward. Polyps of some species propagate vegetatively, forming colonies (if the progeny remain
attached to one another) or clones (if the progeny separate). Polymorphism occurs in colonies of
some species of hydrozoans and anthozoans, the polyps being specialized for functions such as
feeding, defense, and sexual reproduction. Polyps of some taxa form a skeleton within or
external to their tissues; some skeletons are mineralic (of calcium carbonate), others are organic
(of chitin or another carbohydrate), and some are both. The spheroidal or discoidal medusae are
solitary, and those of most species are pelagic. Although typically depicted as living with mouth
and tentacles pointing down, medusae assume all orientations in the water. Medusae of few
species possess the ability to propagate vegetatively. The common name of medusae, jellyfish,
alludes to the massive amount of mesoglea that contributes to their buoyancy.
All cnidarians have hydrostatic skeletons, regardless of whether they also have mineralic and/or
organic exoskeletons or endoskeletons. The muscles of the body wall operate against the fluid in
the coelenteron to extend individual polyps and to effect the swimming of medusae, for example.
The hollow tentacles of anthozoans are extended through hydrostatic action as well.
Discussion of Phylogenetic Relationships
Cnidaria is thought to have one of the longest fossil histories of metazoan phlya with
representatives in the Ediacaran fauna of the late Precambrian (Scrutton 1979). These earliest
fossils are both medusoid and polypoid, and thought to represent all cnidarian classes (Scrutton
1979).
The four extant cnidarian classes are identifiable as early as the Ordovician (Robson 1985), but
evolutionary relationships among them have been the subject of much debate (e.g. Brooks 1886,
Hyman 1940, Jagersten 1955, Hand 1959, Pantin 1960, Werner 1973, Petersen 1979, Barnes
1987, Ax 1989). Anthozoa is alternatively considered the most basal or the most derived group.
The former hypothesis posits that the polyp is the original body form, with the medusa (and
metagenesis) being derived (Fig. 1A). The latter perspective is that, in the "typical" life cycle, the
medusa is gametogenic, and so constitutes the definitive, or adult, stage, with the polyp being a
persistent larva. Thus, it is reasoned, the polyp evolved secondarily, and loss of the original body
form, the medusa, places Anthozoa as the most derived taxon (Fig. 1B). A comprehensive
morphological cladistic analysis by Schuchert (1993) supports the basal position of Anthozoa
with the Scyphozoa and Cubozoa being more closely related to each other than to Hydrozoa.
Morphological, mtDNA, and 18S rDNA data separately and together also support the basal
position of Anthozoa but do not resolve the relationships among Scyphozoa, Cubozoa and
Hydrozoa (Bridge et al. 1995). The phylogenetic tree at the beginning of this page is that of
Bridge et al. (1995). Neither of these treatments attempts to include the extinct class Conulata,
which has been considered by most paleontologists to be related to the Scyphozoa.
Click on an image to view larger version & data in a new window javascript: w =
window.open('/onlinecontributors/app?service=external/ViewImageData&sp=2041', '2041',
'resizable,height=500,width=700,scrollbars=yes'); w.focus();
javascript: w =
window.open('/onlinecontributors/app?service=external/ViewImageData&sp=2041', '2041',
'resizable,height=500,width=700,scrollbars=yes'); w.focus();
Alternative views of cnidarian life-cycle evolution and systematic relationships. (After Bridge et
al. 1995.)
Their diploblastic structure and their single body opening and cavity had been thought to ally
cnidarians with ctenophores. Indeed, until relatively recently the phylum Coelenterata was
considered to include animals now placed in Cnidaria and Ctenophora. However, ctenophores
lack a metagenetic life cycle and cnidae. Cnidae have been found in one ctenophore, but it is
now known that the ctenophore acquires those cnidae from the hydromedusae upon which it
preys (Mills and Miller 1984). Thus, it is generally agreed that the similarity in body form
between pelagic ctenophores and pelagic cnidarians is convergent; benthic ctenophores do not
resemble cnidarians at all. Cnidaria, therefore, is a well circumscribed taxon; it is considered by
many to be a sister group of all metazoans other than sponges.
WoRMS taxon details
Anthoathecata
AphiaID: 13551
Classification: Biota > Animalia (Kingdom) > Cnidaria (Phylum) > Hydrozoa (Class) > Hydroidolina
(Subclass)
Rank
Order
Parent
Hydroidolina
Synonymised
taxa
Anthomedusae (synonym)
Athecata (synonym)
Gymnoblastea (synonym.)
Laingiomedusae (synonym)
Sources
original description: Cornelius, P.F.S., 1992. Medusa loss in leptolid Hydrozoan (Cnidaria)
hydroid rafting, and abbreviated life-cycles among their remote-island faunae: an interim review. In: J.
Bouillon, F. Boero, F. Cicogna, J.M. Gili & R.G. Hughes, eds., Aspects of hydrozoan biology. Scientia
Marina 56 2-3: 245-261.
page(s): 245 [details]
basis of record: Bouillon, J.; Boero, F. (2000). Synopsis of the families and genera of the Hydromedusae
of the world, with a list of the worldwide species. Thalassia Salent. 24: 47-296 (look up in IMIS) [details]
from synonym: Howson, C.M.; Picton, B.E. (Ed.) (1997). The species directory of the marine fauna and
flora of the British Isles and surrounding seas. Ulster Museum Publication, 276. The Ulster Museum:
Belfast, UK. ISBN 0-948150-06-8. vi, 508 (+ cd-rom) pp. (look up in IMIS) [details] [view taxon]
from synonym: Brusca, R.C.; Brusca, G.J. (1990). Invertebrates. Sinauer Associates: Sunderland, MA
(USA). ISBN 0-87893-098-1. 922 pp. (look up in IMIS) [details] [view taxon]
from synonym: Hayward, P.J.; Ryland, J.S. (Ed.) (1990). The marine fauna of the British Isles and NorthWest Europe: 1. Introduction and protozoans to arthropods. Clarendon Press: Oxford, UK. ISBN 0-19857356-1. 627 pp. (look up in IMIS) [details] [view taxon]
Links
To GenBank
To ITIS
Notes
Diagnosis: Hydrozoans that always have a polyp stage. Hydranths either solitary or
colonial, body not covered by firm perisarc. Medusae not colonial, without statocysts, with gonads on
manubrium, with radial canals, with tentacles arising from bell-margin. Cnidome normally includes
desmonemes (not Eudendriidae and Laingiidae). [details]
Taxonomic Remark: The original spelling in Cornelius (1992) was Anthoathecata. Cornelius (1995a)
changed the spelling to Anthoathecatae, which seems unnecessary. [details]
Taxonomic status: The naming of this taxon is disputed, some prefer Anthomedusae, some Athecata
(oldest name). Molecular investigations show that this group is likely not monophyletic, it will thus most
probably
Siphonophorae
Top
Home > Library > Miscellaneous > Wikipedia
Siphonophores
http://en.wikipedia.org/wiki/File:Portuguese_Man-O-War_(Physalia_physalis).jpg
http://en.wikipedia.org/wiki/File:Portuguese_Man-O-War_(Physalia_physalis).jpg
Portuguese Man o' War, Physalia physalis
(Cystonectae: Physaliidae)
Scientific classification http://en.wikipedia.org/wiki/Template:Taxonomy/Siphonophorae
http://en.wikipedia.org/wiki/Template:Taxonomy/Siphonophorae
Kingdom:
Animalia
Phylum:
Cnidaria
Class: Hydrozoa
Subclass:
Leptolinae
Order: Siphonophorae
Eschscholtz, 1829
Suborders
Calycophorae
Cystonectae
Physonectae
Synonyms
Siphonophora Eschscholtz, 1829
Siphonophorae or Siphonophora, the siphonophores, are an order of the Hydrozoa, a class of marine
invertebrates belonging to the phylum Cnidaria. They are colonial, but the colonies can superficially
resemble jellyfish; although they appear to be a single organism, each specimen is actually a colony of
Siphonophora. The best known species is the dangerous Portuguese Man o' War (Physalia physalis).
With a body length of 40–50 m, another species of siphonophore, Praya dubia, is one of the longest
animals in the world.[1]
Contents
1 Description
2 Systematics
3 Footnotes
4 References
5 External links
Description
Siphonophores are especially scientifically interesting because they are composed of medusoid and
polypoid zooids that are morphologically and functionally specialized. Each zooid is an individual, but
their integration with each other is so strong that the colony attains the character of one large organism.
Indeed, most of the zooids are so specialized that they lack the ability to survive on their own.
Siphonophorae thus exist at the boundary between colonial and complex multicellular organisms. Also,
because multicellular organisms have cells which, like zooids, are specialized and interdependent,
siphonophores may provide clues regarding their evolution.[1]
Like other hydrozoans, certain siphonophores can emit light. A siphonophore of the genus Erenna has
been discovered at a depth of around 1,600 meters off the coast of Monterey, California. The individuals
from these colonies are strung together like a feather boa. They prey on small animals using stinging
cells. Among the stinging cells are stalks with red glowing ends. The tips twitch back and forth creating a
twinkling effect. It is theorized that twinkling red light attracts small fish that have been found eaten by
these siphonophores. While many sea animals produce blue and green bioluminescence, this
siphonophore was only the second lifeform found to produce a red light (the first being the scaleless
dragonfish Chirostomias pliopterus).[2]
Systematics
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpg
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpg
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpg
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpgAspects of Physophora hydrostatica
(Physonectae: Physophoridae).
Plate 37 in Kunstformen der Natur by Ernst Haeckel (1904). See also below.
Due to their highly specialized colonies, siphonophores have long misled scientists. They were for a long
time believed to be a highly distinct group, but now are known to have evolved from simpler colonial
hydrozoans similar to Anthomedusae or Leptomedusae. Consequently, they are now united with these
in a subclass Leptolinae.
The Siphonophorae have long fascinated scientists and layfolk alike, due to their dramatic appearance as
well as the large size and dangerous sting of several species. Compared to their relatives, their
systematics are relatively straightforward:[3]
Suborder Calycophorae
Family Abylidae
Family Clausophyidae
Family Diphyidae
Family Hippopodiidae
Family Prayidae
Family Sphaeronectidae
Suborder Cystonectae
Family Physaliidae
Family Rhizophysidae
Suborder Physonectae
Family Agalmatidae
Family Apolemiidae
Family Athorybiidae
Family Erennidae
Family Forskaliidae
Family Physophoridae
Family Pyrostephidae
Family Rhodaliidae
The genus Stepanyantsia is of unclear affiliations; it might belong in the Agalmatidae.
-23.
Top
Home > Library > Miscellaneous > Wikipedia
Siphonophores
http://en.wikipedia.org/wiki/File:Portuguese_Man-O-War_(Physalia_physalis).jpg
http://en.wikipedia.org/wiki/File:Portuguese_Man-O-War_(Physalia_physalis).jpg
Portuguese Man o' War, Physalia physalis
(Cystonectae: Physaliidae)
Scientific classification http://en.wikipedia.org/wiki/Template:Taxonomy/Siphonophorae
http://en.wikipedia.org/wiki/Template:Taxonomy/Siphonophorae
Kingdom:
Animalia
Phylum:
Cnidaria
Class: Hydrozoa
Subclass:
Leptolinae
Order: Siphonophorae
Eschscholtz, 1829
Suborders
Calycophorae
Cystonectae
Physonectae
Synonyms
Siphonophora Eschscholtz, 1829
Siphonophorae or Siphonophora, the siphonophores, are an order of the Hydrozoa, a class of marine
invertebrates belonging to the phylum Cnidaria. They are colonial, but the colonies can superficially
resemble jellyfish; although they appear to be a single organism, each specimen is actually a colony of
Siphonophora. The best known species is the dangerous Portuguese Man o' War (Physalia physalis).
With a body length of 40–50 m, another species of siphonophore, Praya dubia, is one of the longest
animals in the world.[1]
Contents
1 Description
2 Systematics
3 Footnotes
4 References
5 External links
Description
Siphonophores are especially scientifically interesting because they are composed of medusoid and
polypoid zooids that are morphologically and functionally specialized. Each zooid is an individual, but
their integration with each other is so strong that the colony attains the character of one large organism.
Indeed, most of the zooids are so specialized that they lack the ability to survive on their own.
Siphonophorae thus exist at the boundary between colonial and complex multicellular organisms. Also,
because multicellular organisms have cells which, like zooids, are specialized and interdependent,
siphonophores may provide clues regarding their evolution.[1]
Like other hydrozoans, certain siphonophores can emit light. A siphonophore of the genus Erenna has
been discovered at a depth of around 1,600 meters off the coast of Monterey, California. The individuals
from these colonies are strung together like a feather boa. They prey on small animals using stinging
cells. Among the stinging cells are stalks with red glowing ends. The tips twitch back and forth creating a
twinkling effect. It is theorized that twinkling red light attracts small fish that have been found eaten by
these siphonophores. While many sea animals produce blue and green bioluminescence, this
siphonophore was only the second lifeform found to produce a red light (the first being the scaleless
dragonfish Chirostomias pliopterus).[2]
Systematics
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpg
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpg
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpg
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpgAspects of Physophora hydrostatica
(Physonectae: Physophoridae).
Plate 37 in Kunstformen der Natur by Ernst Haeckel (1904). See also below.
Due to their highly specialized colonies, siphonophores have long misled scientists. They were for a long
time believed to be a highly distinct group, but now are known to have evolved from simpler colonial
hydrozoans similar to Anthomedusae or Leptomedusae. Consequently, they are now united with these
in a subclass Leptolinae.
The Siphonophorae have long fascinated scientists and layfolk alike, due to their dramatic appearance as
well as the large size and dangerous sting of several species. Compared to their relatives, their
systematics are relatively straightforward:[3]
Suborder Calycophorae
Family Abylidae
Family Clausophyidae
Family Diphyidae
Family Hippopodiidae
Family Prayidae
Family Sphaeronectidae
Suborder Cystonectae
Family Physaliidae
Family Rhizophysidae
Suborder Physonectae
Family Agalmatidae
Family Apolemiidae
Family Athorybiidae
Family Erennidae
Family Forskaliidae
Family Physophoridae
Family Pyrostephidae
Family Rhodaliidae
The genus Stepanyantsia is of unclear affiliations; it might belong in the Agalmatidae.
ZOO2010 Laboratory Study Guide
Chapter 9, The Radiate Animals
Type your email address in the space provided.
Type your full name in the space provided.
Terms to Know in This Chapter:
germ layers
gastrovascular cavity [gas trow VASS cue lar]
hydrostatic skeleton [high drow STAT ik]
polymorphism [pol eh MORE fiz um]
colloblast (sing.) [COL oh blast]
polyp (sing.) [POL up]
medusa (sing.) [meh DUE sah]
medusae (pl.) [meh DUE see]
jellyfish
sea anemone [ah NEM oh knee]
radially symmetrical
coral
tentacle (sing.) [TEN tah kul]
hypostome [HIGH po stome]
mouth
gonad (sing.) [GO nad]
basal disc
bud
cnidocyte (sing.) [NIDE oh sight]
nematocyst (sing.) [neh MAD oh sist]
cnidocil (sing.) [NIDE oh sill]
symbiont [SIM bee ont]
glutathione [glut ah THIGH on]
extracellular
intracellular
Bouin's fluid [BOW ins]
barb
hypnotoxin [hip no TOX in]
epidermis (sing.) [ep eh DERM iss]
mesoglea [mez oh GLEE ah]
diploblastic [dip low BLAST ik]
epitheliomuscular cell [ep eh THEL ee oh MUSS cue lar]
nerve net
interstitial cell [in tur STEH shul]
nutritive-muscular cell [NEW trah tive]
sensory cell
asexual
budding
monoecious [moe KNEE shuss]
dioecious [die EE shuss]
testis (sing.) [TEST tiss]
testes (pl.) [TEST tees]
egg
spermatozoan (sing.) [spur mat oh ZOE ah]
spermatozoa (pl.) [spur mat oh ZOE an]
zygote [ZIE goat]
planula (sing.) [PLAN you lah]
planulae (pl.) [PLAN you lee]
hydranth (sing.) [HIGH dranth]
gonangium (sing.) [go NAN gee um]
gonangia (pl.) [go NAN gee ah]
hydrorhiza (sing.) [high drow RISE ah]
hydrorhizae (pl.) [high drow RISE ee]
hydrocaulus (sing.) [high drow CALL us]
hydrocauli [high drow CALL eye]
perisarc [PERRY sark]
coenosarc [SIN oh sark]
hydrothecum (sing.) [high drow THEE cum]
hydrotheca (pl.) [high drow THEE kah]
blastostyle [BLAST oh style]
gonothecum (sing.) [go no THEE cum]
gonotheca (pl.) [go no THEE kah]
exumbrella [ex UM brah lah]
subumbrella [sub UM brah lah]
tentacular bulb [ten TACK you lar]
statocyst (sing.) [STAT oh sist]
manubrium (sing.) [mah NUBE ree um]
manubria (pl.) [mah NUBE ree ah]
stomach
radial canal
ring canal
tetramerous [tet rah MEER us]
rhopalium (sing.) [row FALE ee um]
rhophalia (pl.) [row FALE ee ah]
lappet (sing.) [LAP it]
oral arm
gastric pouch
scyphistoma (sing.) [sky FIST oh mah]
scyphistomae (pl.) [sky FIST oh mee]
strobilum (sing.) [STROW bee lum]
strobila (pl.) [STROW bee lah]
ephyra (sing.) [EE frah]
ephyrae (pl.) [EE free]
acontium (sing.) [ah CON tee um]
acontia (pl.) [ah CON ee ah]
columm
siphonoglyph [seh FON oh glif]
peristome (sing.) [PEAR eh stome]
pharynx (sing.) [FAIR inks]
pharynges (pl.) [fair IN geez]
septum (sing.) [SEP tum]
septa (pl.) [SEP tah]
thecum (sing.) [THEE cum]
theca (pl.) [THEE kah]
Portugeuse man-of-war
ocellus (sing.) [oh CELL us]
ocelli (pl.) [oh CELL ee]
larva (sing.) [LAR vah]
larvae (pl.) [LAR vee]
vellum (sing.) [VEL um]
vella (pl.) [VEL ah]
Genera You Need to Know:
Hydra [HIGH drah]
Daphnia [DAFF knee ah]
Obelia [oh BEEL yah]
Gonionemus [go knee oh NEE mus]
Physalia [feh SAIL ee ah]
Aurelia [or REEL yah]
Metridium [meh TRID ee um]
Classification You Need to Know:
Kingdom Animalia [an eh MALE yah]
Class Hydrozoa [high drow ZOE ah]
Class Scyphozoa [sky foe ZOE ah]
Class Anthozoa [an thow ZOE ah]
What You Need to Know:
You should be able to:
1.
name the three classes of Cnidaria, give the characteristics of each and recognize each, on sight,
in lab,
2.
identify living Hydra and under the microscope, be able to identify tentacles, hypostome,
mouth, gonads, nematocysts, and the two layers of cells,
3.
name the basic cell types in the epidermis and gastrodermis of Hydra,
4.
distinguish between Hydra budding and with testes and ovaries,
5.
identify both preserved specimens and prepared slides of Obelia and identify the major parts of
the colony,
6.
identify preserved specimens and prepared slides of Gonionemus and recognize the major
structures,
7.
distinguish among statocysts, tentacular bulbs, rhophalia, and ocelli,
8.
explain what is meant by polymorphism and dimorphism,
9.
explain how you can tell most jellyfish of the class Scyphozoa from jellyfish of the class
Hydrozoa,
10.
identify from preserved specimens Aurelia and recognize the major structures associated with it,
11.
provide life cycles for Hydra, Obelia, Aurelia and the larval types of each,
12.
identify the major structures associated with Metridium from preserved specimens, and,
13.
recognize several stony and soft corals provided by the instructor.
Exercises: Fill in the Blank.
1.
The genus Obelia belongs to the class while Metridium belongs to the class .
2.
Which class of Cnidarians has no medusae?
3.
Which class of Cnidarians typically has a velum?
4.
How many tentacles does a Hydra typically have? .
5.
The stinging cell of Cnidarians is called the while the trigger is called the and the "stinger" itself
is called the .
6.
Which cell type in Cnidarians may give rise to other types of cells?
7.
Balance structures in the Cnidarians are called .
8.
The middle layer of tissue in Cnidarians is called the while the middle layer in sponges is called .
9.
The larval type in sponges is the larva and the larval type in Cnidarians is called the larva.
10.
The gonads of the class Scyphozoa and Anthozoa are found in the .
11.
The name of the medusae produced by Aurelia is and the polyps are called .
12.
This is a tiny opening on either side of the mouth in Metridium used to circulate water while
keeping prey inside the pharynx.
13.
Stinging cells in Metridium are found on tiny threads called
14.
The walls of the cup of stoney corals is called the
15.
Reproductive polyps in Obelia are called .
Exercises: Multiple Choice. Select the Best Answer.
1.
Which genus has no medusae and only polyps?
(1) Hydra
(2) Obelia
(3) Metridium
(4) both 1 and 3
(5) All of the above have no medusae.
2.
Which class has polyps?
(1) Hydrozoa
(2) Scyphozoa
(3) Anthozoa
(4) both 1 and 2
(5) all of the above
3.
Which of the following is a colonial polyp form that floats in the ocean and is carried by winds
and waves?
(1) Obelia
(2) Aurelia
(3) Physalia
(4) Metridium
(5) Gonionemus
4.
Which cell type is found in the gastrodermis of Hydra?
(1) interstitial cell
(2) nutritive-muscle cell
(3) epitheliomuscle cell
(4) cnidocyte
(5) sensory cell
5.
Which terms are correctly matched?
(1) Hydra-medusa
(2) Velum-Aureila
(3) acontium-Obelia
(4) ephyra-Aurelia
(5) Physalia-scyphistoma
l}
View more Animal Life videos
McGraw-Hill Science & Technology Encyclopedia:
Coelom
Top
Home > Library > Science > Sci-Tech Encyclopedia
The mesodermally lined body cavity of most animals above the flatworms and nonsegmented
roundworms. Its manner of origin provides one basis for classifying the major higher groups.
Annelids, arthropods, and mollusks have a coelom which develops from solid mesodermal bands. Within
the trochophore larva of annelids, a single pole cell proliferates two strips of mesoblast lying on either
side of the ventral midline. These bands subdivide transversely into bilateral solid blocks, the somites.
Each somite then splits internally to form a hollow vesicle, the cavity of which is the coelom. The
mollusks also form bands of mesoderm from a single pole cell, but these bands do not segment. They
split internally to form single right and left coelomic sacs, but the cavities are soon reduced and the
surrounding mesoblast disperses as separate cells, many of which become muscle. The only remnants of
the coelom in the adult are the pericardial cavity and the cavities of the gonads and their ducts. In
arthropods paired bands of mesoblast may proliferate from a posterior growth center or may separate
inward from a blastoderm, a superficial layer of cells, on the ventral surface of the egg. These bands
divide into linear series of somites which then hollow out. Their cavities represent the coelom.
Echinoderms and chordates constitute a second major group, characterized by the origin of the coelom
from outpocketings of the primitive gut wall. In echinoderms one pair of bilateral pouches evaginates
and separates from the archenteron or primitive digestive cavity. Each pouch constricts into three
portions, not homologous to the metameres of other animals.
The protochordates of the groups Hemichordata and Cephalochordata have three coelomic pouches
formed by separate evaginations of the archenteral roof. In hemichordates the head cavity remains
single as the cavity of the proboscis and has a pore to the exterior on each side. The second pouches
form cavities within the collar and also acquire external pores. The third pair is contained within the
trunk and forms the major perivisceral cavity.
In cephalochordates the head cavity divides into lateral halves. The left side communicates, by a pore, to
an ectodermal pit called the wheel organ. The second pair of pouches forms the pair of mesoblastic
somites, and the third pouches subdivide transversely to give rise to the remainder of the linear series of
somites. The upper or myotomic portion of each somite remains metameric and forms the segmental
muscles. As it enlarges, the coelomic space is displaced ventrally and expands above and below the gut
to form the perivisceral cavities and mesenteries, as described for annelids.
In vertebrates the mesoderm arises as a solid sheet from surface cells that have been involuted through
the blastopore. Lateral to the notochord, beginning at about the level of the ear, the mesoderm
subdivides into three parts: (1) the somites; (2) the nephrotomic cord, temporarily segmented in lower
vertebrates, which will form excretory organs and ducts; and (3) the unsegmented lateral plate. The
coelom arises as a split within the lateral plate. See also Animal kingdom; Gastrulation
Read more: http://www.answers.com/topic/body-cavity#ixzz1nzNGISn8
Fax: 1- 425- 458- 9358 | Toll free: 1- 877- 252 - 7763
. Forgot Password? Click HereRegister | Account
.HomeOnline TutoringHomework HelpSolution LibraryCareersMore
Pricing
About Us
Contact Us
Test Preparation
Content Development Services
Blog
Testimonials
Homework Answers > Zoology> Coelom of vertebrates Need Zoology Homework Help?
Coelom of Vertebrates
Structure of Coelom:
The lower part of Mesoderm is called Hypomere or Lateral plate. This part is non-segmented and is
continuous with a cavity called Coelom or Splanchnocoel. The puter wall of the cavity forms the lining of
the body wall and is called Parietal Peritoneum. The inner wall forms the outer covering of the
alimentary canal and viscera and so called Visceral Peritoneum. Thus the cavity enclosed within the
Parietal and Visceral Peritoneum is the Coelomic cavity. It contains a clear fluid called Coelomic fluid.
The Parietal and Visceral Peritoneum are connected to each other through a double layered Mesentry.
The ventral Mesentry connects the alimentary canal to an organ and is then called Omentum.
Partitions of Coelom:
The anterior and posterior Coelomic cavity is partitioned by a transverse septum. The anterior part
contains heart and hence called Pericardial cavity which contains Pericardial fluid. The lining of the
cavity is called Pericardial membrane. The posterior cavity contains other visceral organs and so called
Peritoneal or Abdominal cavity and the fluid called as Peritoneal fluid. This cavity is lined by Parietal or
Visceral peritoneum.
Differentiation of Coelom in vertebrates:
In anurans and reptiles the Pericardial cavity lies ventral to the anterior Peritoneal cavity. The Peritoneal
cavity has lungs in its anterior region and other visceral organs in its posterior region and is thus called
as Pleuroperitoneal cavity.
In crocodiles, birds and mammals the transverse section extends to the dorsal body wall to form a new
partition behind the lungs. This is membranous in birds and is called Oblique septum. In mammals it is
the highly muscular Diaphragm. This divides the Pleuroperitoneal cavity into Thoracic and Abdominal
cavities.
The Thoracic region has two Pleural cavities each enclosing a lung. The Pericardial cavity lies in between
the ventral portion of the Pleural cavities. The posterior region of the Diaphragm is called the peritoneal
or Abdominal cavity.
The Coelomic fluid cushions and offer protection to the visceral organs present in the Coelomic cavity.
Share these flashcards
Share on Facebook
Share on Twitter
Link or embed
About these flashcards
Created by:
blondygal007 on November 12, 2011
Log in to favorite or report as inappropriate.
Pop outDiscuss
No MessagesYou must log in to discuss this set.
Flashcards: Zoology CHAPTER 29 DEUTEROSTOMES: THE CHORDATES
Term First
Both Sides
dorsal tubular nerve cord
forms the central nervous system
Click to flip
1/27
Study: SpellerLearnTest
Play Games: ScatterSpace Race
All 27 terms
Print new! Export CombineTerms Definitions
dorsal tubular nerve cord forms the central nervous system
notochord dorsal hollow rod that extends the length of the body and serves as a firm but flexible axis
tunicates sea squirts;sessile filter feeders as adults; larvae are free swimming
tunic an enveloping or covering membrane or layer of body tissue
paedogenesis known as juvenification; the retention of juvenile characteristics in an adult
skull/cranium Bone that protects the brain
kidneys organs that filter nitrogen wastes from blood to make urine
Chrondrichthyes predatory fishes such as sharks, rays, and skates that have jaws, paired fins, skeletons
made of cartilage, and skin covered by a unique kind of scale
Amphibia Name the Class of Vertebrata: bone, tetrapod, external fertilization, anamniotic egg, lungs as
adults, gills as young, three chambered heart, ectothermic, lay eggs in water but live on land, skin is
water proof, toxin producing glands, gas exchange through skin
chordates an animal phylum that has a notochord, a dorsal hollow nerve cord, pharyngeal pouches,
and gill slits at some time in its life cycle
pharyngeal pouches openings in the lining of the upper respiratory tract (from gills in fish and
amphibian larvae)
sea squirts = tunicates; P. Chordata, Subphylum Urochordata. sessile adults w/ 2 tubes. siphons moving
h2o into & out of body. hard outer shell; "tunic". toxic secondary compounds. vibrant colors. tadpole
larvae w/ notochord in body and tail (not head)
lancelet small translucent lancet-shaped burrowing marine animal
vertebrate animals having a bony or cartilaginous skeleton with a segmented spinal column and a large
brain enclosed in a skull or cranium
somites Paired blocks of mesoderm just lateral to the notochord of a vertebrate embryo.
Superclass Agnatha superclass of eel-shaped chordates lacking jaws and pelvic fins: lampreys
Class Osteichthyes a class of fish having a skeleton composed of bone in addition to cartilage
Reptilia first completly successful group of animals to move on land; snakes, crocodilians, turtles, dry
skin/claws/amniote egg that can be layed on land
postanal tail tail that continues past the end of the digestive tract (anus)
Subphylum Urochordata "tail cord" sea squirts (tunicates). possess all chordate characteristics as larvar,
but adults only have the pharyngeal gill slits (pouches). sessile filter feeders. 2 siphons, though tunic
siphon a tube running from the liquid in a vessel to a lower level outside the vessel so that atmospheric
pressure forces the liquid through the tube
Subphylum Cephalochordata "head cord" lancelots, amphioxus (genus name). marine filter feeds,
resembles a fish. burrow into sand with head exposed. retain all chordate characteristics as adults.
segmented muscles in tail. develop from SOMITES
vertebral column the series of vertebrae forming the axis of the skeleton and protecting the spinal cord
endoskeleton internal skeleton or supporting framework in an animal
Placodermi extinct group of bony-plated fishes with primitive jaws
tetrapods vertebrate animals having 4 feet, legs, or leglike appendages
Mammalia Class: Endotherms with hair or fur. mammary glands produce milk. glandular skin with hair
or fur. external ear present. teeth are different types. diaphragm between thorax/abdomen
Home > Library > Science > Sci-Tech Encyclopedia
A class of the phylum Coelenterata which includes the fresh-water hydras, the marine hydroids, many of
the smaller jellyfish, a few special corals, and the Portuguese man-of-war. The Hydrozoa may be divided
into six orders: the Hydroida, Milleporina, Stylasterina, Trachylina, Siphonophora, and Spongiomorphida.
See separate article on each order.
The form of the body varies greatly among the hydrozoans. This diversity is due in part to the existence
of two body types, the polyp and the medusa. A specimen may be a polyp, a medusa, a colony of polyps,
or even a composite of the first two. Polyps are somewhat cylindrical, attached at one end, and have a
mouth surrounded by tentacles at the free end. Medusae are free-swimming jellyfish with tentacles
around the margin of the discoidal body.
In a representative life cycle, the fertilized egg develops into a swimming larva which soon attaches itself
and transforms into a polyp. The polyp develops stolons (which fasten to substrates), stems, and other
polyps to make up a colony of interconnected polyps. Medusae are produced by budding and liberated
to feed, grow, and produce eggs and sperm.
Most hydrozoans are carnivorous and capture animals which come in contact with their tentacles. The
prey is immobilized by poison injected by stinging capsules, the nematocysts. Most animals of
appropriate size can be captured, but small crustaceans are probably the most common food. See also
Coelenterata.
WoRMS taxon details
Anthoathecata
AphiaID: 13551
Classification: Biota > Animalia (Kingdom) > Cnidaria (Phylum) > Hydrozoa (Class) > Hydroidolina
(Subclass)
Authority
Status
Cornelius, 1992
accepted
Record
status
Checked by Taxonomic Editor
Rank
Order
Parent
Hydroidolina
Synonymised
taxa
Anthomedusae (synonym)
Athecata (synonym)
Gymnoblastea (synonym.)
Laingiomedusae (synonym)
Sources
original description: Cornelius, P.F.S., 1992. Medusa loss in leptolid Hydrozoan (Cnidaria)
hydroid rafting, and abbreviated life-cycles among their remote-island faunae: an interim review. In: J.
Bouillon, F. Boero, F. Cicogna, J.M. Gili & R.G. Hughes, eds., Aspects of hydrozoan biology. Scientia
Marina 56 2-3: 245-261.
page(s): 245 [details]
basis of record: Bouillon, J.; Boero, F. (2000). Synopsis of the families and genera of the Hydromedusae
of the world, with a list of the worldwide species. Thalassia Salent. 24: 47-296 (look up in IMIS) [details]
from synonym: Howson, C.M.; Picton, B.E. (Ed.) (1997). The species directory of the marine fauna and
flora of the British Isles and surrounding seas. Ulster Museum Publication, 276. The Ulster Museum:
Belfast, UK. ISBN 0-948150-06-8. vi, 508 (+ cd-rom) pp. (look up in IMIS) [details] [view taxon]
from synonym: Brusca, R.C.; Brusca, G.J. (1990). Invertebrates. Sinauer Associates: Sunderland, MA
(USA). ISBN 0-87893-098-1. 922 pp. (look up in IMIS) [details] [view taxon]
from synonym: Hayward, P.J.; Ryland, J.S. (Ed.) (1990). The marine fauna of the British Isles and NorthWest Europe: 1. Introduction and protozoans to arthropods. Clarendon Press: Oxford, UK. ISBN 0-19857356-1. 627 pp. (look up in IMIS) [details] [view taxon]
Direct child
taxa
Suborder Anthoathecata incertae sedis
Suborder Capitata
Suborder Filifera
Environment
marine, brackish, fresh, terrestrial
Fossil range
recent + fossil
Feedingtypes
omnivore [details]
predator [details]
scavenger [details]
Links
To GenBank
To ITIS
Notes
Diagnosis: Hydrozoans that always have a polyp stage. Hydranths either solitary or
colonial, body not covered by firm perisarc. Medusae not colonial, without statocysts, with gonads on
manubrium, with radial canals, with tentacles arising from bell-margin. Cnidome normally includes
desmonemes (not Eudendriidae and Laingiidae). [details]
Taxonomic Remark: The original spelling in Cornelius (1992) was Anthoathecata. Cornelius (1995a)
changed the spelling to Anthoathecatae, which seems unnecessary. [details]
Taxonomic status: The naming of this taxon is disputed, some prefer Anthomedusae, some Athecata
(oldest name). Molecular investigations show that this group is likely not monophyletic, it will thus most
probably
WoRMS taxon details
Anthoathecata
AphiaID: 13551
Classification: Biota > Animalia (Kingdom) > Cnidaria (Phylum) > Hydrozoa (Class) > Hydroidolina
(Subclass)
Authority
Status
Cornelius, 1992
accepted
Record
status
Checked by Taxonomic Editor
Rank
Order
Parent
Hydroidolina
Synonymised
taxa
Anthomedusae (synonym)
Athecata (synonym)
Gymnoblastea (synonym.)
Laingiomedusae (synonym)
Sources
original description: Cornelius, P.F.S., 1992. Medusa loss in leptolid Hydrozoan (Cnidaria)
hydroid rafting, and abbreviated life-cycles among their remote-island faunae: an interim review. In: J.
Bouillon, F. Boero, F. Cicogna, J.M. Gili & R.G. Hughes, eds., Aspects of hydrozoan biology. Scientia
Marina 56 2-3: 245-261.
page(s): 245 [details]
basis of record: Bouillon, J.; Boero, F. (2000). Synopsis of the families and genera of the Hydromedusae
of the world, with a list of the worldwide species. Thalassia Salent. 24: 47-296 (look up in IMIS) [details]
from synonym: Howson, C.M.; Picton, B.E. (Ed.) (1997). The species directory of the marine fauna and
flora of the British Isles and surrounding seas. Ulster Museum Publication, 276. The Ulster Museum:
Belfast, UK. ISBN 0-948150-06-8. vi, 508 (+ cd-rom) pp. (look up in IMIS) [details] [view taxon]
from synonym: Brusca, R.C.; Brusca, G.J. (1990). Invertebrates. Sinauer Associates: Sunderland, MA
(USA). ISBN 0-87893-098-1. 922 pp. (look up in IMIS) [details] [view taxon]
from synonym: Hayward, P.J.; Ryland, J.S. (Ed.) (1990). The marine fauna of the British Isles and NorthWest Europe: 1. Introduction and protozoans to arthropods. Clarendon Press: Oxford, UK. ISBN 0-19857356-1. 627 pp. (look up in IMIS) [details] [view taxon]
Direct child
taxa
Suborder Anthoathecata incertae sedis
Suborder Capitata
Suborder Filifera
Environment
marine, brackish, fresh, terrestrial
Fossil range
recent + fossil
Feedingtypes
omnivore [details]
predator [details]
scavenger [details]
Links
To GenBank
To ITIS
Notes
Diagnosis: Hydrozoans that always have a polyp stage. Hydranths either solitary or
colonial, body not covered by firm perisarc. Medusae not colonial, without statocysts, with gonads on
manubrium, with radial canals, with tentacles arising from bell-margin. Cnidome normally includes
desmonemes (not Eudendriidae and Laingiidae). [details]
Taxonomic Remark: The original spelling in Cornelius (1992) was Anthoathecata. Cornelius (1995a)
changed the spelling to Anthoathecatae, which seems unnecessary. [details]
Taxonomic status: The naming of this taxon is disputed, some prefer Anthomedusae, some Athecata
(oldest name). Molecular investigations show that this group is likely not monophyletic, it will thus most
probably
WoRMS taxon details
Anthoathecata
AphiaID: 13551
Classification: Biota > Animalia (Kingdom) > Cnidaria (Phylum) > Hydrozoa (Class) > Hydroidolina
(Subclass)
Rank
Order
Parent
Hydroidolina
Synonymised
taxa
Anthomedusae (synonym)
Athecata (synonym)
Gymnoblastea (synonym.)
Laingiomedusae (synonym)
Sources
original description: Cornelius, P.F.S., 1992. Medusa loss in leptolid Hydrozoan (Cnidaria)
hydroid rafting, and abbreviated life-cycles among their remote-island faunae: an interim review. In: J.
Bouillon, F. Boero, F. Cicogna, J.M. Gili & R.G. Hughes, eds., Aspects of hydrozoan biology. Scientia
Marina 56 2-3: 245-261.
page(s): 245 [details]
basis of record: Bouillon, J.; Boero, F. (2000). Synopsis of the families and genera of the Hydromedusae
of the world, with a list of the worldwide species. Thalassia Salent. 24: 47-296 (look up in IMIS) [details]
from synonym: Howson, C.M.; Picton, B.E. (Ed.) (1997). The species directory of the marine fauna and
flora of the British Isles and surrounding seas. Ulster Museum Publication, 276. The Ulster Museum:
Belfast, UK. ISBN 0-948150-06-8. vi, 508 (+ cd-rom) pp. (look up in IMIS) [details] [view taxon]
from synonym: Brusca, R.C.; Brusca, G.J. (1990). Invertebrates. Sinauer Associates: Sunderland, MA
(USA). ISBN 0-87893-098-1. 922 pp. (look up in IMIS) [details] [view taxon]
from synonym: Hayward, P.J.; Ryland, J.S. (Ed.) (1990). The marine fauna of the British Isles and NorthWest Europe: 1. Introduction and protozoans to arthropods. Clarendon Press: Oxford, UK. ISBN 0-19857356-1. 627 pp. (look up in IMIS) [details] [view taxon]
Links
To GenBank
To ITIS
Notes
Diagnosis: Hydrozoans that always have a polyp stage. Hydranths either solitary or
colonial, body not covered by firm perisarc. Medusae not colonial, without statocysts, with gonads on
manubrium, with radial canals, with tentacles arising from bell-margin. Cnidome normally includes
desmonemes (not Eudendriidae and Laingiidae). [details]
Taxonomic Remark: The original spelling in Cornelius (1992) was Anthoathecata. Cornelius (1995a)
changed the spelling to Anthoathecatae, which seems unnecessary. [details]
Taxonomic status: The naming of this taxon is disputed, some prefer Anthomedusae, some Athecata
(oldest name). Molecular investigations show that this group is likely not monophyletic, it will thus most
probably
Siphonophorae
Top
Home > Library > Miscellaneous > Wikipedia
Siphonophores
http://en.wikipedia.org/wiki/File:Portuguese_Man-O-War_(Physalia_physalis).jpg
http://en.wikipedia.org/wiki/File:Portuguese_Man-O-War_(Physalia_physalis).jpg
Portuguese Man o' War, Physalia physalis
(Cystonectae: Physaliidae)
Scientific classification http://en.wikipedia.org/wiki/Template:Taxonomy/Siphonophorae
http://en.wikipedia.org/wiki/Template:Taxonomy/Siphonophorae
Kingdom:
Animalia
Phylum:
Cnidaria
Class: Hydrozoa
Subclass:
Leptolinae
Order: Siphonophorae
Eschscholtz, 1829
Suborders
Calycophorae
Cystonectae
Physonectae
Synonyms
Siphonophora Eschscholtz, 1829
Siphonophorae or Siphonophora, the siphonophores, are an order of the Hydrozoa, a class of marine
invertebrates belonging to the phylum Cnidaria. They are colonial, but the colonies can superficially
resemble jellyfish; although they appear to be a single organism, each specimen is actually a colony of
Siphonophora. The best known species is the dangerous Portuguese Man o' War (Physalia physalis).
With a body length of 40–50 m, another species of siphonophore, Praya dubia, is one of the longest
animals in the world.[1]
Contents
1 Description
2 Systematics
3 Footnotes
4 References
5 External links
Description
Siphonophores are especially scientifically interesting because they are composed of medusoid and
polypoid zooids that are morphologically and functionally specialized. Each zooid is an individual, but
their integration with each other is so strong that the colony attains the character of one large organism.
Indeed, most of the zooids are so specialized that they lack the ability to survive on their own.
Siphonophorae thus exist at the boundary between colonial and complex multicellular organisms. Also,
because multicellular organisms have cells which, like zooids, are specialized and interdependent,
siphonophores may provide clues regarding their evolution.[1]
Like other hydrozoans, certain siphonophores can emit light. A siphonophore of the genus Erenna has
been discovered at a depth of around 1,600 meters off the coast of Monterey, California. The individuals
from these colonies are strung together like a feather boa. They prey on small animals using stinging
cells. Among the stinging cells are stalks with red glowing ends. The tips twitch back and forth creating a
twinkling effect. It is theorized that twinkling red light attracts small fish that have been found eaten by
these siphonophores. While many sea animals produce blue and green bioluminescence, this
siphonophore was only the second lifeform found to produce a red light (the first being the scaleless
dragonfish Chirostomias pliopterus).[2]
Systematics
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpg
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpg
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpghttp://en.wikipedia.org/wiki/File:Haec
kel_Siphonophorae_37.jpgAspects of Physophora hydrostatica (Physonectae: Physophoridae).
Plate 37 in Kunstformen der Natur by Ernst Haeckel (1904). See also below.
Due to their highly specialized colonies, siphonophores have long misled scientists. They were for a long
time believed to be a highly distinct group, but now are known to have evolved from simpler colonial
hydrozoans similar to Anthomedusae or Leptomedusae. Consequently, they are now united with these
in a subclass Leptolinae.
The Siphonophorae have long fascinated scientists and layfolk alike, due to their dramatic appearance as
well as the large size and dangerous sting of several species. Compared to their relatives, their
systematics are relatively straightforward:[3]
Suborder Calycophorae
Family Abylidae
Family Clausophyidae
Family Diphyidae
Family Hippopodiidae
Family Prayidae
Family Sphaeronectidae
Suborder Cystonectae
Family Physaliidae
Family Rhizophysidae
Suborder Physonectae
Family Agalmatidae
Family Apolemiidae
Family Athorybiidae
Family Erennidae
Family Forskaliidae
Family Physophoridae
Family Pyrostephidae
Family Rhodaliidae
The genus Stepanyantsia is of unclear affiliations; it might belong in the Agalmatidae.
-23.
Top
Home > Library > Miscellaneous > Wikipedia
Siphonophores
http://en.wikipedia.org/wiki/File:Portuguese_Man-O-War_(Physalia_physalis).jpg
http://en.wikipedia.org/wiki/File:Portuguese_Man-O-War_(Physalia_physalis).jpg
Portuguese Man o' War, Physalia physalis
(Cystonectae: Physaliidae)
Scientific classification http://en.wikipedia.org/wiki/Template:Taxonomy/Siphonophorae
http://en.wikipedia.org/wiki/Template:Taxonomy/Siphonophorae
Kingdom:
Animalia
Phylum:
Cnidaria
Class: Hydrozoa
Subclass:
Leptolinae
Order: Siphonophorae
Eschscholtz, 1829
Suborders
Calycophorae
Cystonectae
Physonectae
Synonyms
Siphonophora Eschscholtz, 1829
Siphonophorae or Siphonophora, the siphonophores, are an order of the Hydrozoa, a class of marine
invertebrates belonging to the phylum Cnidaria. They are colonial, but the colonies can superficially
resemble jellyfish; although they appear to be a single organism, each specimen is actually a colony of
Siphonophora. The best known species is the dangerous Portuguese Man o' War (Physalia physalis).
With a body length of 40–50 m, another species of siphonophore, Praya dubia, is one of the longest
animals in the world.[1]
Contents
1 Description
2 Systematics
3 Footnotes
4 References
5 External links
Description
Siphonophores are especially scientifically interesting because they are composed of medusoid and
polypoid zooids that are morphologically and functionally specialized. Each zooid is an individual, but
their integration with each other is so strong that the colony attains the character of one large organism.
Indeed, most of the zooids are so specialized that they lack the ability to survive on their own.
Siphonophorae thus exist at the boundary between colonial and complex multicellular organisms. Also,
because multicellular organisms have cells which, like zooids, are specialized and interdependent,
siphonophores may provide clues regarding their evolution.[1]
Like other hydrozoans, certain siphonophores can emit light. A siphonophore of the genus Erenna has
been discovered at a depth of around 1,600 meters off the coast of Monterey, California. The individuals
from these colonies are strung together like a feather boa. They prey on small animals using stinging
cells. Among the stinging cells are stalks with red glowing ends. The tips twitch back and forth creating a
twinkling effect. It is theorized that twinkling red light attracts small fish that have been found eaten by
these siphonophores. While many sea animals produce blue and green bioluminescence, this
siphonophore was only the second lifeform found to produce a red light (the first being the scaleless
dragonfish Chirostomias pliopterus).[2]
Systematics
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpg
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpg
http://en.wikipedia.org/wiki/File:Haeckel_Siphonophorae_37.jpghttp://en.wikipedia.org/wiki/File:Haec
kel_Siphonophorae_37.jpgAspects of Physophora hydrostatica (Physonectae: Physophoridae).
Plate 37 in Kunstformen der Natur by Ernst Haeckel (1904). See also below.
Due to their highly specialized colonies, siphonophores have long misled scientists. They were for a long
time believed to be a highly distinct group, but now are known to have evolved from simpler colonial
hydrozoans similar to Anthomedusae or Leptomedusae. Consequently, they are now united with these
in a subclass Leptolinae.
The Siphonophorae have long fascinated scientists and layfolk alike, due to their dramatic appearance as
well as the large size and dangerous sting of several species. Compared to their relatives, their
systematics are relatively straightforward:[3]
Suborder Calycophorae
Family Abylidae
Family Clausophyidae
Family Diphyidae
Family Hippopodiidae
Family Prayidae
Family Sphaeronectidae
Suborder Cystonectae
Family Physaliidae
Family Rhizophysidae
Suborder Physonectae
Family Agalmatidae
Family Apolemiidae
Family Athorybiidae
Family Erennidae
Family Forskaliidae
Family Physophoridae
Family Pyrostephidae
Family Rhodaliidae
The genus Stepanyantsia is of unclear affiliations; it might belong in the Agalmatidae.
ZOO2010 Laboratory Study Guide
Chapter 9, The Radiate Animals
Type your email address in the space provided.
Type your full name in the space provided.
Terms to Know in This Chapter:
germ layers
gastrovascular cavity [gas trow VASS cue lar]
hydrostatic skeleton [high drow STAT ik]
polymorphism [pol eh MORE fiz um]
colloblast (sing.) [COL oh blast]
polyp (sing.) [POL up]
medusa (sing.) [meh DUE sah]
medusae (pl.) [meh DUE see]
jellyfish
sea anemone [ah NEM oh knee]
radially symmetrical
coral
tentacle (sing.) [TEN tah kul]
hypostome [HIGH po stome]
mouth
gonad (sing.) [GO nad]
basal disc
bud
cnidocyte (sing.) [NIDE oh sight]
nematocyst (sing.) [neh MAD oh sist]
cnidocil (sing.) [NIDE oh sill]
symbiont [SIM bee ont]
glutathione [glut ah THIGH on]
extracellular
intracellular
Bouin's fluid [BOW ins]
barb
hypnotoxin [hip no TOX in]
epidermis (sing.) [ep eh DERM iss]
mesoglea [mez oh GLEE ah]
diploblastic [dip low BLAST ik]
epitheliomuscular cell [ep eh THEL ee oh MUSS cue lar]
nerve net
interstitial cell [in tur STEH shul]
nutritive-muscular cell [NEW trah tive]
sensory cell
asexual
budding
monoecious [moe KNEE shuss]
dioecious [die EE shuss]
testis (sing.) [TEST tiss]
testes (pl.) [TEST tees]
egg
spermatozoan (sing.) [spur mat oh ZOE ah]
spermatozoa (pl.) [spur mat oh ZOE an]
zygote [ZIE goat]
planula (sing.) [PLAN you lah]
planulae (pl.) [PLAN you lee]
hydranth (sing.) [HIGH dranth]
gonangium (sing.) [go NAN gee um]
gonangia (pl.) [go NAN gee ah]
hydrorhiza (sing.) [high drow RISE ah]
hydrorhizae (pl.) [high drow RISE ee]
hydrocaulus (sing.) [high drow CALL us]
hydrocauli [high drow CALL eye]
perisarc [PERRY sark]
coenosarc [SIN oh sark]
hydrothecum (sing.) [high drow THEE cum]
hydrotheca (pl.) [high drow THEE kah]
blastostyle [BLAST oh style]
gonothecum (sing.) [go no THEE cum]
gonotheca (pl.) [go no THEE kah]
exumbrella [ex UM brah lah]
subumbrella [sub UM brah lah]
tentacular bulb [ten TACK you lar]
statocyst (sing.) [STAT oh sist]
manubrium (sing.) [mah NUBE ree um]
manubria (pl.) [mah NUBE ree ah]
stomach
radial canal
ring canal
tetramerous [tet rah MEER us]
rhopalium (sing.) [row FALE ee um]
rhophalia (pl.) [row FALE ee ah]
lappet (sing.) [LAP it]
oral arm
gastric pouch
scyphistoma (sing.) [sky FIST oh mah]
scyphistomae (pl.) [sky FIST oh mee]
strobilum (sing.) [STROW bee lum]
strobila (pl.) [STROW bee lah]
ephyra (sing.) [EE frah]
ephyrae (pl.) [EE free]
acontium (sing.) [ah CON tee um]
acontia (pl.) [ah CON ee ah]
columm
siphonoglyph [seh FON oh glif]
peristome (sing.) [PEAR eh stome]
pharynx (sing.) [FAIR inks]
pharynges (pl.) [fair IN geez]
septum (sing.) [SEP tum]
septa (pl.) [SEP tah]
thecum (sing.) [THEE cum]
theca (pl.) [THEE kah]
Portugeuse man-of-war
ocellus (sing.) [oh CELL us]
ocelli (pl.) [oh CELL ee]
larva (sing.) [LAR vah]
larvae (pl.) [LAR vee]
vellum (sing.) [VEL um]
vella (pl.) [VEL ah]
Genera You Need to Know:
Hydra [HIGH drah]
Daphnia [DAFF knee ah]
Obelia [oh BEEL yah]
Gonionemus [go knee oh NEE mus]
Physalia [feh SAIL ee ah]
Aurelia [or REEL yah]
Metridium [meh TRID ee um]
Classification You Need to Know:
Kingdom Animalia [an eh MALE yah]
Class Hydrozoa [high drow ZOE ah]
Class Scyphozoa [sky foe ZOE ah]
Class Anthozoa [an thow ZOE ah]
What You Need to Know:
You should be able to:
name the three classes of Cnidaria, give the characteristics of each and recognize each, on sight, in lab,
identify living Hydra and under the microscope, be able to identify tentacles, hypostome, mouth,
gonads, nematocysts, and the two layers of cells,
name the basic cell types in the epidermis and gastrodermis of Hydra,
distinguish between Hydra budding and with testes and ovaries,
identify both preserved specimens and prepared slides of Obelia and identify the major parts of the
colony,
identify preserved specimens and prepared slides of Gonionemus and recognize the major structures,
distinguish among statocysts, tentacular bulbs, rhophalia, and ocelli,
explain what is meant by polymorphism and dimorphism,
explain how you can tell most jellyfish of the class Scyphozoa from jellyfish of the class Hydrozoa,
identify from preserved specimens Aurelia and recognize the major structures associated with it,
provide life cycles for Hydra, Obelia, Aurelia and the larval types of each,
identify the major structures associated with Metridium from preserved specimens, and,
recognize several stony and soft corals provided by the instructor.
Exercises: Fill in the Blank.
The genus Obelia belongs to the class while Metridium belongs to the class .
Which class of Cnidarians has no medusae?
Which class of Cnidarians typically has a velum?
How many tentacles does a Hydra typically have? .
The stinging cell of Cnidarians is called the while the trigger is called the and the "stinger" itself is called
the .
Which cell type in Cnidarians may give rise to other types of cells?
Balance structures in the Cnidarians are called .
The middle layer of tissue in Cnidarians is called the while the middle layer in sponges is called .
The larval type in sponges is the larva and the larval type in Cnidarians is called the larva.
The gonads of the class Scyphozoa and Anthozoa are found in the .
The name of the medusae produced by Aurelia is and the polyps are called .
This is a tiny opening on either side of the mouth in Metridium used to circulate water while keeping
prey inside the pharynx.
Stinging cells in Metridium are found on tiny threads called
The walls of the cup of stoney corals is called the
Reproductive polyps in Obelia are called .
Exercises: Multiple Choice. Select the Best Answer.
Which genus has no medusae and only polyps?
(1) Hydra
(2) Obelia
(3) Metridium
(4) both 1 and 3
(5) All of the above have no medusae.
Which class has polyps?
(1) Hydrozoa
(2) Scyphozoa
(3) Anthozoa
(4) both 1 and 2
(5) all of the above
Which of the following is a colonial polyp form that floats in the ocean and is carried by winds and
waves?
(1) Obelia
(2) Aurelia
(3) Physalia
(4) Metridium
(5) Gonionemus
Which cell type is found in the gastrodermis of Hydra?
(1) interstitial cell
(2) nutritive-muscle cell
(3) epitheliomuscle cell
(4) cnidocyte
(5) sensory cell
Which terms are correctly matched?
(1) Hydra-medusa
(2) Velum-Aureila
(3) acontium-Obelia
(4) ephyra-Aurelia
(5) Physalia-scyphistoma
l}
Huber (2010). Marine Biology. New York: McGraw-Hill. p. 121. ^ "Sea Anemones – info and
games". sheppardsoftware.com.
http://sheppardsoftware.com/content/animals/animals/invertebrates/seaanemone.htm. Retrieved
November 20, 2010. ^ a b c d Robert D. Barnes (1982). Invertebrate Zoology. Philadelphia, PA:
Holt-Saunders International. pp. 150–157. ISBN 0-03-056747-5.
1. About.com
2. Education
3. Animals / Wildlife
http://www.about.com/
Animals / Wildlife
Search
http://www.about.com/
Animals / Wildlife
Animal Facts
Habitat Facts
Animal Pictures
Share
Print
Free Animals / Wildlife Newsletter!Sign Up
Discuss in my forum
A Guide to Vertebrates and Invertebrates
A Backbone Makes All the Difference
By Laura Klappenbach, About.com Guide
See More About:
invertebrates
vertebrates
An assortment of vertebrates and invertebrates.
Photos © Shutterstock.
Ads
OxyPlates for AnaerobesNo bags, jars, or chambers needed Free Samplewww.oxyrase.com
Mycoplasma TestingResults in 2 Days High Sensitivitywww.ABSbio.com
Wildlife AnimalsSee Wildlife in South Africa. SA's Official Tourism
Website.www.SouthAfrica.net
Animals / Wildlife Ads
Animals
Invertebrates
Wildlife Animals
Sea Animals
Wild Life Animals
Animal classification is a matter of sorting out similarities and differences, of placing animals in
groups and then breaking those groups apart into subgroups. The whole endeavor creates a
structure—a hierarchy in which the large high-level groups sort out bold and obvious
differences, while the low-level groups tease apart subtle, almost imperceptible, variations. This
sorting process enables scientists to describe evolutionary relationships, identify shared traits,
and highlight unique characteristics down through the various levels of animal groups and
subgroups.
Among the most basic criteria by which animals are sorted is whether or not they possess a
backbone. This single trait places an animal into one of just two groups: the vertebrates or the
invertebrates and represents a fundamental division among all animals alive today as well as
those that have long ago disappeared. If we are to know anything about an animal, we should
first aim to determine whether it is an invertebrate or a vertebrate. We'll then be on our way to
understanding its place within the animal world.
What are Vertebrates?
Vertebrates (Subphylum Vertebrata) are animals that possess an internal skeleton (endoskeleton)
that includes a backbone made up of a column of vertebrae (Keeton, 1986:1150). The
Subphylum Vertebrata is a group within the Phylum Chordata (commonly called the 'chordates')
and as such inherits the characteristics of all chordates:
bilateral symmetry
body segmentation
endoskeleton (bony or cartilaginous)
pharyngeal pouches (present during some stage of development)
complete digestive system
ventral heart
closed blood system
tail (at some stage of development)
In addition to the traits listed above, vertebrates possess one additional trait that makes them
unique among chordates: the presence of a backbone. There are a few groups of chordates that
do not possess a backbone (these organisms are not vertebrates and are instead referred to as
invertebrate chordates).
The animal classes that are vertebrates include:
Jawless fish (Class Agnatha)
Armored fish (Class Placodermi) - extinct
Cartilaginous fish (Class Chondrichthyes)
Bony fish (Class Osteichthyes)
Amphibians (Class Amphibia)
Reptiles (Class Reptilia)
Birds (Class Aves)
Mammals (Class Mammalia)
What are Invertebrates?
Invertebrates are a broad collection of animal groups (they do not belong to a single subphylum
like the vertebrates) all of which lack a backbone. Some (not all) of the animal groups that are
invertebrates include:
Sponges (Phylum Porifera)
Jellyfish, hydras, sea anemones, corals (Phylum Cnidaria)
Comb jellies (Phylum Ctenophora)
Flatworms (Phylum Platyhelminthes)
Molluscs (Phylum Mollusca)
Arthropods (Phylum Arthropoda)
Segmented worms (Phylum Annelida)
Echinoderms (Phylum Echinodermata
l} javascript:popup_window_1();
javascript:popup_window_1();
Scientific
Heteractis malu
Name
Comments A sea anemone (Anthozoa)
Reference
Creator
From D. G. Fautin and G. R. Allen. 1992. Field Guide to Anemonefishes and their
Host Sea Anemones. Western Australia Museum.
photographed by Art Reed
Specimen
Live Specimen
Condition
Copyright © 1992 Western Australia Museum
javascript:popup_window_2();
javascript:popup_window_2();
Scientific
Aglantha digitale
Name
Comment A direct-developing holoplanktonic hydromedusa (Hydrozoa) that has no polyp. The
gonads are visible through the transparent bell.
s
Copyright © 1998 Claudia E. Mills
Sea anemone
From Wikipedia, the free encyclopedia
Jump to: navigation, search
Sea anemones
/wiki/File:Actiniaria.jpg
/wiki/File:Actiniaria.jpg
Various examples of sea anemones
Scientific classification /wiki/Template:Taxonomy/Actiniaria
/wiki/Template:Taxonomy/Actiniaria
Kingdom:
Animalia
Phylum:
Cnidaria
Class:
Anthozoa
Subclass:
Hexacorallia
Order:
Actiniaria
Suborders
Endocoelantheae
Nyantheae
Protantheae
Ptychodacteae
Diversity
46 families
/wiki/File:Haeckel_Actiniae.jpg
/wiki/File:Haeckel_Actiniae.jpg
/wiki/File:Haeckel_Actiniae.jpg /wiki/File:Haeckel_Actiniae.jpgThe 49th plate from Ernst
Haeckel's Kunstformen der Natur, 1904, showing various sea anemones classified as Actiniae
Sea anemones are a group of water-dwelling, predatory animals of the order Actiniaria; they
are named after the anemone, a terrestrial flower. Sea anemones are classified in the phylum
Cnidaria, class Anthozoa, subclass Zoantharia.[1] Anthozoa often have large polyps that allow
for digestion of larger prey and also lack a medusa stage.[2] As cnidarians, sea anemones are
closely related to corals, jellyfish, tube-dwelling anemones, and Hydra.
Contents
[hide]
1 Anatomy
1.1 Digestive system
1.2 Nerve system
2 Life cycle
3 Ecology
4 Exploitation
5 Fossil record
6 See also
7 References
8 External links
[edit] Anatomy
A sea anemone is a polyp attached at the bottom to the surface beneath it by an adhesive foot,
called a basal disc, with a column shaped body ending in an oral disc. Most are from 1.8 to 3
centimetres (0.71 to 1.2 in) in diameter, but anemones as small as 4 millimetres (0.16 in) or as
large as nearly 2 metres (6.6 ft) are known.[3] They can have anywhere from a few tens to a few
hundred tentacles.
A few species are pelagic, and are not attached to the bottom; instead they have a gas chamber
within the pedal disc, allowing them to float upside down in the water.[4]
The mouth is in the middle of the oral disc surrounded by tentacles armed with many cnidocytes,
which are cells that function as a defense and as a means to capture prey. Cnidocytes contain
nematocyst, capsule-like organelles capable of everting, giving phylum Cnidaria its name.[5]
The cnidae that sting are called nematocysts. Each nematocyst contains a small vesicle filled
with toxins (actinoporins), an inner filament, and an external sensory hair. When the hair is
touched it mechanically triggers the cell explosion, a harpoon-like structure which attaches to
organisms that trigger it, and injects a dose of poison in the flesh of the aggressor or prey. This
gives the anemone its characteristic sticky feeling. The sea anemone eats small fish and shrimp.
The poison is a mix of toxins, including neurotoxins, which paralyzes the prey and allows it to be
moved to the mouth for digestion inside the gastrovascular cavity. Actinoporins have been
reported as highly toxic to fish and crustaceans, which are the natural prey of sea anemones. In
addition to their role in predation, it has been suggested that actinoporins could act, when
released in water, as repellents against potential predators[citation needed]. Anemonefish
(clownfish), small banded fish in various colors, are not affected by their host anemone's sting
and shelter themselves from predators within its tentacles.[6]
The internal anatomy of anemones is quite complex.
[edit] Digestive system
There is a gastrovascular cavity (which functions as a stomach) with a single opening to the
outside which functions as both a mouth and an anus; waste and undigested matter is excreted
through the mouth/anus, which can be described as an incomplete gut. The mouth is typically
slit-like in shape, and bears a groove at one or both ends. The groove, termed a siphonophore, is
ciliated, and helps to circulate water through the gastrovascular cavity.[4] Some anemones feed
on small particles, which are caught with the aid of a mucus secretion and moving currents that
are set up by the tentacles. Most sea anemones are predacious, immobilizing their prey with the
aid of their nematocysts.[1]
The mouth opens into a flattened pharynx. This consists of an in-folding of the body wall, and is
therefore lined by the animal's epidermis. The pharynx typically runs for about two-thirds the
length of the body before opening into the gastrovascular cavity that fills the remainder of the
body.
The gastrovascular cavity itself is divided into a number of chambers by mesenteries radiating
inwards from the body wall. Some of the mesenteries form complete partitions with a free edge
at the base of the pharynx, to which they connect, but others reach only partway across. The
mesenteries are usually found in multiples of twelve, and are symmetrically arranged around the
central pharynx. They have stomach lining on both sides, separated by a thin layer of mesoglea,
and includes filaments of tissue specialised for secreting digestive enzymes. In some species
these filaments extend below the lower margin of the mesentery, hanging free in the
gastovascular cavity as acontial filaments.[4]
[edit] Nerve system
A primitive nervous system, without centralization, coordinates the processes involved in
maintaining homeostasis as well as biochemical and physical responses to various stimuli. There
are no specialized sense organs.
The muscles and nerves are much simpler than those of most other animals, although more
specialised than in other cnidarians, such as corals. Cells in the outer layer (epidermis) and the
inner layer (gastrodermis) have microfilaments that group into contractile fibers. These fibers are
not true muscles because they are not freely suspended in the body cavity as they are in more
developed animals. Longitudinal fibres are found in the tentacles and oral disc, and also within
the mesenteries, where they can contract the whole length of the body. Circular fibers are found
in the body wall and, in some species, around the oral disc, allowing the animal to retract its
tentacles into a protective sphincter.[4]
Since the anemone lacks a skeleton, the contractile cells pull against the gastrovascular cavity,
which acts as a hydrostatic skeleton. The anemone stabilizes itself by shutting its mouth, which
keeps the gastrovascular cavity at a constant volume, making it more rigid. Although generally
sessile, sea anemones are capable of slow movements using their pedal disc, or of swimming,
using either their tentacles or by flexing their body.
[edit] Life cycle
/wiki/File:Brooding_sea_anemone_Epiactis_prolifera_1.jpg
/wiki/File:Brooding_sea_anemone_Epiactis_prolifera_1.jpg
/wiki/File:Brooding_sea_anemone_Epiactis_prolifera_1.jpg
/wiki/File:Brooding_sea_anemone_Epiactis_prolifera_1.jpgBrooding anemone (Epiactis
prolifera) with developing young.
Unlike other cnidarians, anemones (and other anthozoans) entirely lack the free-swimming
medusa stage of the life cycle; the polyp produces eggs and sperm, and the fertilized egg
develops into a planula that develops directly into another polyp.
Anemones tend to stay in the same spot until conditions become unsuitable (prolonged dryness,
for example), or a predator attacks them. In that case anemones can release themselves from the
substrate and use flexing motions to swim to a new location. Most sea anemones attach
temporarily to submerged objects; a few thrust themselves into the sand or live in burrows; a few
are parasitic on other marine organisms [1] and some have symbiotic relationships with hermit
crabs.
The sexes in sea anemones are separate in some species, while other species, like the brooding
anemone (Epiactis prolifera), are protandric hermaphrodites. The gonads are strips of tissue
within the mesenteries. Both sexual and asexual reproduction can occur. In sexual reproduction
males release sperm to stimulate females to release eggs, and fertilization occurs. Anemones
eject eggs and sperm through the mouth. The fertilized egg develops into a planula, which settles
and grows into a single polyp.
Anemones can also reproduce asexually, by budding, binary fission (the polyp separates into two
halves), and pedal laceration, in which small pieces of the pedal disc break off and regenerate
into small anemones.
[edit] Ecology
/wiki/File:Actinoscyphia_aurelia_1.jpg
/wiki/File:Actinoscyphia_aurelia_1.jpg
/wiki/File:Actinoscyphia_aurelia_1.jpg /wiki/File:Actinoscyphia_aurelia_1.jpgVenus' fly-trap
anemone (Actinoscyphia) in the Gulf of Mexico
The sea anemone has a pedal disc, which the organism uses to attach itself to rocks or which it
anchors in the sand. Others also burrow into a stronger object. Some species attach to kelp while
others are free-swimming. Although not plants and therefore incapable of photosynthesis
themselves, many sea anemones form an important facultative symbiotic relationship with
certain single-celled green algae species which reside in the animals' gastrodermal cells. These
algae may be either zooxanthellae, zoochlorellae or both. The sea anemone benefits from the
products of the algae's photosynthesis, namely oxygen and food in the form of glycerol, glucose
and alanine; the algae in turn are assured a reliable exposure to sunlight and protection from
micro-feeders, which the sea anemones actively maintain. The algae also benefit by being
protected due to the presence of stinging cells called nematocysts, reducing the likelihood of
being eaten by herbivores. Most species inhabit tropical reefs, although there are species adapted
to relatively cold waters, intertidal reefs, and sand/kelp environments.
[edit] Exploitation
/wiki/File:Actinodendron.jpg
/wiki/File:Actinodendron.jpg
/wiki/File:Actinodendron.jpg /wiki/File:Actinodendron.jpgActinodendron sp.
The global trade of marine ornamentals has been a rapidly expanding industry involving
numerous countries worldwide. In the early 1980s, the estimated value of imported marine fish
and invertebrates was US$24–40 million annually.[7] Current estimates place that value at
US$200–330 million,[8] with the United States accounting for 80% of the industry imports.[9]
Despite advances and the expansion of aquaculture, post-larval capture and rearing, the majority
of marine ornamentals are collected in the wild as adults or juveniles.[10] Anemones are
susceptible to overexploitation due to their long life spans, slower relative growth rates, and
lower reproductive rates than their resident fish, which are also affected due to the fact that they
settle exclusively and are restricted to specific host sea anemones. The demand for these
organisms is reflected in fishermen's catch records, which document the value they are paid per
catch, and on average sea anemones were valued at five times the average value of anemonefish,
and ten times the value of the most abundant anemonefish, and in fact only made up 4.1% of the
total value of the catch. Research has shown that aquarium fishing activities significantly impact
the populations of anemones and anemonefish by drastically reducing the densities of each in
exploited areas,[10] and could also negatively impact anemone shrimp, and any organisms
obligately associated with sea anemones. It should be noted that anemonefish can survive alone
in captivity, as has been shown by multiple research efforts.[11][12]
In southern Italy and southwestern Spain the anemone Anemonia sulcata is consumed as a
delicacy.[13]. The whole animal is marinated in vinegar, then coated in a tempura-like batter and
deep-fried in olive oil. They are similar in appearance and texture to croquettes, but have an
intense seafood taste.
[edit] Fossil record
Most Actiniaria do not form hard parts that can be recognized as fossils but a few fossils do
exist; Mackenzia, from the Middle Cambrian Burgess Shale of Canada, is the oldest fossil
identified as a sea anemone.<!contradicted in reference>
Sea anemone From Wikipedia, the free encyclopediaJump to: navigation, search Sea anemones
Various examples of sea anemones
Scientific classification
Kingdom: Animalia
Phylum: Cnidaria
Class: Anthozoa
Subclass: Hexacorallia
Order: Actiniaria
Suborders
Endocoelantheae
Nyantheae
Protantheae
Ptychodacteae
Diversity
46 families
The 49th plate from Ernst Haeckel's Kunstformen der Natur, 1904, showing various sea anemones
classified as ActiniaeSea anemones are a group of water-dwelling, predatory animals of the order
Actiniaria; they are named after the anemone, a terrestrial flower. Sea anemones are classified in the
phylum Cnidaria, class Anthozoa, subclass Zoantharia.[1] Anthozoa often have large polyps that allow for
digestion of larger prey and also lack a medusa stage.[2] As cnidarians, sea anemones are closely related
to corals, jellyfish, tube-dwelling anemones, and Hydra.
Contents [hide]
1 Anatomy
1.1 Digestive system
1.2 Nerve system
2 Life cycle
3 Ecology
4 Exploitation
5 Fossil record
6 See also
7 References
8 External links
[edit] AnatomyA sea anemone is a polyp attached at the bottom to the surface beneath it by an
adhesive foot, called a basal disc, with a column shaped body ending in an oral disc. Most are from 1.8
to 3 centimetres (0.71 to 1.2 in) in diameter, but anemones as small as 4 millimetres (0.16 in) or as large
as nearly 2 metres (6.6 ft) are known.[3] They can have anywhere from a few tens to a few hundred
tentacles.
A few species are pelagic, and are not attached to the bottom; instead they have a gas chamber within
the pedal disc, allowing them to float upside down in the water.[4]
The mouth is in the middle of the oral disc surrounded by tentacles armed with many cnidocytes, which
are cells that function as a defense and as a means to capture prey. Cnidocytes contain nematocyst,
capsule-like organelles capable of everting, giving phylum Cnidaria its name.[5] The cnidae that sting are
called nematocysts. Each nematocyst contains a small vesicle filled with toxins (actinoporins), an inner
filament, and an external sensory hair. When the hair is touched it mechanically triggers the cell
explosion, a harpoon-like structure which attaches to organisms that trigger it, and injects a dose of
poison in the flesh of the aggressor or prey. This gives the anemone its characteristic sticky feeling. The
sea anemone eats small fish and shrimp.
The poison is a mix of toxins, including neurotoxins, which paralyzes the prey and allows it to be moved
to the mouth for digestion inside the gastrovascular cavity. Actinoporins have been reported as highly
toxic to fish and crustaceans, which are the natural prey of sea anemones. In addition to their role in
predation, it has been suggested that actinoporins could act, when released in water, as repellents
against potential predators[citation needed]. Anemonefish (clownfish), small banded fish in various
colors, are not affected by their host anemone's sting and shelter themselves from predators within its
tentacles.[6]
The internal anatomy of anemones is quite complex.
[edit] Digestive systemThere is a gastrovascular cavity (which functions as a stomach) with a single
opening to the outside which functions as both a mouth and an anus; waste and undigested matter is
excreted through the mouth/anus, which can be described as an incomplete gut. The mouth is typically
slit-like in shape, and bears a groove at one or both ends. The groove, termed a siphonophore, is
ciliated, and helps to circulate water through the gastrovascular cavity.[4] Some anemones feed on
small particles, which are caught with the aid of a mucus secretion and moving currents that are set up
by the tentacles. Most sea anemones are predacious, immobilizing their prey with the aid of their
nematocysts.[1]
The mouth opens into a flattened pharynx. This consists of an in-folding of the body wall, and is
therefore lined by the animal's epidermis. The pharynx typically runs for about two-thirds the length of
the body before opening into the gastrovascular cavity that fills the remainder of the body.
The gastrovascular cavity itself is divided into a number of chambers by mesenteries radiating inwards
from the body wall. Some of the mesenteries form complete partitions with a free edge at the base of
the pharynx, to which they connect, but others reach only partway across. The mesenteries are usually
found in multiples of twelve, and are symmetrically arranged around the central pharynx. They have
stomach lining on both sides, separated by a thin layer of mesoglea, and includes filaments of tissue
specialised for secreting digestive enzymes. In some species these filaments extend below the lower
margin of the mesentery, hanging free in the gastovascular cavity as acontial filaments.[4]
[edit] Nerve systemA primitive nervous system, without centralization, coordinates the processes
involved in maintaining homeostasis as well as biochemical and physical responses to various stimuli.
There are no specialized sense organs.
The muscles and nerves are much simpler than those of most other animals, although more specialised
than in other cnidarians, such as corals. Cells in the outer layer (epidermis) and the inner layer
(gastrodermis) have microfilaments that group into contractile fibers. These fibers are not true muscles
because they are not freely suspended in the body cavity as they are in more developed animals.
Longitudinal fibres are found in the tentacles and oral disc, and also within the mesenteries, where they
can contract the whole length of the body. Circular fibers are found in the body wall and, in some
species, around the oral disc, allowing the animal to retract its tentacles into a protective sphincter.[4]
Since the anemone lacks a skeleton, the contractile cells pull against the gastrovascular cavity, which
acts as a hydrostatic skeleton. The anemone stabilizes itself by shutting its mouth, which keeps the
gastrovascular cavity at a constant volume, making it more rigid. Although generally sessile, sea
anemones are capable of slow movements using their pedal disc, or of swimming, using either their
tentacles or by flexing their body.
[edit] Life cycle
Brooding anemone (Epiactis prolifera) with developing young.Unlike other cnidarians, anemones (and
other anthozoans) entirely lack the free-swimming medusa stage of the life cycle; the polyp produces
eggs and sperm, and the fertilized egg develops into a planula that develops directly into another polyp.
Anemones tend to stay in the same spot until conditions become unsuitable (prolonged dryness, for
example), or a predator attacks them. In that case anemones can release themselves from the substrate
and use flexing motions to swim to a new location. Most sea anemones attach temporarily to
submerged objects; a few thrust themselves into the sand or live in burrows; a few are parasitic on
other marine organisms [1] and some have symbiotic relationships with hermit crabs.
The sexes in sea anemones are separate in some species, while other species, like the brooding
anemone (Epiactis prolifera), are protandric hermaphrodites. The gonads are strips of tissue within the
mesenteries. Both sexual and asexual reproduction can occur. In sexual reproduction males release
sperm to stimulate females to release eggs, and fertilization occurs. Anemones eject eggs and sperm
through the mouth. The fertilized egg develops into a planula, which settles and grows into a single
polyp.
Anemones can also reproduce asexually, by budding, binary fission (the polyp separates into two
halves), and pedal laceration, in which small pieces of the pedal disc break off and regenerate into small
anemones.
[edit] Ecology
Venus' fly-trap anemone (Actinoscyphia) in the Gulf of MexicoThe sea anemone has a pedal disc, which
the organism uses to attach itself to rocks or which it anchors in the sand. Others also burrow into a
stronger object. Some species attach to kelp while others are free-swimming. Although not plants and
therefore incapable of photosynthesis themselves, many sea anemones form an important facultative
symbiotic relationship with certain single-celled green algae species which reside in the animals'
gastrodermal cells. These algae may be either zooxanthellae, zoochlorellae or both. The sea anemone
benefits from the products of the algae's photosynthesis, namely oxygen and food in the form of
glycerol, glucose and alanine; the algae in turn are assured a reliable exposure to sunlight and protection
from micro-feeders, which the sea anemones actively maintain. The algae also benefit by being
protected due to the presence of stinging cells called nematocysts, reducing the likelihood of being
eaten by herbivores. Most species inhabit tropical reefs, although there are species adapted to relatively
cold waters, intertidal reefs, and sand/kelp environments.
[edit] Exploitation
Actinodendron sp.The global trade of marine ornamentals has been a rapidly expanding industry
involving numerous countries worldwide. In the early 1980s, the estimated value of imported marine
fish and invertebrates was US$24–40 million annually.[7] Current estimates place that value at US$200–
330 million,[8] with the United States accounting for 80% of the industry imports.[9] Despite advances
and the expansion of aquaculture, post-larval capture and rearing, the majority of marine ornamentals
are collected in the wild as adults or juveniles.[10] Anemones are susceptible to overexploitation due to
their long life spans, slower relative growth rates, and lower reproductive rates than their resident fish,
which are also affected due to the fact that they settle exclusively and are restricted to specific host sea
anemones. The demand for these organisms is reflected in fishermen's catch records, which document
the value they are paid per catch, and on average sea anemones were valued at five times the average
value of anemonefish, and ten times the value of the most abundant anemonefish, and in fact only
made up 4.1% of the total value of the catch. Research has shown that aquarium fishing activities
significantly impact the populations of anemones and anemonefish by drastically reducing the densities
of each in exploited areas,[10] and could also negatively impact anemone shrimp, and any organisms
obligately associated with sea anemones. It should be noted that anemonefish can survive alone in
captivity, as has been shown by multiple research efforts.[11][12]
In southern Italy and southwestern Spain the anemone Anemonia sulcata is consumed as a delicacy.[13].
The whole animal is marinated in vinegar, then coated in a tempura-like batter and deep-fried in olive
oil. They are similar in appearance and texture to croquettes, but have an intense seafood taste.
[edit] Fossil recordMost Actiniaria do not form hard parts that can be recognized as fossils but a few
fossils do exist; Mackenzia, from the Middle Cambrian Burgess Shale of Canada, is the oldest fossil
identified as a sea anemone.<!contradicted in reference>
[edit] See
Also
Cnidaria
From Wikipedia, the free encyclopedia
(Redirected from Cnideria)
Jump to: navigation, search
Cnidaria
Pacific sea nettles, Chrysaora fuscescens
Scientific classification
Domain:
Eukaryota
Kingdom:
Animalia
Phylum:
Cnidaria
Hatschek, 1888
Subphylum/Classes[3]
Anthozoa—corals and sea anemones
Medusozoa—jellyfish:[1]
Cubozoa—box jellyfish, sea wasps
Hydrozoa—hydroids, hydra-like animals
Scyphozoa—true jellyfish
Staurozoa—stalked jellyfish
Unranked, may not be scyphozoans[2]
Myxozoa—parasites
Polypodiozoa—parasites
Cnidaria ( /naɪˈdɛəriə/ with a silent c) is a phylum containing over 10,000[4] species of
animals found exclusively in aquatic and mostly marine environments. Their distinguishing
feature is cnidocytes, specialized cells that they use mainly for capturing prey. Their bodies
consist of mesoglea, a non-living jelly-like substance, sandwiched between two layers of
epithelium that are mostly one cell thick. They have two basic body forms: swimming medusae
and sessile polyps, both of which are radially symmetrical with mouths surrounded by tentacles
that bear cnidocytes. Both forms have a single orifice and body cavity that are used for digestion
and respiration. Many cnidarian species produce colonies that are single organisms composed of
medusa-like or polyp-like zooids, or both. Cnidarians' activities are coordinated by a
decentralized nerve net and simple receptors. Several free-swimming Cubozoa and Scyphozoa
possess balance-sensing statocysts, and some have simple eyes. Not all cnidarians reproduce
sexually. Many have complex lifecycles with asexual polyp stages and sexual medusae, but some
omit either the polyp or the medusa stage.
Cnidarians were for a long time grouped with Ctenophores in the phylum Coelenterata, but
increasing awareness of their differences caused them to be placed in separate phyla. Cnidarians
are classified into four main groups: the almost wholly sessile Anthozoa (sea anemones, corals,
sea pens); swimming Scyphozoa (jellyfish); Cubozoa (box jellies); and Hydrozoa, a diverse
group that includes all the freshwater cnidarians as well as many marine forms, and has both
sessile members such as Hydra and colonial swimmers such as the Portuguese Man o' War.
Staurozoa have recently been recognised as a class in their own right rather than a sub-group of
Scyphozoa, and there is debate about whether Myxozoa and Polypodiozoa are cnidarians or
closer to bilaterians (more complex animals).
Most cnidarians prey on organisms ranging in size from plankton to animals several times larger
than themselves, but many obtain much of their nutrition from endosymbiotic algae, and a few
are parasites. Many are preyed upon by other animals including starfish, sea slugs, fish and
turtles. Coral reefs, whose polyps are rich in endosymbiotic algae, support some of the world's
most productive ecosystems, and protect vegetation in tidal zones and on shorelines from strong
currents and tides. While corals are almost entirely restricted to warm, shallow marine waters,
other cnidarians live in the depths, in polar seas and in freshwater.
Fossil cnidarians have been found in rocks formed about 580 million years ago, and other fossils
show that corals may have been present shortly before 490 million years ago and diversified a
few million years later. Fossils of cnidarians that do not build mineralized structures are very
rare. Scientists currently think that cnidarians, ctenophores and bilaterians are more closely
related to calcareous sponges than these are to other sponges, and that anthozoans are the
evolutionary "aunts" or "sisters" of other cnidarians, and the most closely related to bilaterians.
Recent analyses have concluded that cnidarians, although considered more "primitive" than
bilaterians, have a wider range of genes.
Jellyfish stings killed several hundred people in the 20th century, and cubozoans are particularly
dangerous. On the other hand, some large jellyfish are considered a delicacy in eastern and
southern Asia. Coral reefs have long been economically important as providers of fishing
grounds, protectors of shore buildings against currents and tides, and more recently as centers of
tourism. However, they are vulnerable to over-fishing, mining for construction materials,
pollution, and damage caused by tourism.
Contents
[hide]
1 Distinguishing features
2 Description
o 2.1 Basic body forms
o 2.2 Colonial forms
o 2.3 Skeletons
o 2.4 Main cell layers
o 2.5 Cnidocytes
o 2.6 Locomotion
o 2.7 Nervous system and senses
o 2.8 Feeding and excretion
o 2.9 Respiration
o 2.10 Regeneration
3 Reproduction
o 3.1 Sexual
o 3.2 Asexual
4 Classification
5 Ecology
6 Evolutionary history
o 6.1 Fossil record
o 6.2 Family tree
7 Interaction with humans
8 Notes
9 Further reading
o
o
9.1 Books
9.2 Journal articles
10 External links
[edit] Distinguishing features
Further information: Sponge, Ctenophore, and Bilateria
Cnidarians form an animal phylum that is more complex than sponges, about as complex as
ctenophores (comb jellies), and less complex than bilaterians, which include almost all other
animals. However, both cnidarians and ctenophores are more complex than sponges as they
have: cells bound by inter-cell connections and carpet-like basement membranes; muscles;
nervous systems; and some have sensory organs. Cnidarians are distinguished from all other
animals by having cnidocytes that fire like harpoons and are used mainly to capture prey but also
as anchors in some species.[5]
Like sponges and ctenophores, cnidarians have two main layers of cells that sandwich a middle
layer of jelly-like material, which is called the mesoglea in cnidarians; more complex animals
have three main cell layers and no intermediate jelly-like layer. Hence, cnidarians and
ctenophores have traditionally been labelled diploblastic, along with sponges.[5][6] However, both
cnidarians and ctenophores have a type of muscle that, in more complex animals, arises from the
middle cell layer.[7] As a result some recent text books classify ctenophores as triploblastic,[8] and
it has been suggested that cnidarians evolved from triploblastic ancestors.[7]
Cnidocytes
Sponges[9][10]
Cnidarians[5][6]
Ctenophores[5][8] Bilateria[5]
No
Yes
No
Colloblasts
No
Digestive and
circulatory organs
Number of main cell
layers
Yes
No
Two, with jelly-like layer between them
No
Yes
Two[5] or
Three[7][8]
Three
Cells in each layer
bound together
No, except that
Homoscleromorpha have
basement membranes.[11]
Yes: inter-cell connections; basement membranes
Sensory organs
No
Yes
Number of cells in
middle "jelly" layer
Many
Few
(Not
applicable)
Cells in outer layers
can move inwards
and change
functions
Yes
No
(Not
applicable)
Nervous system
No
Yes, simple
Simple to
complex
Muscles
None
[edit] Description
[edit] Basic body forms
Aboral end
Oral end
Mouth
Oral end
Aboral end
Exoderm
Gastroderm (Endoderm)
Mesoglea
Digestive cavity
Mostly
epitheliomuscular
Mostly
myoepithelial
Mostly
myocytes
Medusa (left) and polyp (right)[6]
Oral end of actinodiscus polyp, with close-up of the mouth
Adult cnidarians appear as either swimming medusae or sessile polyps. Both are radially
symmetrical, like a wheel and a tube respectively. Since these animals have no heads, their ends
are described as "oral" (nearest the mouth) and "aboral" (furthest from the mouth). Most have
fringes of tentacles equipped with cnidocytes around their edges, and medusae generally have an
inner ring of tentacles around the mouth. The mesoglea of polyps is usually thin and often soft,
but that of medusae is usually thick and springy, so that it returns to its original shape after
muscles around the edge have contracted to squeeze water out, enabling medusae to swim by a
sort of jet propulsion.[6]
[edit] Colonial forms
Tree-like polyp colony[6]
Cnidaria produce a variety of colonial forms, each of which is one organism but consists of
polyp-like zooids. The simplest is a connecting tunnel that runs over the substrate (rock or
seabed) and from which single zooids sprout. In some cases the tunnels form visible webs, and in
others they are enclosed in a fleshy mat. More complex forms are also based on connecting
tunnels but produce "tree-like" groups of zooids. The "trees" may be formed either by a central
zooid that functions as a "trunk" with later zooids growing to the sides as "branches", or in a zigzag shape as a succession of zooids, each of which grows to full size and then produces a single
bud at an angle to itself. In many cases the connecting tunnels and the "stems" are covered in
periderm, a protective layer of chitin.[6] Some colonial forms have other specialized types of
zooid, for example, to pump water through their tunnels.[12]
Siphonophores form complex colonies that consist of: an upside-down polyp that forms a central
stem with a gas-filled float at the top; one or more sets of medusa-like zooids that provide
propulsion; leaf-like bracts that give some protection to other parts; sets of tentacles that bear
nematocytes that capture prey; other tentacles that act as sensors; near the base of each set of
tentacles, a polyp-like zooid that acts as a stomach for the colony; medusa-like zooids that serve
as gonads. Although some of these zooids resemble polyps or medusae in shape, they lack
features that are not relevant to their specific functions, for example the swimming "medusae"
have no digestive, sensory or reproductive cells. The best-known siphonophore is the Portuguese
Man o' War (Physalia physalis).[12][13][14]
[edit] Skeletons
In medusae the only supporting structure is the mesoglea. Hydra and most sea anemones close
their mouths when they are not feeding, and the water in the digestive cavity then acts as a
hydrostatic skeleton, rather like a water-filled balloon. Other polyps such as Tubularia use
columns of water-filled cells for support. Sea pens stiffen the mesoglea with calcium carbonate
spicules and tough fibrous proteins, rather like sponges.[6]
In some colonial polyps a chitinous periderm gives support and some protection to the
connecting sections and to the lower parts of individual polyps. Stony corals secrete massive
calcium carbonate exoskeletons. A few polyps collect materials such as sand grains and shell
fragments, which they attach to their outsides. Some colonial sea anemones stiffen the mesoglea
with sediment particles.[6]
[edit] Main cell layers
Cnidaria are diploblastic animals, in other words they have two main cell layers, while more
complex animals are triploblasts having three main layers. The two main cell layers of cnidarians
form epithelia that are mostly one cell thick, and are attached to a fibrous basement membrane,
which they secrete. They also secrete the jelly-like mesoglea that separates the layers. The layer
that faces outwards, known as the ectoderm ("outside skin"), generally contains the following
types of cells:[5]
Epitheliomuscular cells whose bodies form part of the epithelium but whose bases extend to
form muscle fibers in parallel rows.[15] The fibers of the outward-facing cell layer generally run at
right angles to the fibers of the inward-facing one. In Anthozoa (anemones, corals, etc.) and
Scyphozoa (jellyfish), the mesoglea also contains some muscle cells.[6]
Cnidocytes, the harpoon-like "nettle cells" that give the phylum Cnidaria its name. These appear
between or sometimes on top of the muscle cells.[5]
Nerve cells. Sensory cells appear between or sometimes on top of the muscle cells,[5] and
communicate via synapses (gaps across which chemical signals flow) with motor nerve cells,
which lie mostly between the bases of the muscle cells.[6]
Interstitial cells, which are unspecialized and can replace lost or damaged cells by transforming
into the appropriate types. These are found between the bases of muscle cells.[5]
In addition to epitheliomuscular, nerve and interstitial cells, the inward-facing gastroderm
("stomach skin") contains gland cells that secrete digestive enzymes. In some species it also
contains low concentrations of cnidocytes, which are used to subdue prey that is still
struggling.[5][6]
The mesoglea contains small numbers of amoeba-like cells,[6] and muscle cells in some
species.[5] However the number of middle-layer cells and types are much lower than in
sponges.[6]
[edit] Cnidocytes
A hydra's nematocyst, before firing.
"trigger" cilium[6]
Firing sequence of the cnida in a hydra's nematocyst[6]
Operculum (lid)
"Finger" that turns inside out
/ / / Barbs
Venom
Victim's skin
Victim's tissues
These "nettle cells" function as harpoons, since their payloads remain connected to the bodies of
the cells by threads. Three types of cnidocytes are known:[5][6]
Nematocysts inject venom into prey, and usually have barbs to keep them embedded in the
victims. Most species have nematocysts.[5]
Spirocysts do not penetrate the victim or inject venom, but entangle it by means of small sticky
hairs on the thread.
Ptychocysts are not used for prey capture — instead the threads of discharged ptychocysts are
used for building protective tubes in which their owners live. Ptychocysts are found only in the
order Cerianthria, tube anemones.[6]
The main components of a cnidocyte are:[5][6]
A cilium (fine hair) which projects above the surface and acts as a trigger. Spirocysts do not have
cilia.
A tough capsule, the cnida, which houses the thread, its payload and a mixture of chemicals
which may include venom or adhesives or both. ("cnida" is derived from the Greek word κνίδη,
which means "nettle"[16])
A tube-like extension of the wall of the cnida that points into the cnida, like the finger of a
rubber glove pushed inwards. When a cnidocyte fires, the finger pops out. If the cell is a
venomous nematocyte, the "finger"'s tip reveals a set of barbs that anchor it in the prey.
The thread, which is an extension of the "finger" and coils round it until the cnidocyte fires. The
thread is usually hollow and delivers chemicals from the cnida to the target.
An operculum (lid) over the end of the cnida. The lid may be a single hinged flap or three flaps
arranged like slices of pie.
The cell body which produces all the other parts.
It is difficult to study the firing mechanisms of cnidocytes as these structures are small but very
complex. At least four hypotheses have been proposed:[5]
Rapid contraction of fibers round the cnida may increase its internal pressure.
The thread may be like a coiled spring that extends rapidly when released.
In the case of Chironex (the "sea wasp"), chemical changes in the cnida's contents may cause
them to expand rapidly by polymerization.
Chemical changes in the liquid in the cnida make it a much more concentrated solution, so that
osmotic pressure forces water in very rapidly to dilute it. This mechanism has been observed in
nematocysts of the class Hydrozoa, sometimes producing pressures as high as 140 atmospheres,
similar to that of scuba air tanks, and fully extending the thread in as little as 2 milliseconds
(0.002 second).[6]
Cnidocytes can only fire once, and about 25% of a hydra's nematocysts are lost from its tentacles
when capturing a brine shrimp. Used cnidocytes have to be replaced, which takes about 48 hours.
To minimise wasteful firing, two types of stimulus are generally required to trigger cnidocytes:
their cilia detect contact, and nearby sensory cells "smell" chemicals in the water. This
combination prevents them from firing at distant or non-living objects. Groups of cnidocytes are
usually connected by nerves and, if one fires, the rest of the group requires a weaker minimum
stimulus than the cells that fire first.[5][6]
[edit] Locomotion
Chrysaora quinquecirrha ("sea nettle") swimming
Medusae swim by a form of jet propulsion: muscles, especially inside the rim of the bell, squeeze
water out of the cavity inside the bell, and the springiness of the mesoglea powers the recovery
stroke. Since the tissue layers are very thin, they provide too little power to swim against currents
and just enough to control movement within currents.[6]
Hydras and some sea anemones can move slowly over rocks and sea or stream beds by various
means: creeping like snails, crawling like inchworms, or by somersaulting. A few can swim
clumsily by waggling their bases.[6]
[edit] Nervous system and senses
Cnidaria have no brains or even central nervous systems. Instead they have decentralized nerve
nets consisting of : sensory neurons that generate signals in response to various types of stimulus,
such as odors; motor neurons that tell muscles to contract; all connected by "cobwebs" of
intermediate neurons. As well as forming the "signal cables", intermediate neurons also form
ganglia that act as local coordination centers. The cilia of the cnidocytes detect physical contact.
Nerves inform cnidocytes when odors from prey or attackers are detected and when
neighbouring cnidocytes fire. Most of the communications between nerve cells are via chemical
synapses, small gaps across which chemicals flow. As this process is too slow to ensure that the
muscles round the rim of a medusa's bell contract simultaneously in swimming the neurons
which control this communicate by much faster electrical signals across gap junctions.[6]
Medusae and complex swimming colonies such as siphonophores and chondrophores sense tilt
and acceleration by means of statocysts, chambers lined with hairs which detect the movements
of internal mineral grains called statoliths. If the body tilts in the wrong direction, the animal
rights itself by increasing the strength of the swimming movements on the side that is too low.
They also have ocelli ("little eyes"), which can detect the direction from which light is coming.
Box jellies have camera eyes, although these probably do not form images, and their lenses
simply produce a clearer indication of the direction from which light is coming.[5]
[edit] Feeding and excretion
Cnidarians feed in several ways: predation, absorbing dissolved organic chemicals, filtering food
particles out of the water, and obtaining nutrients from symbiotic algae within their cells. Most
obtain the majority of their food from predation but some, including the corals Hetroxenia and
Leptogorgia, depend almost completely on their endosymbionts and on absorbing dissolved
nutrients.[5] Cnidaria give their symbiotic algae carbon dioxide, some nutrients and a place in the
sun.[6]
Predatory species use their cnidocytes to poison or entangle prey, and those with venomous
nematocysts may start digestion by injecting digestive enzymes. The "smell" of fluids from
wounded prey makes the tentacles fold inwards and wipe the prey off into the mouth. In medusae
the tentacles round the edge of the bell are often short and most of the prey capture is done by
"oral arms", which are extensions of the edge of the mouth and are often frilled and sometimes
branched to increase their surface area. Medusae often trap prey or suspended food particles by
swimming upwards, spreading their tentacles and oral arms and then sinking. In species for
which suspended food particles are important, the tentacles and oral arms often have rows of
cilia whose beating creates currents that flow towards the mouth, and some produce nets of
mucus to trap particles.[5]
Once the food is in the digestive cavity, gland cells in the gastroderm release enzymes that
reduce the prey to slurry, usually within a few hours. This circulates through the digestive cavity
and, in colonial cnidarians, through the connecting tunnels, so that gastroderm cells can absorb
the nutrients. Absorption may take a few hours, and digestion within the cells may take a few
days. The circulation of nutrients is driven by water currents produced by cilia in the gastroderm
or by muscular movements or both, so that nutrients reach all parts of the digestive cavity.[6]
Nutrients reach the outer cell layer by diffusion or, for animals or zooids such as medusae which
have thick mesogleas, are transported by mobile cells in the mesoglea.[5]
Indigestible remains of prey are expelled through the mouth. The main waste product of cells'
internal processes is ammonia, which is removed by the external and internal water currents.[6]
[edit] Respiration
There are no respiratory organs, and both cell layers absorb oxygen from and expel carbon
dioxide into the surrounding water. When the water in the digestive cavity becomes stale it must
be replaced, and nutrients that have not been absorbed will be expelled with it. Some Anthozoa
have ciliated grooves on their tentacles, allowing them to pump water out of and into the
digestive cavity without opening the mouth. This improves respiration after feeding and allows
these animals, which use the cavity as a hydrostatic skeleton, to control the water pressure in the
cavity without expelling undigested food.[5]
Cnidaria that carry photosynthetic symbionts may have the opposite problem, an excess of
oxygen, which may prove toxic. The animals produce large quantities of antioxidants to
neutralize the excess oxygen.[5]
[edit] Regeneration
All cnidarians can regenerate, allowing them to recover from injury and to reproduce asexually.
Medusae have limited ability to regenerate, but polyps can do so from small pieces or even
collections of separated cells. This enables corals to recover even after apparently being
destroyed by predators.[5]
[edit] Reproduction
1
2
3
4
5
6
7
8
9
10
11
12
13
14
Life cycle of a jellyfish:[5][6]
1–3 Larva searches for site
4–8 Polyp grows
9–11 Polyp strobilates
12–14 Medusa grows
[edit] Sexual
In the Cnidaria sexual reproduction often involves a complex life cycle with both polyp and
medusa stages. For example in Scyphozoa (jellyfish) and Cubozoa (box jellies) a larva swims
until it finds a good site, and then becomes a polyp. This grows normally but then absorbs its
tentacles and splits horizontally into a series of disks that become juvenile medusae, a process
called strobilation. The juveniles swim off and slowly grow to maturity, while the polyp regrows and may continue strobilating periodically. The adults have gonads in the gastroderm, and
these release ova and sperm into the water in the breeding season.[5][6]
Shortened forms of this life cycle are common, for example some oceanic scyphozoans omit the
polyp stage completely, and cubozoan polyps produce only one medusa. Hydrozoa have a
variety of life cycles. Some have no polyp stages and some (e.g. hydra) have no medusae. In
some species the medusae remain attached to the polyp and are responsible for sexual
reproduction; in extreme cases these reproductive zooids may not look much like medusae.
Anthozoa have no medusa stage at all and the polyps are responsible for sexual reproduction.[5]
Spawning is generally driven by environmental factors such as changes in the water temperature,
and their release is triggered by lighting conditions such as sunrise, sunset or the phase of the
moon. Many species of Cnidaria may spawn simultaneously in the same location, so that there
are too many ova and sperm for predators to eat more than a tiny percentage — one famous
example is the Great Barrier Reef, where at least 110 corals and a few non-cnidarian
invertebrates produce enough to turn the water cloudy. These mass spawnings may produce
hybrids, some of which can settle and form polyps, but it is not known how long these can
survive. In some species the ova release chemicals that attract sperm of the same species.[5]
The fertilized eggs develop into larvae by dividing until there are enough cells to form a hollow
sphere (blastula) and then a depression forms at one end (gastrulation) and eventually become
the digestive cavity. However in cnidarians the depression forms at the end further from the yolk
(at the animal pole), while in bilaterians it forms at the other end (vegetal pole).[6] The larvae,
called planulae, swim or crawl by means of cilia.[5] They are cigar-shaped but slightly broader at
the "front" end, which is the aboral, vegetal-pole end and eventually attaches to a substrate if the
species has a polyp stage.[6]
Anthozoan larvae either have large yolks or are capable of feeding on plankton, and some
already have endosymbiotic algae that help to feed them. Since the parents are immobile, these
feeding capabilities extend the larvae's range and avoid overcrowding of sites. Scyphozoan and
hydrozoan larvae have little yolk and most lack endosymbiotic algae, and therefore have to settle
quickly and metamorphose into polyps. Instead these species rely on their medusae to extend
their ranges.[6]
[edit] Asexual
All known cnidaria can reproduce asexually by various means, in addition to regenerating after
being fragmented. Hydrozoan polyps only bud, while the medusae of some hydrozoans can
divide down the middle. Scyphozoan polyps can both bud and split down the middle. In addition
to both of these methods, Anthozoa can split horizontally just above the base.[5][6]
[edit] Classification
Cnidarians were for a long time grouped with Ctenophores in the phylum Coelenterata, but
increasing awareness of their differences caused them to be placed in separate phyla. Cnidarians
are classified into four main groups: sessile Anthozoa (sea anemones, corals, sea pens);
swimming Scyphozoa (jellyfish); Cubozoa (box jellies); and Hydrozoa, a diverse group that
includes all the freshwater cnidarians as well as many marine forms, and has both sessile
members such as Hydra and colonial swimmers such as the Portuguese Man o' War. Staurozoa
have recently been recognised as a class in their own right rather than a sub-group of Scyphozoa,
and there is debate about whether Myxozoa and Polypodiozoa are cnidarians or closer to
bilaterians.
Modern cnidarians are generally classified into four classes:[5]
Hydrozoa
Number of species[4]
Scyphozoa
Cubozoa
Anthozoa
3,600
228
42
6,100
Hydra,
siphonophores
Jellyfish
Box
jellies
Sea anemones,
corals, sea pens
Yes
Yes
Yes
Yes
Yes
Yes
Medusa phase in life
In some species
cycle
Yes, except for Stauromedusae
Yes
if they are scyphozoans
No
Number of medusae
Many
produced per polyp
Many
(not applicable)
Examples
Cells found in mesoglea No
Nematocysts in
exodermis
No
One
Stauromedusae, small sessile cnidarians with stalks and no medusa stage, have traditionally been
classified as members of the Scyphozoa, but recent research suggests they should be regarded as
a separate class, Staurozoa.[17]
The Myxozoa, microscopic parasites, were first classified as protozoans,[18] but recently as
heavily modified cnidarians, and more closely related to Hydrozoa and Scyphozoa than to
Anthozoa.[19] However other recent research suggests that Polypodium hydriforme, a parasite
within the egg cells of sturgeon, is closely related to the Myxozoa and that both Polypodium and
the Myxozoa are intermediate between cnidarians and bilaterian animals.[20]
Some researchers classify the extinct conulariids as cnidarians, while others propose that they
form a completely separate phylum.[21]
[edit] Ecology
Coral reefs support rich ecosystems
Many cnidarians are limited to shallow waters because they depend on endosymbiotic algae for
much of their nutrients. The life cycles of most have polyp stages, which are limited to locations
that offer stable substrates. Nevertheless major cnidarian groups contain species that have
escaped these limitations. Hydrozoans have a worldwide range: some, such as Hydra, live in
freshwater; Obelia appears in the coastal waters of all the oceans; and Liriope can form large
shoals near the surface in mid-ocean. Among anthozoans, a few scleractinian corals, sea pens
and sea fans live in deep, cold waters, and some sea anemones inhabit polar seabeds while others
live near hydrothermal vents over 10 kilometres (6.2 mi) below sea-level. Reef-building corals
are limited to tropical seas between 30°N and 30°S with a maximum depth of 46 metres (151 ft),
temperatures between 20°C and 28°C, high salinity and low carbon dioxide levels.
Stauromedusae, although usually classified as jellyfish, are stalked, sessile animals that live in
cool to Arctic waters.[12] Cnidarians range in size from Hydra, 5–20 millimetres (0.20–0.79 in)
long,[22] to the Lion's mane jellyfish, which may exceed 2 metres (6.6 ft) in diameter and 75
metres (246 ft) in length.[23]
Prey of cnidarians ranges from plankton to animals several times larger than themselves.[12][24]
Some cnidarians are parasites, mainly on jellyfish but a few are major pests of fish.[12] Others
obtain most of their nourishment from endosymbiotic algae or dissolved nutrients.[5] Predators of
cnidarians include: sea slugs, which can incorporate nematocysts into their own bodies for selfdefense;[25] starfish, notably the crown of thorns starfish, which can devastate corals;[12] butterfly
fish and parrot fish, which eat corals;[26] and marine turtles, which eat jellyfish.[23] Some sea
anemones and jellyfish have a symbiotic relationship with some fish; for example clown fish live
among the tentacles of sea anemones, and each partner protects the other against predators.[12]
Coral reefs form some of the world's most productive ecosystems. Common coral reef cnidarians
include both Anthozoans (hard corals, octocorals, anemones) and Hydrozoans (fire corals, lace
corals) The endosymbiotic algae of many cnidarian species are very effective primary producers,
in other words converters of inorganic chemicals into organic ones that other organisms can use,
and their coral hosts use these organic chemicals very efficiently. In addition reefs provide
complex and varied habitats that support a wide range of other organisms.[27] Fringing reefs just
below low-tide level also have a mutually beneficial relationship with mangrove forests at high-
tide level and sea grass meadows in between: the reefs protect the mangroves and seagrass from
strong currents and waves that would damage them or erode the sediments in which they are
rooted, while the mangroves and seagrass protect the coral from large influxes of silt, fresh water
and pollutants. This additional level of variety in the environment is beneficial to many types of
coral reef animals, which for example may feed in the sea grass and use the reefs for protection
or breeding.[28]
[edit] Evolutionary history
[edit] Fossil record
The fossil coral Cladocora from Pliocene rocks in Cyprus
The earliest widely accepted animal fossils are rather modern-looking cnidarians, possibly from
around 580 million years ago, although fossils from the Doushantuo Formation can only be dated
approximately.[29] The identification of some of these as embryos of animals has been contested,
but other fossils from these rocks strongly resemble tubes and other mineralized structures made
by corals.[30] Their presence implies that the cnidarian and bilaterian lineages had already
diverged.[31] Although the Ediacaran fossil Charnia used to be classified as a jellyfish or sea
pen,[32] more recent study of growth patterns in Charnia and modern cnidarians has cast doubt on
this hypothesis,[33][34] and there are now no bona-fide cnidarian body fossils in the Ediacaran.
Few fossils of cnidarians without mineralized skeletons are known from more recent rocks,
except in lagerstätten that preserved soft-bodied animals.[35]
A few mineralized fossils that resemble corals have been found in rocks from the Cambrian
period, and corals diversified in the Early Ordovician.[35] These corals, which were wiped out in
the Permian-Triassic extinction about 251 million years ago,[35] did not dominate reef
construction since sponges and algae also played a major part.[36] During the Mesozoic era rudist
bivalves were the main reef-builders, but they were wiped out in the Cretaceous-Tertiary
extinction 65 million years ago,[37] and since then the main reef-builders have been scleractinian
corals.[35]
[edit] Family tree
Further information: Phylogeny
Metazoa
Glass sponges
Demosponges
Calcareous sponges
Eumetazoa
Ctenophora (comb jellies)
Planulozoa Cnidaria
Anthozoa
(sea anemones and corals)
Medusozoa
Hydrozoa
(Hydra, siphonophores, etc.)
Cubozoa
(box jellies)
Staurozoa
"Scyphozoa"
(jellyfish, excluding
Staurozoa)
Placozoa
Bilateria
Myxozoa
Other Bilateria
(more complex)
Family tree of Cnidaria and the origins of animals[2][38][39][40]
It is difficult to reconstruct the early stages in the evolutionary "family tree" of animals using
only morphology (their shapes and structures), because the large differences between Porifera
(sponges), Cnidaria plus Ctenophora (comb jellies), Placozoa and Bilateria (all the more complex
animals) make comparisons difficult. Hence reconstructions now rely largely or entirely on
molecular phylogenetics, which groups organisms according to similarities and differences in
their biochemistry, usually in their DNA or RNA.[41]
It is now generally thought that the Calcarea (sponges with calcium carbonate spicules) are more
closely related to Cnidaria, Ctenophora (comb jellies) and Bilateria (all the more complex
animals) than they are to the other groups of sponges.[38][42][43] In 1866 it was proposed that
Cnidaria and Ctenophora were more closely related to each other than to Bilateria and formed a
group called Coelenterata ("hollow guts"), because Cnidaria and Ctenophora both rely on the
flow of water in and out of a single cavity for feeding, excretion and respiration. In 1881 it was
proposed that Ctenophora and Bilateria were more closely related to each other, since they
shared features that Cnidaria lack, for example muscles in the middle layer (mesoglea in
Ctenophora, mesoderm in Bilateria). However more recent analyses indicate that these
similarities are rather vague, and the current view, based on molecular phylogenetics, is that
Cnidaria and Bilateria are more closely related to each other than either is to Ctenophora. This
grouping of Cnidaria and Bilateria has been labelled "Planulozoa" because it suggests that the
earliest Bilateria were similar to the planula larvae of Cnidaria.[2][39]
Within the Cnidaria, the Anthozoa (sea anemones and corals) are regarded as the sister-group of
the rest, which suggests that the earliest cnidarians were sessile polyps with no medusa stage.
However it is unclear how the other groups acquired the medusa stage, since Hydrozoa form
medusae by budding from the side of the polyp while the other Medusozoa do so by splitting
them off from the tip of the polyp. The traditional grouping of Scyphozoa included the
Staurozoa, but morphology and molecular phylogenetics indicate that Staurozoa are more closely
related to Cubozoa (box jellies) than to other "Scyphozoa". Similarities in the double body walls
of Staurozoa and the extinct Conulariida suggest that they are closely related. The position of
Anthozoa nearest the beginning of the cnidarian family tree also implies that Anthozoa are the
cnidarians most closely related to Bilateria, and this is supported by the fact that Anthozoa and
Bilateria share some genes that determine the main axes of the body.[2][44]
However in 2005 Katja Seipel and Volker Schmid suggested that cnidarians and ctenophores are
simplified descendants of triploblastic animals, since ctenophores and the medusa stage of some
cnidarians have striated muscle, which in bilaterians arises from the mesoderm. They did not
commit themselves on whether bilaterians evolved from early cnidarians or from the
hypothesized triploblastic ancestors of cnidarians.[7]
In molecular phylogenetics analyses from 2005 onwards, important groups of developmental
genes show the same variety in cnidarians as in chordates.[45] In fact cnidarians, and especially
anthozoans (sea anemones and corals), retain some genes that are present in bacteria, protists,
plants and fungi but not in bilaterians.[46]
The mitochondial genomes in the medusozoan cnidarians unlike that of other animals is linear
with fragmented genes.[47] The reason for this difference is unknown.
[edit] Interaction with humans
Jellyfish stings killed about 1,500 people in the 20th century,[48] and cubozoans are particularly
dangerous. On the other hand, some large jellyfish are considered a delicacy in eastern and
southern Asia. Coral reefs have long been economically important as providers of fishing
grounds, protectors of shore buildings against currents and tides, and more recently as centers of
tourism. However, they are vulnerable to over-fishing, mining for construction materials,
pollution, and damage caused by tourism.
Beaches protected from tides and storms by coral reefs are often the best places for housing in
tropical countries. Reefs are an important food source for low-technology fishing, both on the
reefs themselves and in the adjacent seas.[49] However despite their great productivity reefs are
vulnerable to over-fishing, because much of the organic carbon they produce is exhaled as
carbon dioxide by organisms at the middle levels of the food chain and never reaches the larger
species that are of interest to fishermen.[27] Tourism centered on reefs provides much of the
income of some tropical islands, attracting photographers, divers and sports fishermen. However
human activities damage reefs in several ways: mining for construction materials; pollution,
including large influxes of fresh water from storm drains; commercial fishing, including the use
of dynamite to stun fish and the capture of young fish for aquariums; and tourist damage caused
by boat anchors and the cumulative effect of walking on the reefs.[49] Coral, mainly from the
Pacific Ocean has long been used in jewellery, and demand rose sharply in the 1980s.[50]
The dangerous "sea wasp" Chironex fleckeri
Some large jellyfish species have been used in Chinese cuisine at least since 200 AD, and are
now fished in the seas around most of South East Asia. Japan is the largest single consumer of
edible jellyfish, importing at first only from China but now from all of South East Asia as prices
rose in the 1970s. This fishing industry is restricted to daylight hours and calm conditions in two
short seasons, from March to May and August to November.[51] The commercial value of
jellyfish food products depends on the skill with which they are prepared, and "Jellyfish
Masters" guard their trade secrets carefully. Jellyfish is very low in cholesterol and sugars, but
cheap preparation can introduce undesirable amounts of heavy metals.[52]
The "sea wasp" Chironex fleckeri has been described as the world's most venomous animal and
is held responsible for 67 deaths, although it is difficult to identify the animal as it is almost
transparent. Most stingings by C. fleckeri cause only mild symptoms.[53] Seven other box jellies
can cause a set of symptoms called Irukandji syndrome,[54] which takes about 30 minutes to
develop,[55] and from a few hours to two weeks to disappear.[56] Hospital treatment is usually
required, and there have been a few deaths.[54]
[edit] Notes
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
^ Classes in Medusozoa based on "The Taxonomicon - Taxon: Subphylum Medusozoa". Universal
Taxonomic Services. http://www.taxonomy.nl/Taxonomicon/TaxonTree.aspx?id=11582. Retrieved 200901-26.
^ a b c d Collins, A.G. (2002). "Phylogeny of Medusozoa and the Evolution of Cnidarian Life Cycles" (PDF).
Journal of Evolutionary Biology 15 (3): 418–432. doi:10.1046/j.1420-9101.2002.00403.x.
http://cima.uprm.edu/~n_schizas/CMOB_8676/Collins2002.pdf. Retrieved 2008-11-27.
^ Subphyla Anthozoa and Medusozoa based on "The Taxonomicon - Taxon: Phylum Cnidaria". Universal
Taxonomic Services. http://www.taxonomy.nl/Taxonomicon/TaxonTree.aspx?id=11551. Retrieved 200707-10.
^ a b Zhang, Z.-Q. (2011). "Animal biodiversity: An introduction to higher-level classification and taxonomic
richness". Zootaxa 3148: 7–12. http://mapress.com/zootaxa/2011/f/zt03148p012.pdf.
^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af Hinde, R.T., (1998). "The Cnidaria and Ctenophora". In
Anderson, D.T.,. Invertebrate Zoology. Oxford University Press. pp. 28–57. ISBN 0195513681.
^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004).
Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 111–124. ISBN 0030259827.
^ a b c d Seipel, K., and Schmid, V. (June 2005). "Evolution of striated muscle: Jellyfish and the origin of
triploblasty". Developmental Biology 282 (1): 14–26. doi:10.1016/j.ydbio.2005.03.032. PMID 15936326.
^ a b c Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole.
pp. 182–195. ISBN 0030259827.
^ Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 76–97.
ISBN 0030259827.
^ Bergquist, P.R., (1998). "Porifera". In Anderson, D.T.,. Invertebrate Zoology. Oxford University Press.
pp. 10–27. ISBN 0195513681.
^ Exposito, J-Y., Cluzel, C., Garrone, R., and Lethias, C. (2002). "Evolution of collagens". The Anatomical
Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology 268 (3): 302–316.
doi:10.1002/ar.10162. PMID 12382326.
^ a b c d e f g Shostak, S. (2006). "Cnidaria (Coelenterates)". Encyclopedia of Life Sciences. John Wiley & Sons.
doi:10.1038/npg.els.0004117.
^ Bhamrah, H.S., and Juneja, K. (2002). A Textbook of Invertebrates. Anmol Publications. pp. 278–280.
ISBN 8126104163.
http://books.google.com/?id=05So4shx9tIC&pg=PA279&dq=siphonophore+siphonophora. Retrieved
2008-11-17.
^ Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 167–
170. ISBN 0030259827.
^ Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). "Introduction to Metazoa". Invertebrate Zoology (7 ed.).
Brooks / Cole. pp. 103–104. ISBN 0030259827.
^ Trumble, W., and Brown, L. (2002). "Cnida". Shorter Oxford English Dictionary. Oxford University Press.
^ Collins, A.G., Cartwright, P., McFadden, C.S., and Schierwater, B. (2005). "Phylogenetic Context and
Basal Metazoan Model Systems". Integrative and Comparative Biology 45 (4): 585–594.
doi:10.1093/icb/45.4.585.
^ Štolc, A. (1899). "Actinomyxidies, nouveau groupe de Mesozoaires parent des Myxosporidies". Bull. Int.
L'Acad. Sci. Bohème 12: 1–12.
^ E. Jímenez-Guri; Philippe, H; Okamura, B; Holland, PW (July 2007). "Buddenbrockia is a cnidarian worm".
Science 317 (116): 116–118. doi:10.1126/science.1142024. PMID 17615357.
20. ^ Zrzavý, J. and Hypša, V. (2003). "Myxozoa, Polypodium, and the origin of the Bilateria: The phylogenetic
position of "Endocnidozoa" in light of the rediscovery of Buddenbrockia". Cladistics 19 (2): 164–169.
doi:10.1111/j.1096-0031.2003.tb00305.x.
21. ^ "The Conulariida". University of California Museum of Paleontology.
http://www.ucmp.berkeley.edu/cnidaria/conulariida.html. Retrieved 2008-11-27.
22. ^ Blaise, C., and Férard, J-F. (2005). Small-scale Freshwater Toxicity Investigations: Toxicity Test Methods.
Springer. p. 398. ISBN 140203119X. http://books.google.com/?id=Ibew5SLx2oMC&dq=hydra+size+length.
Retrieved 2008-11-21.
23. ^ a b Safina, C. (2007). Voyage of the Turtle: In Pursuit of the Earth's Last Dinosaur. Macmillan. p. 154.
ISBN 0805083189. http://books.google.com/?id=dQD883dAv6YC&pg=PA154&dq=cnidaria+turtle.
Retrieved 2008-11-21.
24. ^ Cowen, R. (2000). History of Life (3 ed.). Blackwell. p. 54. ISBN 0632044446.
http://books.google.com/?id=qvyBS4gwPF4C&pg=PA54&dq=cnidaria+prey. Retrieved 2008-11-21.
25. ^ Frick, K (2003). "Predator Suites and Flabellinid Nudibranch Nematocyst Complements in the Gulf of
Maine.". In: SF Norton (ed). Diving for Science...2003. Proceedings of the American Academy of
Underwater Sciences (22nd Annual Scientific Diving Symposium). http://archive.rubiconfoundation.org/4744. Retrieved 2008-07-03.
26. ^ Choat, J.H. and Bellwood, D.R. (1998). Paxton, J.R. and Eschmeyer, W.N.. ed. Encyclopedia of Fishes. San
Diego: Academic Press. pp. 209–211. ISBN 0-12-547665-5.
27. ^ a b Barnes, R.S.K., and Mann, K.H. (1991). Fundamentals of Aquatic Ecology. Blackwell Publishing.
pp. 217–227. ISBN 0632029838.
http://books.google.com/?id=mOZZlzgdTrwC&pg=PA227&dq=%22Coral+Reef%22+productivity. Retrieved
2008-11-26.
28. ^ Hatcher, B.G. Johannes, R.E., and Robertson, A.J. (1989). "Conservation of Shallow-water Marine
Ecosystems". Oceanography and Marine Biology: An Annual Review: Volume 27. Routledge. p. 320.
ISBN 0080377181.
http://books.google.com/?id=XpmNqFaDZ7cC&pg=PA320&dq=%22Coral+Reef%22+mangrove+%22seagra
ss%22. Retrieved 2008-11-21.
29. ^ Chen, J-Y.; Oliveri, P; Li, CW; Zhou, GQ; Gao, F; Hagadorn, JW; Peterson, KJ; Davidson, EH (2000).
"Putative phosphatized embryos from the Doushantuo Formation of China". Proceedings of the National
Academy of Sciences 97 (9): 4457–4462. doi:10.1073/pnas.97.9.4457. PMC 18256. PMID 10781044.
http://www.pnas.org/content/97/9/4457.full. Retrieved 2009-04-30.
30. ^ Xiao, S., Yuan, X., and Knoll, A.H. (2000). "Eumetazoan fossils in terminal Proterozoic phosphorites?".
Proceedings of the National Academy of Sciences 97 (25): 13684–13689. doi:10.1073/pnas.250491697.
PMC 17636. PMID 11095754.
http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=17636.
31. ^ Chen, J.-Y., Oliveri, P., Gao, F., Dornbos, S.Q., Li, C-W., Bottjer, D.J. and Davidson, E.H. (August 2002).
"Precambrian Animal Life: Probable Developmental and Adult Cnidarian Forms from Southwest China"
(PDF). Developmental Biology 248 (1): 182–196. doi:10.1006/dbio.2002.0714. PMID 12142030.
http://www.uwm.edu/~sdornbos/PDF's/Chen%20et%20al.%202002.pdf. Retrieved 2008-09-03.
32. ^ Donovan, Stephen K., Lewis, David N. (2001). "Fossils explained 35. The Ediacaran biota" (abstract).
Geology Today 17 (3): 115–120. doi:10.1046/j.0266-6979.2001.00285.x.
33. ^ Antcliffe, J.B.; Brasier, M. D. (2007). "Charnia and sea pens are poles apart". Journal of the Geological
Society 164 (1): 49–51. doi:10.1144/0016-76492006-080.
34. ^ Antcliffe, J.B.; Brasier, Martin D. (2007). "Charnia At 50: Developmental Models For Ediacaran Fronds".
Palaeontology 51 (1): 11–26. doi:10.1111/j.1475-4983.2007.00738.x.
35. ^ a b c d "Cnidaria: Fossil Record". University of California Museum of Paleontology.
http://www.ucmp.berkeley.edu/cnidaria/cnidariafr.html. Retrieved 2008-11-27.
36. ^ Copper, P. (January 1994). "Ancient reef ecosystem expansion and collapse". Coral Reefs 13 (1): 3–11.
Bibcode 1994CorRe..13....3C. doi:10.1007/BF00426428.
37. ^ "The Rudists". University of California Museum of Paleontology.
http://www.ucmp.berkeley.edu/taxa/inverts/mollusca/rudists.php. Retrieved 2008-11-27.
38. ^ a b Borchiellini, C., Manuel, M., Alivon, E., Boury-Esnault, N., Vacelet J., and Le Parco, Y. (2001). "Sponge
paraphyly and the origin of Metazoa". Journal of Evolutionary Biology 14 (1): 171–179.
doi:10.1046/j.1420-9101.2001.00244.x.
39. ^ a b Wallberg, A., Thollesson, M., , Farris, J.S., and Jondelius, U. (2004). "The phylogenetic position of the
comb jellies (Ctenophora) and the importance of taxonomic sampling". Cladistics 20 (6): 558–578.
doi:10.1111/j.1096-0031.2004.00041.x.
40. ^ Philippe, H. (April 2009). "Phylogenomics Revives Traditional Views on Deep Animal Relationships".
Current Biology 19: 706–712. doi:10.1016/j.cub.2009.02.052. PMID 19345102.
http://www.cell.com/current-biology/retrieve/pii/S0960982209008057. Retrieved 2011-09-25.
41. ^ Halanych, K.M. (December 2004). "The New View of Animal Phylogeny" (PDF). Annual Review of
Ecology, Evolution, and Systematics 35: 229–256. doi:10.1146/annurev.ecolsys.35.112202.130124.
http://gump.auburn.edu/halanych/lab/Pub.pdfs/Halanych2004.pdf. Retrieved 2008-11-27.
42. ^ Medina, M., Collins, A.G., Silberman, J.D., and Sogin, M.L. (August 2001). "Evaluating hypotheses of
basal animal phylogeny using complete sequences of large and small subunit rRNA". Proceedings of the
National Academy of Sciences 98 (17): 9707–9712. doi:10.1073/pnas.171316998. PMC 55517.
PMID 11504944. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=55517.
43. ^ Müller, W.E.G., Li, J., Schröder, H.C., Qiao, L., and Wang, X. (2007). "The unique skeleton of siliceous
sponges (Porifera; Hexactinellida and Demospongiae) that evolved first from the Urmetazoa during the
Proterozoic: a review". Biogeosciences 4 (2): 219–232. doi:10.5194/bg-4-219-2007.
44. ^ Marques, A.C., and Collins, A.G. (2004). "Cladistic analysis of Medusozoa and cnidarian evolution".
Invertebrate Biology 123 (1): 23–42. doi:10.1111/j.1744-7410.2004.tb00139.x.
http://www.marinespecies.org/aphia.php?p=sourceget&id=38492. Retrieved 2008-11-27.
45. ^ Miller, D.J., Ball, E.E., and Technau, U. (October 2005). "Cnidarians and ancestral genetic complexity in
the animal kingdom". Trends in Genetics 21 (10): 536–539. doi:10.1016/j.tig.2005.08.002.
PMID 16098631.
46. ^ Technau, U., Rudd, S., and Maxwell, P (December 2005). "Maintenance of ancestral complexity and nonmetazoan genes in two basal cnidarians". Trends in Genetics 21 (12): 633–639.
doi:10.1016/j.tig.2005.09.007. PMID 16226338.
47. ^ Smith DR, Kayal E, Yanagihara AA, Collins AG, Pirro S, Keeling PJ (2011) First complete mitochondrial
genome sequence from a box jellyfish reveals a highly fragmented, linear architecture and insights into
telomere evolution. Genome Biol Evol
48. ^ Williamson, J.A., Fenner, P.J., Burnett, J.W., and Rifkin, J. (1996). Venomous and Poisonous Marine
Animals: A Medical and Biological Handbook. UNSW Press. pp. 65–68. ISBN 0868402796.
http://books.google.com/?id=YsZ3GryFIzEC&pg=PA75&lpg=PA75&dq=mollusc+venom+fatal. Retrieved
2008-10-03.
49. ^ a b Clark, J.R. (1998). Coastal Seas: The Conservation Challenge. Blackwell. pp. 8–9. ISBN 0632049553.
http://books.google.com/?id=H82xdtuLxDMC&pg=PA8&dq=%22Coral+Reef%22+productivity. Retrieved
2008-11-28.
50. ^ Cronan, D.S., (1991). Marine Minerals in Exclusive Economic Zones. Springer. pp. 63–65.
ISBN 041229270X. http://books.google.com/?id=4g4nhd8USO8C&pg=PA63&dq=coral+jewellery.
Retrieved 2008-11-28.
51. ^ Omori, M. and Nakano, E. (2001). "Jellyfish fisheries in southeast Asia". In Purcell, J.E.,. Jellyfish Blooms:
Ecological and Societal Importance. Springer. pp. 19–26. ISBN 0792369645.
52. ^ Hsieh, Y-H.P. Leong, F-M., and Rudloe, J. (2001). "Jellyfish as food". In Purcell, J.E.,. Jellyfish Blooms:
Ecological and Societal Importance. Springer. pp. 11–17. ISBN 0792369645.
53. ^ Greenberg, M.I., Hendrickson, R.G., Silverberg, M., Campbell, C., and Morocco, A. (2004). "Box Jellyfish
Envenomation". Greenberg's Text-atlas of Emergency Medicine. Lippincott Williams & Wilkins. p. 875.
ISBN 0781745861.
54. ^ a b Little, M., Pereira, P., Carrette, T., and Seymour, J. (2006). "Jellyfish Responsible for Irukandji
Syndrome". QJM (Quarterly Journal of Medicine) 99 (6): 425–427. doi:10.1093/qjmed/hcl057.
PMID 16687419.
55. ^ Barnes, J. (1964). "Cause and effect in Irukandji stingings". Medical Journal of Australia 1: 897–904.
PMID 14172390.
56. ^ Grady J, Burnett J (2003). "Irukandji-like syndrome in South Florida divers". Annals of Emergency
Medicine 42 (6): 763–6. doi:10.1016/S0196-0644(03)00513-4. PMID 14634600.
[edit] Further reading
[edit] Books
Arai, M.N. (1997). A Functional Biology of Scyphozoa. London: Chapman & Hall [p. 316]. ISBN 0412-45110-7.
Ax, P. (1999). Das System der Metazoa I. Ein Lehrbuch der phylogenetischen Systematik. Gustav
Fischer, Stuttgart-Jena: Gustav Fischer. ISBN 3-437-30803-3.
Barnes, R.S.K., P. Calow, P. J. W. Olive, D. W. Golding & J. I. Spicer (2001). The invertebrates—a
synthesis. Oxford: Blackwell. 3rd edition [chapter 3.4.2, p. 54]. ISBN 0-632-04761-5.
Brusca, R.C., G.J. Brusca (2003). Invertebrates. Sunderland, Mass.: Sinauer Associates. 2nd
edition [chapter 8, p. 219]. ISBN 0-87893-097-3.
Dalby, A. (2003). Food in the Ancient World: from A to Z. London: Routledge.
Moore, J.(2001). An Introduction to the Invertebrates. Cambridge: Cambridge University Press
[chapter 4, p. 30]. ISBN 0-521-77914-6.
Schäfer, W. (1997). Cnidaria, Nesseltiere. In Rieger, W. (ed.) Spezielle Zoologie. Teil 1. Einzeller
und Wirbellose Tiere. Stuttgart-Jena: Gustav Fischer. Spektrum Akademischer Verl., Heidelberg,
2004. ISBN 3-8274-1482-2.
Werner, B. 4. Stamm Cnidaria. In: V. Gruner (ed.) Lehrbuch der speziellen Zoologie. Begr. von
Kaestner. 2 Bde. Stuttgart-Jena: Gustav Fischer, Stuttgart-Jena. 1954, 1980, 1984, Spektrum
Akad. Verl., Heidelberg-Berlin, 1993. 5th edition. ISBN 3-334-60474-8.
[edit] Journal articles
D. Bridge, B. Schierwater, C. W. Cunningham, R. DeSalle R, L. W. Buss: Mitochondrial DNA
structure and the molecular phylogeny of recent cnidaria classes. in: Proceedings of the Academy
of Natural Sciences of Philadelphia. Philadelphia USA 89.1992, p. 8750. ISSN 0097-3157
D. Bridge, C. W. Cunningham, R. DeSalle, L. W. Buss: Class-level relationships in the phylum
Cnidaria—Molecular and morphological evidence. in: Molecular biology and evolution. Oxford
University Press, Oxford 12.1995, p. 679. ISSN 0737-4038
D. G. Fautin: Reproduction of Cnidaria. in: Canadian Journal of Zoology. Ottawa Ont. 80.2002,
p. 1735. (PDF, online) ISSN 0008-4301
G. O. Mackie: What's new in cnidarian biology? in: Canadian Journal of Zoology. Ottawa Ont.
80.2002, p. 1649. (PDF, online) ISSN 0008-4301
P. Schuchert: Phylogenetic analysis of the Cnidaria. in: Zeitschrift für zoologische Systematik und
Evolutionsforschung. Paray, Hamburg-Berlin 31.1993, p. 161. ISSN 0044-3808
G. Kass-Simon, A. A. Scappaticci Jr.: The behavioral and developmental physiology of
nematocysts. in: Canadian Journal of Zoology. Ottawa Ont. 80.2002, p. 1772. (PDF, online)
ISSN 0044-3808
J. Zrzavý (2001). "The interrelationships of metazoan parasites: a review of phylum- and higherlevel hypotheses from recent morphological and molecular phylogenetic analyses" (PDF). Folia
Parasitologica 48 (2): 81–103. PMID 11437135. Archived from the original on 2007-10-25.
http://web.archive.org/web/20071025220832/http://www.paru.cas.cz/folia/pdf/2-01/Zrz.pdf.
Retrieved 2009-01-26.
[edit] External links
Wikispecies has information related to: Cnidaria
The Wikibook Dichotomous Key has a page on the topic of
Cnidaria
Wikimedia Commons has media related to: Cnidaria
Look up Cnidaria in Wiktionary, the free dictionary.
YouTube: Nematocysts Firing
YouTube:My Anemone Eat Meat Defensive and feeding behaviour of sea anemone
Cnidaria - Guide to the Marine Zooplankton of south eastern Australia, Tasmanian Aquaculture
& Fisheries Institute
A Cnidaria homepage maintained by University of California, Irvine
Cnidaria page at Tree of Life
Fossil Gallery: Cnidarians
The Hydrozoa Directory
Hexacorallians of the World
[hide]
v
t
e
Eukaryota
Domain : Archaea · Bacteria · Eukaryota
Bikonta
Archaeplastida, or Plantae sensu lato
Viridiplantae/Plantae sensu stricto ·
Rhodophyta · Glaucocystophyceae
Hacrobia, or non-SAR chromalveolata
Haptophyta · Cryptophyta ·
Centroheliozoa
AH/SAR AH
Heterokont ("S") Ochrophyta · Bigyra · Pseudofungi
Halvaria
SAR
Alveolata Ciliates · Myzozoa (Apicomplexa, Dinoflagellata)
Rhizaria Cercozoa · Retaria (Foraminifera, Radiolaria)
Excavata Discoba (Euglenozoa, Percolozoa) · Metamonad · Malawimonas
Apusozoa
Apusomonadida (Apusomonas, Amastigomonas) · Ancyromonadida
(Ancyromonas) · Hemimastigida (Hemimastix, Spironema, Stereonema)
Amoebozoa Lobosea · Conosa · Phalansterium · Breviata
Mesomycetozoea Dermocystida · Ichthyophonida
Filasterea Capsaspora · Ministeria
Choanoflagellate Codonosigidae
Holozoa
Filozoa
Unikonta
Opisthokonta
Holomycota
Eumetazoa (Bilateria,
Metazoa Cnidaria, Ctenophora) ·
or "Animalia" Mesozoa · Parazoa
(Placozoa, Porifera)
Dikarya (Ascomycota, Basidiomycota) ·
Glomeromycota · Zygomycota ·
Fungi Blastocladiomycota ·
Chytridiomycota/Neocallimastigomycota ·
Microsporidia
Nucleariidae
Nuclearia · Micronuclearia · Rabdiophrys ·
Pinaciophora · Pompholyxophrys · Fonticula
[show]
v
t
e
Extant phyla of kingdom Animalia by subkingdom
o
Radiata
Ctenophora
Cnidaria
o Anthozoa
o Hydrozoa
o Scyphozoa
o Cubozoa
o Staurozoa
o Myxozoa
o Polypodiozoa
Scalidophor
a
Kinorhyncha
Loricifera
Priapulida
Nematoida
Nematoda
Nematomorpha
Cycloneuralia
Ecdysozoa
Panarthropod
a
Onychophora
Tardigrada
Arthropoda
Lobopodia
Bilateria Protostomia
Platyhelminthes
Gastrotricha
Platyzoa
Lophotrochoz
oa
Gnathife
ra
Spiralia
Trochoz
oa
Lophophora
Rotifera
Acanthocephala
Gnathostomulid
a
Micrognathozoa
Cycliophora
Sipuncula
Nemertea
Mollusca
Annelida
Phoronida
Brachiopoda
ta
Ambulacraria
Deuterosto
mia
Basal/disput
ed
Bryozoa (?)
Entoprocta (?)
Hemichordata
Echinodermata
Xenoturbellida
Chordata
o Craniata
Vertebrata
Myxini
o Cephalochordata
o Tunicata
Acoelomorpha
o Acoela
o Nemertodermatida
Chaetognatha
Retrieved from "http://en.wikipedia.org/w/index.php?title=Cnidaria&oldid=479630448"
View page ratings
Rate this page
What's this?
Trustworthy
Objective
Complete
Well-written
I am highly knowledgeable about this topic (optional)
Submit ratings
Saved successfully
Your ratings have not been submitted yet
Categories:
Venomous animals
Cnidarians
Hidden categories:
Good articles
Articles with 'species' microformats
Personal tools
Log in / create account
Namespaces
Article
Talk
Variants
Views
Read
Edit
View history
Actions
Search
Search
Special:Search
Navigation
Main page
Contents
Featured content
Current events
Random article
Donate to Wikipedia
Interaction
Help
About Wikipedia
Community portal
Recent changes
Contact Wikipedia
Toolbox
What links here
Related changes
Upload file
Special pages
Permanent link
Cite this page
Rate this page
Print/export
Create a book
Download as PDF
Printable version
Languages
ال عرب ية
Armãneashce
Azərbaycanca
Български
Bosanski
Brezhoneg
Català
Česky
Cymraeg
Dansk
Deutsch
Eesti
Español
Esperanto
Euskara
ف ار سی
Français
Gaelg
Galego
한국어
हिन्दी
Hrvatski
Ido
Bahasa Indonesia
Íslenska
Italiano
עברית
Basa Jawa
Latina
Latviešu
Lëtzebuergesch
Lietuvių
Magyar
Македонски
Nederlands
日本語
Norsk (bokmål)
Norsk (nynorsk)
Occitan
Plattdüütsch
Polski
Português
Runa Simi
Русский
Sicilianu
Simple English
Slovenčina
Slovenščina
Српски / Srpski
Srpskohrvatski / Српскохрватски
Suomi
Svenska
Tagalog
తెలుగు
ไทย
Türkçe
Українська
Zazaki
Zeêuws
中文
This page was last modified on 1 March 2012 at 10:48.
Text is available under the Creative Commons Attribution-ShareAlike License; additional terms
may apply. See Terms of use for details.
Wikipedia® is a registered trademark of the Wikimedia Foundation, Inc., a non-profit
organization.
Cnidaria
From Wikipedia, the free encyclopedia
(Redirected from Cnideria)
Jump to: navigation, search
Cnidaria
Pacific sea nettles, Chrysaora fuscescens
Scientific classification
Domain:
Eukaryota
Kingdom:
Animalia
Phylum:
Cnidaria
Hatschek, 1888
Subphylum/Classes[3]
Anthozoa—corals and sea anemones
Medusozoa—jellyfish:[1]
Cubozoa—box jellyfish, sea wasps
Hydrozoa—hydroids, hydra-like animals
Scyphozoa—true jellyfish
Staurozoa—stalked jellyfish
Unranked, may not be scyphozoans[2]
Myxozoa—parasites
Polypodiozoa—parasites
Cnidaria ( /naɪˈdɛəriə/ with a silent c) is a phylum containing over 10,000[4] species of
animals found exclusively in aquatic and mostly marine environments. Their distinguishing
feature is cnidocytes, specialized cells that they use mainly for capturing prey. Their bodies
consist of mesoglea, a non-living jelly-like substance, sandwiched between two layers of
epithelium that are mostly one cell thick. They have two basic body forms: swimming medusae
and sessile polyps, both of which are radially symmetrical with mouths surrounded by tentacles
that bear cnidocytes. Both forms have a single orifice and body cavity that are used for digestion
and respiration. Many cnidarian species produce colonies that are single organisms composed of
medusa-like or polyp-like zooids, or both. Cnidarians' activities are coordinated by a
decentralized nerve net and simple receptors. Several free-swimming Cubozoa and Scyphozoa
possess balance-sensing statocysts, and some have simple eyes. Not all cnidarians reproduce
sexually. Many have complex lifecycles with asexual polyp stages and sexual medusae, but some
omit either the polyp or the medusa stage.
Cnidarians were for a long time grouped with Ctenophores in the phylum Coelenterata, but
increasing awareness of their differences caused them to be placed in separate phyla. Cnidarians
are classified into four main groups: the almost wholly sessile Anthozoa (sea anemones, corals,
sea pens); swimming Scyphozoa (jellyfish); Cubozoa (box jellies); and Hydrozoa, a diverse
group that includes all the freshwater cnidarians as well as many marine forms, and has both
sessile members such as Hydra and colonial swimmers such as the Portuguese Man o' War.
Staurozoa have recently been recognised as a class in their own right rather than a sub-group of
Scyphozoa, and there is debate about whether Myxozoa and Polypodiozoa are cnidarians or
closer to bilaterians (more complex animals).
Most cnidarians prey on organisms ranging in size from plankton to animals several times larger
than themselves, but many obtain much of their nutrition from endosymbiotic algae, and a few
are parasites. Many are preyed upon by other animals including starfish, sea slugs, fish and
turtles. Coral reefs, whose polyps are rich in endosymbiotic algae, support some of the world's
most productive ecosystems, and protect vegetation in tidal zones and on shorelines from strong
currents and tides. While corals are almost entirely restricted to warm, shallow marine waters,
other cnidarians live in the depths, in polar seas and in freshwater.
Fossil cnidarians have been found in rocks formed about 580 million years ago, and other fossils
show that corals may have been present shortly before 490 million years ago and diversified a
few million years later. Fossils of cnidarians that do not build mineralized structures are very
rare. Scientists currently think that cnidarians, ctenophores and bilaterians are more closely
related to calcareous sponges than these are to other sponges, and that anthozoans are the
evolutionary "aunts" or "sisters" of other cnidarians, and the most closely related to bilaterians.
Recent analyses have concluded that cnidarians, although considered more "primitive" than
bilaterians, have a wider range of genes.
Jellyfish stings killed several hundred people in the 20th century, and cubozoans are particularly
dangerous. On the other hand, some large jellyfish are considered a delicacy in eastern and
southern Asia. Coral reefs have long been economically important as providers of fishing
grounds, protectors of shore buildings against currents and tides, and more recently as centers of
tourism. However, they are vulnerable to over-fishing, mining for construction materials,
pollution, and damage caused by tourism.
Contents
[hide]
1 Distinguishing features
2 Description
o 2.1 Basic body forms
o 2.2 Colonial forms
o 2.3 Skeletons
o 2.4 Main cell layers
o 2.5 Cnidocytes
o 2.6 Locomotion
o 2.7 Nervous system and senses
o 2.8 Feeding and excretion
o 2.9 Respiration
o 2.10 Regeneration
3 Reproduction
o 3.1 Sexual
o 3.2 Asexual
4 Classification
5 Ecology
6 Evolutionary history
o 6.1 Fossil record
o 6.2 Family tree
7 Interaction with humans
8 Notes
9 Further reading
o 9.1 Books
o 9.2 Journal articles
10 External links
[edit] Distinguishing features
Further information: Sponge, Ctenophore, and Bilateria
Cnidarians form an animal phylum that is more complex than sponges, about as complex as
ctenophores (comb jellies), and less complex than bilaterians, which include almost all other
animals. However, both cnidarians and ctenophores are more complex than sponges as they
have: cells bound by inter-cell connections and carpet-like basement membranes; muscles;
nervous systems; and some have sensory organs. Cnidarians are distinguished from all other
animals by having cnidocytes that fire like harpoons and are used mainly to capture prey but also
as anchors in some species.[5]
Like sponges and ctenophores, cnidarians have two main layers of cells that sandwich a middle
layer of jelly-like material, which is called the mesoglea in cnidarians; more complex animals
have three main cell layers and no intermediate jelly-like layer. Hence, cnidarians and
ctenophores have traditionally been labelled diploblastic, along with sponges.[5][6] However, both
cnidarians and ctenophores have a type of muscle that, in more complex animals, arises from the
middle cell layer.[7] As a result some recent text books classify ctenophores as triploblastic,[8] and
it has been suggested that cnidarians evolved from triploblastic ancestors.[7]
Cnidocytes
Sponges[9][10]
Cnidarians[5][6]
Ctenophores[5][8] Bilateria[5]
No
Yes
No
Colloblasts
No
Digestive and
circulatory organs
Number of main cell
layers
Yes
No
No
Yes
Two[5] or
Three[7][8]
Two, with jelly-like layer between them
Three
Cells in each layer
bound together
No, except that
Homoscleromorpha have
basement membranes.[11]
Yes: inter-cell connections; basement membranes
Sensory organs
No
Yes
Number of cells in
middle "jelly" layer
Many
Few
(Not
applicable)
Cells in outer layers
can move inwards
and change
functions
Yes
No
(Not
applicable)
Nervous system
No
Muscles
None
[edit] Description
[edit] Basic body forms
Aboral end
Oral end
Mouth
Oral end
Aboral end
Exoderm
Gastroderm (Endoderm)
Mesoglea
Digestive cavity
Yes, simple
Mostly
epitheliomuscular
Mostly
myoepithelial
Simple to
complex
Mostly
myocytes
Medusa (left) and polyp (right)[6]
Oral end of actinodiscus polyp, with close-up of the mouth
Adult cnidarians appear as either swimming medusae or sessile polyps. Both are radially
symmetrical, like a wheel and a tube respectively. Since these animals have no heads, their ends
are described as "oral" (nearest the mouth) and "aboral" (furthest from the mouth). Most have
fringes of tentacles equipped with cnidocytes around their edges, and medusae generally have an
inner ring of tentacles around the mouth. The mesoglea of polyps is usually thin and often soft,
but that of medusae is usually thick and springy, so that it returns to its original shape after
muscles around the edge have contracted to squeeze water out, enabling medusae to swim by a
sort of jet propulsion.[6]
[edit] Colonial forms
Tree-like polyp colony[6]
Cnidaria produce a variety of colonial forms, each of which is one organism but consists of
polyp-like zooids. The simplest is a connecting tunnel that runs over the substrate (rock or
seabed) and from which single zooids sprout. In some cases the tunnels form visible webs, and in
others they are enclosed in a fleshy mat. More complex forms are also based on connecting
tunnels but produce "tree-like" groups of zooids. The "trees" may be formed either by a central
zooid that functions as a "trunk" with later zooids growing to the sides as "branches", or in a zig-
zag shape as a succession of zooids, each of which grows to full size and then produces a single
bud at an angle to itself. In many cases the connecting tunnels and the "stems" are covered in
periderm, a protective layer of chitin.[6] Some colonial forms have other specialized types of
zooid, for example, to pump water through their tunnels.[12]
Siphonophores form complex colonies that consist of: an upside-down polyp that forms a central
stem with a gas-filled float at the top; one or more sets of medusa-like zooids that provide
propulsion; leaf-like bracts that give some protection to other parts; sets of tentacles that bear
nematocytes that capture prey; other tentacles that act as sensors; near the base of each set of
tentacles, a polyp-like zooid that acts as a stomach for the colony; medusa-like zooids that serve
as gonads. Although some of these zooids resemble polyps or medusae in shape, they lack
features that are not relevant to their specific functions, for example the swimming "medusae"
have no digestive, sensory or reproductive cells. The best-known siphonophore is the Portuguese
Man o' War (Physalia physalis).[12][13][14]
[edit] Skeletons
In medusae the only supporting structure is the mesoglea. Hydra and most sea anemones close
their mouths when they are not feeding, and the water in the digestive cavity then acts as a
hydrostatic skeleton, rather like a water-filled balloon. Other polyps such as Tubularia use
columns of water-filled cells for support. Sea pens stiffen the mesoglea with calcium carbonate
spicules and tough fibrous proteins, rather like sponges.[6]
In some colonial polyps a chitinous periderm gives support and some protection to the
connecting sections and to the lower parts of individual polyps. Stony corals secrete massive
calcium carbonate exoskeletons. A few polyps collect materials such as sand grains and shell
fragments, which they attach to their outsides. Some colonial sea anemones stiffen the mesoglea
with sediment particles.[6]
[edit] Main cell layers
Cnidaria are diploblastic animals, in other words they have two main cell layers, while more
complex animals are triploblasts having three main layers. The two main cell layers of cnidarians
form epithelia that are mostly one cell thick, and are attached to a fibrous basement membrane,
which they secrete. They also secrete the jelly-like mesoglea that separates the layers. The layer
that faces outwards, known as the ectoderm ("outside skin"), generally contains the following
types of cells:[5]
Epitheliomuscular cells whose bodies form part of the epithelium but whose bases extend to
form muscle fibers in parallel rows.[15] The fibers of the outward-facing cell layer generally run at
right angles to the fibers of the inward-facing one. In Anthozoa (anemones, corals, etc.) and
Scyphozoa (jellyfish), the mesoglea also contains some muscle cells.[6]
Cnidocytes, the harpoon-like "nettle cells" that give the phylum Cnidaria its name. These appear
between or sometimes on top of the muscle cells.[5]
Nerve cells. Sensory cells appear between or sometimes on top of the muscle cells,[5] and
communicate via synapses (gaps across which chemical signals flow) with motor nerve cells,
which lie mostly between the bases of the muscle cells.[6]
Interstitial cells, which are unspecialized and can replace lost or damaged cells by transforming
into the appropriate types. These are found between the bases of muscle cells.[5]
In addition to epitheliomuscular, nerve and interstitial cells, the inward-facing gastroderm
("stomach skin") contains gland cells that secrete digestive enzymes. In some species it also
contains low concentrations of cnidocytes, which are used to subdue prey that is still
struggling.[5][6]
The mesoglea contains small numbers of amoeba-like cells,[6] and muscle cells in some
species.[5] However the number of middle-layer cells and types are much lower than in
sponges.[6]
[edit] Cnidocytes
A hydra's nematocyst, before firing.
"trigger" cilium[6]
Firing sequence of the cnida in a hydra's nematocyst[6]
Operculum (lid)
"Finger" that turns inside out
/ / / Barbs
Venom
Victim's skin
Victim's tissues
These "nettle cells" function as harpoons, since their payloads remain connected to the bodies of
the cells by threads. Three types of cnidocytes are known:[5][6]
Nematocysts inject venom into prey, and usually have barbs to keep them embedded in the
victims. Most species have nematocysts.[5]
Spirocysts do not penetrate the victim or inject venom, but entangle it by means of small sticky
hairs on the thread.
Ptychocysts are not used for prey capture — instead the threads of discharged ptychocysts are
used for building protective tubes in which their owners live. Ptychocysts are found only in the
order Cerianthria, tube anemones.[6]
The main components of a cnidocyte are:[5][6]
A cilium (fine hair) which projects above the surface and acts as a trigger. Spirocysts do not have
cilia.
A tough capsule, the cnida, which houses the thread, its payload and a mixture of chemicals
which may include venom or adhesives or both. ("cnida" is derived from the Greek word κνίδη,
which means "nettle"[16])
A tube-like extension of the wall of the cnida that points into the cnida, like the finger of a
rubber glove pushed inwards. When a cnidocyte fires, the finger pops out. If the cell is a
venomous nematocyte, the "finger"'s tip reveals a set of barbs that anchor it in the prey.
The thread, which is an extension of the "finger" and coils round it until the cnidocyte fires. The
thread is usually hollow and delivers chemicals from the cnida to the target.
An operculum (lid) over the end of the cnida. The lid may be a single hinged flap or three flaps
arranged like slices of pie.
The cell body which produces all the other parts.
It is difficult to study the firing mechanisms of cnidocytes as these structures are small but very
complex. At least four hypotheses have been proposed:[5]
Rapid contraction of fibers round the cnida may increase its internal pressure.
The thread may be like a coiled spring that extends rapidly when released.
In the case of Chironex (the "sea wasp"), chemical changes in the cnida's contents may cause
them to expand rapidly by polymerization.
Chemical changes in the liquid in the cnida make it a much more concentrated solution, so that
osmotic pressure forces water in very rapidly to dilute it. This mechanism has been observed in
nematocysts of the class Hydrozoa, sometimes producing pressures as high as 140 atmospheres,
similar to that of scuba air tanks, and fully extending the thread in as little as 2 milliseconds
(0.002 second).[6]
Cnidocytes can only fire once, and about 25% of a hydra's nematocysts are lost from its tentacles
when capturing a brine shrimp. Used cnidocytes have to be replaced, which takes about 48 hours.
To minimise wasteful firing, two types of stimulus are generally required to trigger cnidocytes:
their cilia detect contact, and nearby sensory cells "smell" chemicals in the water. This
combination prevents them from firing at distant or non-living objects. Groups of cnidocytes are
usually connected by nerves and, if one fires, the rest of the group requires a weaker minimum
stimulus than the cells that fire first.[5][6]
[edit] Locomotion
Chrysaora quinquecirrha ("sea nettle") swimming
Medusae swim by a form of jet propulsion: muscles, especially inside the rim of the bell, squeeze
water out of the cavity inside the bell, and the springiness of the mesoglea powers the recovery
stroke. Since the tissue layers are very thin, they provide too little power to swim against currents
and just enough to control movement within currents.[6]
Hydras and some sea anemones can move slowly over rocks and sea or stream beds by various
means: creeping like snails, crawling like inchworms, or by somersaulting. A few can swim
clumsily by waggling their bases.[6]
[edit] Nervous system and senses
Cnidaria have no brains or even central nervous systems. Instead they have decentralized nerve
nets consisting of : sensory neurons that generate signals in response to various types of stimulus,
such as odors; motor neurons that tell muscles to contract; all connected by "cobwebs" of
intermediate neurons. As well as forming the "signal cables", intermediate neurons also form
ganglia that act as local coordination centers. The cilia of the cnidocytes detect physical contact.
Nerves inform cnidocytes when odors from prey or attackers are detected and when
neighbouring cnidocytes fire. Most of the communications between nerve cells are via chemical
synapses, small gaps across which chemicals flow. As this process is too slow to ensure that the
muscles round the rim of a medusa's bell contract simultaneously in swimming the neurons
which control this communicate by much faster electrical signals across gap junctions.[6]
Medusae and complex swimming colonies such as siphonophores and chondrophores sense tilt
and acceleration by means of statocysts, chambers lined with hairs which detect the movements
of internal mineral grains called statoliths. If the body tilts in the wrong direction, the animal
rights itself by increasing the strength of the swimming movements on the side that is too low.
They also have ocelli ("little eyes"), which can detect the direction from which light is coming.
Box jellies have camera eyes, although these probably do not form images, and their lenses
simply produce a clearer indication of the direction from which light is coming.[5]
[edit] Feeding and excretion
Cnidarians feed in several ways: predation, absorbing dissolved organic chemicals, filtering food
particles out of the water, and obtaining nutrients from symbiotic algae within their cells. Most
obtain the majority of their food from predation but some, including the corals Hetroxenia and
Leptogorgia, depend almost completely on their endosymbionts and on absorbing dissolved
nutrients.[5] Cnidaria give their symbiotic algae carbon dioxide, some nutrients and a place in the
sun.[6]
Predatory species use their cnidocytes to poison or entangle prey, and those with venomous
nematocysts may start digestion by injecting digestive enzymes. The "smell" of fluids from
wounded prey makes the tentacles fold inwards and wipe the prey off into the mouth. In medusae
the tentacles round the edge of the bell are often short and most of the prey capture is done by
"oral arms", which are extensions of the edge of the mouth and are often frilled and sometimes
branched to increase their surface area. Medusae often trap prey or suspended food particles by
swimming upwards, spreading their tentacles and oral arms and then sinking. In species for
which suspended food particles are important, the tentacles and oral arms often have rows of
cilia whose beating creates currents that flow towards the mouth, and some produce nets of
mucus to trap particles.[5]
Once the food is in the digestive cavity, gland cells in the gastroderm release enzymes that
reduce the prey to slurry, usually within a few hours. This circulates through the digestive cavity
and, in colonial cnidarians, through the connecting tunnels, so that gastroderm cells can absorb
the nutrients. Absorption may take a few hours, and digestion within the cells may take a few
days. The circulation of nutrients is driven by water currents produced by cilia in the gastroderm
or by muscular movements or both, so that nutrients reach all parts of the digestive cavity.[6]
Nutrients reach the outer cell layer by diffusion or, for animals or zooids such as medusae which
have thick mesogleas, are transported by mobile cells in the mesoglea.[5]
Indigestible remains of prey are expelled through the mouth. The main waste product of cells'
internal processes is ammonia, which is removed by the external and internal water currents.[6]
[edit] Respiration
There are no respiratory organs, and both cell layers absorb oxygen from and expel carbon
dioxide into the surrounding water. When the water in the digestive cavity becomes stale it must
be replaced, and nutrients that have not been absorbed will be expelled with it. Some Anthozoa
have ciliated grooves on their tentacles, allowing them to pump water out of and into the
digestive cavity without opening the mouth. This improves respiration after feeding and allows
these animals, which use the cavity as a hydrostatic skeleton, to control the water pressure in the
cavity without expelling undigested food.[5]
Cnidaria that carry photosynthetic symbionts may have the opposite problem, an excess of
oxygen, which may prove toxic. The animals produce large quantities of antioxidants to
neutralize the excess oxygen.[5]
[edit] Regeneration
All cnidarians can regenerate, allowing them to recover from injury and to reproduce asexually.
Medusae have limited ability to regenerate, but polyps can do so from small pieces or even
collections of separated cells. This enables corals to recover even after apparently being
destroyed by predators.[5]
[edit] Reproduction
1
2
3
4
5
6
7
8
9
10
11
12
13
14
Life cycle of a jellyfish:[5][6]
1–3 Larva searches for site
4–8 Polyp grows
9–11 Polyp strobilates
12–14 Medusa grows
[edit] Sexual
In the Cnidaria sexual reproduction often involves a complex life cycle with both polyp and
medusa stages. For example in Scyphozoa (jellyfish) and Cubozoa (box jellies) a larva swims
until it finds a good site, and then becomes a polyp. This grows normally but then absorbs its
tentacles and splits horizontally into a series of disks that become juvenile medusae, a process
called strobilation. The juveniles swim off and slowly grow to maturity, while the polyp regrows and may continue strobilating periodically. The adults have gonads in the gastroderm, and
these release ova and sperm into the water in the breeding season.[5][6]
Shortened forms of this life cycle are common, for example some oceanic scyphozoans omit the
polyp stage completely, and cubozoan polyps produce only one medusa. Hydrozoa have a
variety of life cycles. Some have no polyp stages and some (e.g. hydra) have no medusae. In
some species the medusae remain attached to the polyp and are responsible for sexual
reproduction; in extreme cases these reproductive zooids may not look much like medusae.
Anthozoa have no medusa stage at all and the polyps are responsible for sexual reproduction.[5]
Spawning is generally driven by environmental factors such as changes in the water temperature,
and their release is triggered by lighting conditions such as sunrise, sunset or the phase of the
moon. Many species of Cnidaria may spawn simultaneously in the same location, so that there
are too many ova and sperm for predators to eat more than a tiny percentage — one famous
example is the Great Barrier Reef, where at least 110 corals and a few non-cnidarian
invertebrates produce enough to turn the water cloudy. These mass spawnings may produce
hybrids, some of which can settle and form polyps, but it is not known how long these can
survive. In some species the ova release chemicals that attract sperm of the same species.[5]
The fertilized eggs develop into larvae by dividing until there are enough cells to form a hollow
sphere (blastula) and then a depression forms at one end (gastrulation) and eventually become
the digestive cavity. However in cnidarians the depression forms at the end further from the yolk
(at the animal pole), while in bilaterians it forms at the other end (vegetal pole).[6] The larvae,
called planulae, swim or crawl by means of cilia.[5] They are cigar-shaped but slightly broader at
the "front" end, which is the aboral, vegetal-pole end and eventually attaches to a substrate if the
species has a polyp stage.[6]
Anthozoan larvae either have large yolks or are capable of feeding on plankton, and some
already have endosymbiotic algae that help to feed them. Since the parents are immobile, these
feeding capabilities extend the larvae's range and avoid overcrowding of sites. Scyphozoan and
hydrozoan larvae have little yolk and most lack endosymbiotic algae, and therefore have to settle
quickly and metamorphose into polyps. Instead these species rely on their medusae to extend
their ranges.[6]
[edit] Asexual
All known cnidaria can reproduce asexually by various means, in addition to regenerating after
being fragmented. Hydrozoan polyps only bud, while the medusae of some hydrozoans can
divide down the middle. Scyphozoan polyps can both bud and split down the middle. In addition
to both of these methods, Anthozoa can split horizontally just above the base.[5][6]
[edit] Classification
Cnidarians were for a long time grouped with Ctenophores in the phylum Coelenterata, but
increasing awareness of their differences caused them to be placed in separate phyla. Cnidarians
are classified into four main groups: sessile Anthozoa (sea anemones, corals, sea pens);
swimming Scyphozoa (jellyfish); Cubozoa (box jellies); and Hydrozoa, a diverse group that
includes all the freshwater cnidarians as well as many marine forms, and has both sessile
members such as Hydra and colonial swimmers such as the Portuguese Man o' War. Staurozoa
have recently been recognised as a class in their own right rather than a sub-group of Scyphozoa,
and there is debate about whether Myxozoa and Polypodiozoa are cnidarians or closer to
bilaterians.
Modern cnidarians are generally classified into four classes:[5]
Hydrozoa
Number of species[4]
Scyphozoa
Cubozoa
Anthozoa
3,600
228
42
6,100
Hydra,
siphonophores
Jellyfish
Box
jellies
Sea anemones,
corals, sea pens
Yes
Yes
Yes
Yes
Yes
Yes
Medusa phase in life
In some species
cycle
Yes, except for Stauromedusae
Yes
if they are scyphozoans
No
Number of medusae
Many
produced per polyp
Many
(not applicable)
Examples
Cells found in mesoglea No
Nematocysts in
exodermis
No
One
Stauromedusae, small sessile cnidarians with stalks and no medusa stage, have traditionally been
classified as members of the Scyphozoa, but recent research suggests they should be regarded as
a separate class, Staurozoa.[17]
The Myxozoa, microscopic parasites, were first classified as protozoans,[18] but recently as
heavily modified cnidarians, and more closely related to Hydrozoa and Scyphozoa than to
Anthozoa.[19] However other recent research suggests that Polypodium hydriforme, a parasite
within the egg cells of sturgeon, is closely related to the Myxozoa and that both Polypodium and
the Myxozoa are intermediate between cnidarians and bilaterian animals.[20]
Some researchers classify the extinct conulariids as cnidarians, while others propose that they
form a completely separate phylum.[21]
[edit] Ecology
Coral reefs support rich ecosystems
Many cnidarians are limited to shallow waters because they depend on endosymbiotic algae for
much of their nutrients. The life cycles of most have polyp stages, which are limited to locations
that offer stable substrates. Nevertheless major cnidarian groups contain species that have
escaped these limitations. Hydrozoans have a worldwide range: some, such as Hydra, live in
freshwater; Obelia appears in the coastal waters of all the oceans; and Liriope can form large
shoals near the surface in mid-ocean. Among anthozoans, a few scleractinian corals, sea pens
and sea fans live in deep, cold waters, and some sea anemones inhabit polar seabeds while others
live near hydrothermal vents over 10 kilometres (6.2 mi) below sea-level. Reef-building corals
are limited to tropical seas between 30°N and 30°S with a maximum depth of 46 metres (151 ft),
temperatures between 20°C and 28°C, high salinity and low carbon dioxide levels.
Stauromedusae, although usually classified as jellyfish, are stalked, sessile animals that live in
cool to Arctic waters.[12] Cnidarians range in size from Hydra, 5–20 millimetres (0.20–0.79 in)
long,[22] to the Lion's mane jellyfish, which may exceed 2 metres (6.6 ft) in diameter and 75
metres (246 ft) in length.[23]
Prey of cnidarians ranges from plankton to animals several times larger than themselves.[12][24]
Some cnidarians are parasites, mainly on jellyfish but a few are major pests of fish.[12] Others
obtain most of their nourishment from endosymbiotic algae or dissolved nutrients.[5] Predators of
cnidarians include: sea slugs, which can incorporate nematocysts into their own bodies for selfdefense;[25] starfish, notably the crown of thorns starfish, which can devastate corals;[12] butterfly
fish and parrot fish, which eat corals;[26] and marine turtles, which eat jellyfish.[23] Some sea
anemones and jellyfish have a symbiotic relationship with some fish; for example clown fish live
among the tentacles of sea anemones, and each partner protects the other against predators.[12]
Coral reefs form some of the world's most productive ecosystems. Common coral reef cnidarians
include both Anthozoans (hard corals, octocorals, anemones) and Hydrozoans (fire corals, lace
corals) The endosymbiotic algae of many cnidarian species are very effective primary producers,
in other words converters of inorganic chemicals into organic ones that other organisms can use,
and their coral hosts use these organic chemicals very efficiently. In addition reefs provide
complex and varied habitats that support a wide range of other organisms.[27] Fringing reefs just
below low-tide level also have a mutually beneficial relationship with mangrove forests at high-
tide level and sea grass meadows in between: the reefs protect the mangroves and seagrass from
strong currents and waves that would damage them or erode the sediments in which they are
rooted, while the mangroves and seagrass protect the coral from large influxes of silt, fresh water
and pollutants. This additional level of variety in the environment is beneficial to many types of
coral reef animals, which for example may feed in the sea grass and use the reefs for protection
or breeding.[28]
[edit] Evolutionary history
[edit] Fossil record
The fossil coral Cladocora from Pliocene rocks in Cyprus
The earliest widely accepted animal fossils are rather modern-looking cnidarians, possibly from
around 580 million years ago, although fossils from the Doushantuo Formation can only be dated
approximately.[29] The identification of some of these as embryos of animals has been contested,
but other fossils from these rocks strongly resemble tubes and other mineralized structures made
by corals.[30] Their presence implies that the cnidarian and bilaterian lineages had already
diverged.[31] Although the Ediacaran fossil Charnia used to be classified as a jellyfish or sea
pen,[32] more recent study of growth patterns in Charnia and modern cnidarians has cast doubt on
this hypothesis,[33][34] and there are now no bona-fide cnidarian body fossils in the Ediacaran.
Few fossils of cnidarians without mineralized skeletons are known from more recent rocks,
except in lagerstätten that preserved soft-bodied animals.[35]
A few mineralized fossils that resemble corals have been found in rocks from the Cambrian
period, and corals diversified in the Early Ordovician.[35] These corals, which were wiped out in
the Permian-Triassic extinction about 251 million years ago,[35] did not dominate reef
construction since sponges and algae also played a major part.[36] During the Mesozoic era rudist
bivalves were the main reef-builders, but they were wiped out in the Cretaceous-Tertiary
extinction 65 million years ago,[37] and since then the main reef-builders have been scleractinian
corals.[35]
[edit] Family tree
Further information: Phylogeny
Metazoa
Glass sponges
Demosponges
Calcareous sponges
Eumetazoa
Ctenophora (comb jellies)
Planulozoa Cnidaria
Anthozoa
(sea anemones and corals)
Medusozoa
Hydrozoa
(Hydra, siphonophores, etc.)
Cubozoa
(box jellies)
Staurozoa
"Scyphozoa"
(jellyfish, excluding
Staurozoa)
Placozoa
Bilateria
Myxozoa
Other Bilateria
(more complex)
Family tree of Cnidaria and the origins of animals[2][38][39][40]
It is difficult to reconstruct the early stages in the evolutionary "family tree" of animals using
only morphology (their shapes and structures), because the large differences between Porifera
(sponges), Cnidaria plus Ctenophora (comb jellies), Placozoa and Bilateria (all the more complex
animals) make comparisons difficult. Hence reconstructions now rely largely or entirely on
molecular phylogenetics, which groups organisms according to similarities and differences in
their biochemistry, usually in their DNA or RNA.[41]
It is now generally thought that the Calcarea (sponges with calcium carbonate spicules) are more
closely related to Cnidaria, Ctenophora (comb jellies) and Bilateria (all the more complex
animals) than they are to the other groups of sponges.[38][42][43] In 1866 it was proposed that
Cnidaria and Ctenophora were more closely related to each other than to Bilateria and formed a
group called Coelenterata ("hollow guts"), because Cnidaria and Ctenophora both rely on the
flow of water in and out of a single cavity for feeding, excretion and respiration. In 1881 it was
proposed that Ctenophora and Bilateria were more closely related to each other, since they
shared features that Cnidaria lack, for example muscles in the middle layer (mesoglea in
Ctenophora, mesoderm in Bilateria). However more recent analyses indicate that these
similarities are rather vague, and the current view, based on molecular phylogenetics, is that
Cnidaria and Bilateria are more closely related to each other than either is to Ctenophora. This
grouping of Cnidaria and Bilateria has been labelled "Planulozoa" because it suggests that the
earliest Bilateria were similar to the planula larvae of Cnidaria.[2][39]
Within the Cnidaria, the Anthozoa (sea anemones and corals) are regarded as the sister-group of
the rest, which suggests that the earliest cnidarians were sessile polyps with no medusa stage.
However it is unclear how the other groups acquired the medusa stage, since Hydrozoa form
medusae by budding from the side of the polyp while the other Medusozoa do so by splitting
them off from the tip of the polyp. The traditional grouping of Scyphozoa included the
Staurozoa, but morphology and molecular phylogenetics indicate that Staurozoa are more closely
related to Cubozoa (box jellies) than to other "Scyphozoa". Similarities in the double body walls
of Staurozoa and the extinct Conulariida suggest that they are closely related. The position of
Anthozoa nearest the beginning of the cnidarian family tree also implies that Anthozoa are the
cnidarians most closely related to Bilateria, and this is supported by the fact that Anthozoa and
Bilateria share some genes that determine the main axes of the body.[2][44]
However in 2005 Katja Seipel and Volker Schmid suggested that cnidarians and ctenophores are
simplified descendants of triploblastic animals, since ctenophores and the medusa stage of some
cnidarians have striated muscle, which in bilaterians arises from the mesoderm. They did not
commit themselves on whether bilaterians evolved from early cnidarians or from the
hypothesized triploblastic ancestors of cnidarians.[7]
In molecular phylogenetics analyses from 2005 onwards, important groups of developmental
genes show the same variety in cnidarians as in chordates.[45] In fact cnidarians, and especially
anthozoans (sea anemones and corals), retain some genes that are present in bacteria, protists,
plants and fungi but not in bilaterians.[46]
The mitochondial genomes in the medusozoan cnidarians unlike that of other animals is linear
with fragmented genes.[47] The reason for this difference is unknown.
[edit] Interaction with humans
Jellyfish stings killed about 1,500 people in the 20th century,[48] and cubozoans are particularly
dangerous. On the other hand, some large jellyfish are considered a delicacy in eastern and
southern Asia. Coral reefs have long been economically important as providers of fishing
grounds, protectors of shore buildings against currents and tides, and more recently as centers of
tourism. However, they are vulnerable to over-fishing, mining for construction materials,
pollution, and damage caused by tourism.
Beaches protected from tides and storms by coral reefs are often the best places for housing in
tropical countries. Reefs are an important food source for low-technology fishing, both on the
reefs themselves and in the adjacent seas.[49] However despite their great productivity reefs are
vulnerable to over-fishing, because much of the organic carbon they produce is exhaled as
carbon dioxide by organisms at the middle levels of the food chain and never reaches the larger
species that are of interest to fishermen.[27] Tourism centered on reefs provides much of the
income of some tropical islands, attracting photographers, divers and sports fishermen. However
human activities damage reefs in several ways: mining for construction materials; pollution,
including large influxes of fresh water from storm drains; commercial fishing, including the use
of dynamite to stun fish and the capture of young fish for aquariums; and tourist damage caused
by boat anchors and the cumulative effect of walking on the reefs.[49] Coral, mainly from the
Pacific Ocean has long been used in jewellery, and demand rose sharply in the 1980s.[50]
The dangerous "sea wasp" Chironex fleckeri
Some large jellyfish species have been used in Chinese cuisine at least since 200 AD, and are
now fished in the seas around most of South East Asia. Japan is the largest single consumer of
edible jellyfish, importing at first only from China but now from all of South East Asia as prices
rose in the 1970s. This fishing industry is restricted to daylight hours and calm conditions in two
short seasons, from March to May and August to November.[51] The commercial value of
jellyfish food products depends on the skill with which they are prepared, and "Jellyfish
Masters" guard their trade secrets carefully. Jellyfish is very low in cholesterol and sugars, but
cheap preparation can introduce undesirable amounts of heavy metals.[52]
The "sea wasp" Chironex fleckeri has been described as the world's most venomous animal and
is held responsible for 67 deaths, although it is difficult to identify the animal as it is almost
transparent. Most stingings by C. fleckeri cause only mild symptoms.[53] Seven other box jellies
can cause a set of symptoms called Irukandji syndrome,[54] which takes about 30 minutes to
develop,[55] and from a few hours to two weeks to disappear.[56] Hospital treatment is usually
required, and there have been a few deaths.[54]
[edit] Notes
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
^ Classes in Medusozoa based on "The Taxonomicon - Taxon: Subphylum Medusozoa". Universal
Taxonomic Services. http://www.taxonomy.nl/Taxonomicon/TaxonTree.aspx?id=11582. Retrieved 200901-26.
^ a b c d Collins, A.G. (2002). "Phylogeny of Medusozoa and the Evolution of Cnidarian Life Cycles" (PDF).
Journal of Evolutionary Biology 15 (3): 418–432. doi:10.1046/j.1420-9101.2002.00403.x.
http://cima.uprm.edu/~n_schizas/CMOB_8676/Collins2002.pdf. Retrieved 2008-11-27.
^ Subphyla Anthozoa and Medusozoa based on "The Taxonomicon - Taxon: Phylum Cnidaria". Universal
Taxonomic Services. http://www.taxonomy.nl/Taxonomicon/TaxonTree.aspx?id=11551. Retrieved 200707-10.
^ a b Zhang, Z.-Q. (2011). "Animal biodiversity: An introduction to higher-level classification and taxonomic
richness". Zootaxa 3148: 7–12. http://mapress.com/zootaxa/2011/f/zt03148p012.pdf.
^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af Hinde, R.T., (1998). "The Cnidaria and Ctenophora". In
Anderson, D.T.,. Invertebrate Zoology. Oxford University Press. pp. 28–57. ISBN 0195513681.
^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004).
Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 111–124. ISBN 0030259827.
^ a b c d Seipel, K., and Schmid, V. (June 2005). "Evolution of striated muscle: Jellyfish and the origin of
triploblasty". Developmental Biology 282 (1): 14–26. doi:10.1016/j.ydbio.2005.03.032. PMID 15936326.
^ a b c Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole.
pp. 182–195. ISBN 0030259827.
^ Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 76–97.
ISBN 0030259827.
^ Bergquist, P.R., (1998). "Porifera". In Anderson, D.T.,. Invertebrate Zoology. Oxford University Press.
pp. 10–27. ISBN 0195513681.
^ Exposito, J-Y., Cluzel, C., Garrone, R., and Lethias, C. (2002). "Evolution of collagens". The Anatomical
Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology 268 (3): 302–316.
doi:10.1002/ar.10162. PMID 12382326.
^ a b c d e f g Shostak, S. (2006). "Cnidaria (Coelenterates)". Encyclopedia of Life Sciences. John Wiley & Sons.
doi:10.1038/npg.els.0004117.
^ Bhamrah, H.S., and Juneja, K. (2002). A Textbook of Invertebrates. Anmol Publications. pp. 278–280.
ISBN 8126104163.
http://books.google.com/?id=05So4shx9tIC&pg=PA279&dq=siphonophore+siphonophora. Retrieved
2008-11-17.
^ Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 167–
170. ISBN 0030259827.
^ Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). "Introduction to Metazoa". Invertebrate Zoology (7 ed.).
Brooks / Cole. pp. 103–104. ISBN 0030259827.
^ Trumble, W., and Brown, L. (2002). "Cnida". Shorter Oxford English Dictionary. Oxford University Press.
^ Collins, A.G., Cartwright, P., McFadden, C.S., and Schierwater, B. (2005). "Phylogenetic Context and
Basal Metazoan Model Systems". Integrative and Comparative Biology 45 (4): 585–594.
doi:10.1093/icb/45.4.585.
^ Štolc, A. (1899). "Actinomyxidies, nouveau groupe de Mesozoaires parent des Myxosporidies". Bull. Int.
L'Acad. Sci. Bohème 12: 1–12.
^ E. Jímenez-Guri; Philippe, H; Okamura, B; Holland, PW (July 2007). "Buddenbrockia is a cnidarian worm".
Science 317 (116): 116–118. doi:10.1126/science.1142024. PMID 17615357.
20. ^ Zrzavý, J. and Hypša, V. (2003). "Myxozoa, Polypodium, and the origin of the Bilateria: The phylogenetic
position of "Endocnidozoa" in light of the rediscovery of Buddenbrockia". Cladistics 19 (2): 164–169.
doi:10.1111/j.1096-0031.2003.tb00305.x.
21. ^ "The Conulariida". University of California Museum of Paleontology.
http://www.ucmp.berkeley.edu/cnidaria/conulariida.html. Retrieved 2008-11-27.
22. ^ Blaise, C., and Férard, J-F. (2005). Small-scale Freshwater Toxicity Investigations: Toxicity Test Methods.
Springer. p. 398. ISBN 140203119X. http://books.google.com/?id=Ibew5SLx2oMC&dq=hydra+size+length.
Retrieved 2008-11-21.
23. ^ a b Safina, C. (2007). Voyage of the Turtle: In Pursuit of the Earth's Last Dinosaur. Macmillan. p. 154.
ISBN 0805083189. http://books.google.com/?id=dQD883dAv6YC&pg=PA154&dq=cnidaria+turtle.
Retrieved 2008-11-21.
24. ^ Cowen, R. (2000). History of Life (3 ed.). Blackwell. p. 54. ISBN 0632044446.
http://books.google.com/?id=qvyBS4gwPF4C&pg=PA54&dq=cnidaria+prey. Retrieved 2008-11-21.
25. ^ Frick, K (2003). "Predator Suites and Flabellinid Nudibranch Nematocyst Complements in the Gulf of
Maine.". In: SF Norton (ed). Diving for Science...2003. Proceedings of the American Academy of
Underwater Sciences (22nd Annual Scientific Diving Symposium). http://archive.rubiconfoundation.org/4744. Retrieved 2008-07-03.
26. ^ Choat, J.H. and Bellwood, D.R. (1998). Paxton, J.R. and Eschmeyer, W.N.. ed. Encyclopedia of Fishes. San
Diego: Academic Press. pp. 209–211. ISBN 0-12-547665-5.
27. ^ a b Barnes, R.S.K., and Mann, K.H. (1991). Fundamentals of Aquatic Ecology. Blackwell Publishing.
pp. 217–227. ISBN 0632029838.
http://books.google.com/?id=mOZZlzgdTrwC&pg=PA227&dq=%22Coral+Reef%22+productivity. Retrieved
2008-11-26.
28. ^ Hatcher, B.G. Johannes, R.E., and Robertson, A.J. (1989). "Conservation of Shallow-water Marine
Ecosystems". Oceanography and Marine Biology: An Annual Review: Volume 27. Routledge. p. 320.
ISBN 0080377181.
http://books.google.com/?id=XpmNqFaDZ7cC&pg=PA320&dq=%22Coral+Reef%22+mangrove+%22seagra
ss%22. Retrieved 2008-11-21.
29. ^ Chen, J-Y.; Oliveri, P; Li, CW; Zhou, GQ; Gao, F; Hagadorn, JW; Peterson, KJ; Davidson, EH (2000).
"Putative phosphatized embryos from the Doushantuo Formation of China". Proceedings of the National
Academy of Sciences 97 (9): 4457–4462. doi:10.1073/pnas.97.9.4457. PMC 18256. PMID 10781044.
http://www.pnas.org/content/97/9/4457.full. Retrieved 2009-04-30.
30. ^ Xiao, S., Yuan, X., and Knoll, A.H. (2000). "Eumetazoan fossils in terminal Proterozoic phosphorites?".
Proceedings of the National Academy of Sciences 97 (25): 13684–13689. doi:10.1073/pnas.250491697.
PMC 17636. PMID 11095754.
http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=17636.
31. ^ Chen, J.-Y., Oliveri, P., Gao, F., Dornbos, S.Q., Li, C-W., Bottjer, D.J. and Davidson, E.H. (August 2002).
"Precambrian Animal Life: Probable Developmental and Adult Cnidarian Forms from Southwest China"
(PDF). Developmental Biology 248 (1): 182–196. doi:10.1006/dbio.2002.0714. PMID 12142030.
http://www.uwm.edu/~sdornbos/PDF's/Chen%20et%20al.%202002.pdf. Retrieved 2008-09-03.
32. ^ Donovan, Stephen K., Lewis, David N. (2001). "Fossils explained 35. The Ediacaran biota" (abstract).
Geology Today 17 (3): 115–120. doi:10.1046/j.0266-6979.2001.00285.x.
33. ^ Antcliffe, J.B.; Brasier, M. D. (2007). "Charnia and sea pens are poles apart". Journal of the Geological
Society 164 (1): 49–51. doi:10.1144/0016-76492006-080.
34. ^ Antcliffe, J.B.; Brasier, Martin D. (2007). "Charnia At 50: Developmental Models For Ediacaran Fronds".
Palaeontology 51 (1): 11–26. doi:10.1111/j.1475-4983.2007.00738.x.
35. ^ a b c d "Cnidaria: Fossil Record". University of California Museum of Paleontology.
http://www.ucmp.berkeley.edu/cnidaria/cnidariafr.html. Retrieved 2008-11-27.
36. ^ Copper, P. (January 1994). "Ancient reef ecosystem expansion and collapse". Coral Reefs 13 (1): 3–11.
Bibcode 1994CorRe..13....3C. doi:10.1007/BF00426428.
37. ^ "The Rudists". University of California Museum of Paleontology.
http://www.ucmp.berkeley.edu/taxa/inverts/mollusca/rudists.php. Retrieved 2008-11-27.
38. ^ a b Borchiellini, C., Manuel, M., Alivon, E., Boury-Esnault, N., Vacelet J., and Le Parco, Y. (2001). "Sponge
paraphyly and the origin of Metazoa". Journal of Evolutionary Biology 14 (1): 171–179.
doi:10.1046/j.1420-9101.2001.00244.x.
39. ^ a b Wallberg, A., Thollesson, M., , Farris, J.S., and Jondelius, U. (2004). "The phylogenetic position of the
comb jellies (Ctenophora) and the importance of taxonomic sampling". Cladistics 20 (6): 558–578.
doi:10.1111/j.1096-0031.2004.00041.x.
40. ^ Philippe, H. (April 2009). "Phylogenomics Revives Traditional Views on Deep Animal Relationships".
Current Biology 19: 706–712. doi:10.1016/j.cub.2009.02.052. PMID 19345102.
http://www.cell.com/current-biology/retrieve/pii/S0960982209008057. Retrieved 2011-09-25.
41. ^ Halanych, K.M. (December 2004). "The New View of Animal Phylogeny" (PDF). Annual Review of
Ecology, Evolution, and Systematics 35: 229–256. doi:10.1146/annurev.ecolsys.35.112202.130124.
http://gump.auburn.edu/halanych/lab/Pub.pdfs/Halanych2004.pdf. Retrieved 2008-11-27.
42. ^ Medina, M., Collins, A.G., Silberman, J.D., and Sogin, M.L. (August 2001). "Evaluating hypotheses of
basal animal phylogeny using complete sequences of large and small subunit rRNA". Proceedings of the
National Academy of Sciences 98 (17): 9707–9712. doi:10.1073/pnas.171316998. PMC 55517.
PMID 11504944. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=55517.
43. ^ Müller, W.E.G., Li, J., Schröder, H.C., Qiao, L., and Wang, X. (2007). "The unique skeleton of siliceous
sponges (Porifera; Hexactinellida and Demospongiae) that evolved first from the Urmetazoa during the
Proterozoic: a review". Biogeosciences 4 (2): 219–232. doi:10.5194/bg-4-219-2007.
44. ^ Marques, A.C., and Collins, A.G. (2004). "Cladistic analysis of Medusozoa and cnidarian evolution".
Invertebrate Biology 123 (1): 23–42. doi:10.1111/j.1744-7410.2004.tb00139.x.
http://www.marinespecies.org/aphia.php?p=sourceget&id=38492. Retrieved 2008-11-27.
45. ^ Miller, D.J., Ball, E.E., and Technau, U. (October 2005). "Cnidarians and ancestral genetic complexity in
the animal kingdom". Trends in Genetics 21 (10): 536–539. doi:10.1016/j.tig.2005.08.002.
PMID 16098631.
46. ^ Technau, U., Rudd, S., and Maxwell, P (December 2005). "Maintenance of ancestral complexity and nonmetazoan genes in two basal cnidarians". Trends in Genetics 21 (12): 633–639.
doi:10.1016/j.tig.2005.09.007. PMID 16226338.
47. ^ Smith DR, Kayal E, Yanagihara AA, Collins AG, Pirro S, Keeling PJ (2011) First complete mitochondrial
genome sequence from a box jellyfish reveals a highly fragmented, linear architecture and insights into
telomere evolution. Genome Biol Evol
48. ^ Williamson, J.A., Fenner, P.J., Burnett, J.W., and Rifkin, J. (1996). Venomous and Poisonous Marine
Animals: A Medical and Biological Handbook. UNSW Press. pp. 65–68. ISBN 0868402796.
http://books.google.com/?id=YsZ3GryFIzEC&pg=PA75&lpg=PA75&dq=mollusc+venom+fatal. Retrieved
2008-10-03.
49. ^ a b Clark, J.R. (1998). Coastal Seas: The Conservation Challenge. Blackwell. pp. 8–9. ISBN 0632049553.
http://books.google.com/?id=H82xdtuLxDMC&pg=PA8&dq=%22Coral+Reef%22+productivity. Retrieved
2008-11-28.
50. ^ Cronan, D.S., (1991). Marine Minerals in Exclusive Economic Zones. Springer. pp. 63–65.
ISBN 041229270X. http://books.google.com/?id=4g4nhd8USO8C&pg=PA63&dq=coral+jewellery.
Retrieved 2008-11-28.
51. ^ Omori, M. and Nakano, E. (2001). "Jellyfish fisheries in southeast Asia". In Purcell, J.E.,. Jellyfish Blooms:
Ecological and Societal Importance. Springer. pp. 19–26. ISBN 0792369645.
52. ^ Hsieh, Y-H.P. Leong, F-M., and Rudloe, J. (2001). "Jellyfish as food". In Purcell, J.E.,. Jellyfish Blooms:
Ecological and Societal Importance. Springer. pp. 11–17. ISBN 0792369645.
53. ^ Greenberg, M.I., Hendrickson, R.G., Silverberg, M., Campbell, C., and Morocco, A. (2004). "Box Jellyfish
Envenomation". Greenberg's Text-atlas of Emergency Medicine. Lippincott Williams & Wilkins. p. 875.
ISBN 0781745861.
54. ^ a b Little, M., Pereira, P., Carrette, T., and Seymour, J. (2006). "Jellyfish Responsible for Irukandji
Syndrome". QJM (Quarterly Journal of Medicine) 99 (6): 425–427. doi:10.1093/qjmed/hcl057.
PMID 16687419.
55. ^ Barnes, J. (1964). "Cause and effect in Irukandji stingings". Medical Journal of Australia 1: 897–904.
PMID 14172390.
56. ^ Grady J, Burnett J (2003). "Irukandji-like syndrome in South Florida divers". Annals of Emergency
Medicine 42 (6): 763–6. doi:10.1016/S0196-0644(03)00513-4. PMID 14634600.
[edit] Further reading
[edit] Books
Arai, M.N. (1997). A Functional Biology of Scyphozoa. London: Chapman & Hall [p. 316]. ISBN 0412-45110-7.
Ax, P. (1999). Das System der Metazoa I. Ein Lehrbuch der phylogenetischen Systematik. Gustav
Fischer, Stuttgart-Jena: Gustav Fischer. ISBN 3-437-30803-3.
Barnes, R.S.K., P. Calow, P. J. W. Olive, D. W. Golding & J. I. Spicer (2001). The invertebrates—a
synthesis. Oxford: Blackwell. 3rd edition [chapter 3.4.2, p. 54]. ISBN 0-632-04761-5.
Brusca, R.C., G.J. Brusca (2003). Invertebrates. Sunderland, Mass.: Sinauer Associates. 2nd
edition [chapter 8, p. 219]. ISBN 0-87893-097-3.
Dalby, A. (2003). Food in the Ancient World: from A to Z. London: Routledge.
Moore, J.(2001). An Introduction to the Invertebrates. Cambridge: Cambridge University Press
[chapter 4, p. 30]. ISBN 0-521-77914-6.
Schäfer, W. (1997). Cnidaria, Nesseltiere. In Rieger, W. (ed.) Spezielle Zoologie. Teil 1. Einzeller
und Wirbellose Tiere. Stuttgart-Jena: Gustav Fischer. Spektrum Akademischer Verl., Heidelberg,
2004. ISBN 3-8274-1482-2.
Werner, B. 4. Stamm Cnidaria. In: V. Gruner (ed.) Lehrbuch der speziellen Zoologie. Begr. von
Kaestner. 2 Bde. Stuttgart-Jena: Gustav Fischer, Stuttgart-Jena. 1954, 1980, 1984, Spektrum
Akad. Verl., Heidelberg-Berlin, 1993. 5th edition. ISBN 3-334-60474-8.
[edit] Journal articles
D. Bridge, B. Schierwater, C. W. Cunningham, R. DeSalle R, L. W. Buss: Mitochondrial DNA
structure and the molecular phylogeny of recent cnidaria classes. in: Proceedings of the Academy
of Natural Sciences of Philadelphia. Philadelphia USA 89.1992, p. 8750. ISSN 0097-3157
D. Bridge, C. W. Cunningham, R. DeSalle, L. W. Buss: Class-level relationships in the phylum
Cnidaria—Molecular and morphological evidence. in: Molecular biology and evolution. Oxford
University Press, Oxford 12.1995, p. 679. ISSN 0737-4038
D. G. Fautin: Reproduction of Cnidaria. in: Canadian Journal of Zoology. Ottawa Ont. 80.2002,
p. 1735. (PDF, online) ISSN 0008-4301
G. O. Mackie: What's new in cnidarian biology? in: Canadian Journal of Zoology. Ottawa Ont.
80.2002, p. 1649. (PDF, online) ISSN 0008-4301
P. Schuchert: Phylogenetic analysis of the Cnidaria. in: Zeitschrift für zoologische Systematik und
Evolutionsforschung. Paray, Hamburg-Berlin 31.1993, p. 161. ISSN 0044-3808
G. Kass-Simon, A. A. Scappaticci Jr.: The behavioral and developmental physiology of
nematocysts. in: Canadian Journal of Zoology. Ottawa Ont. 80.2002, p. 1772. (PDF, online)
ISSN 0044-3808
J. Zrzavý (2001). "The interrelationships of metazoan parasites: a review of phylum- and higherlevel hypotheses from recent morphological and molecular phylogenetic analyses" (PDF). Folia
Parasitologica 48 (2): 81–103. PMID 11437135. Archived from the original on 2007-10-25.
http://web.archive.org/web/20071025220832/http://www.paru.cas.cz/folia/pdf/2-01/Zrz.pdf.
Retrieved 2009-01-26.
[edit] External links
Wikispecies has information related to: Cnidaria
The Wikibook Dichotomous Key has a page on the topic of
Cnidaria
Wikimedia Commons has media related to: Cnidaria
Look up Cnidaria in Wiktionary, the free dictionary.
YouTube: Nematocysts Firing
YouTube:My Anemone Eat Meat Defensive and feeding behaviour of sea anemone
Cnidaria - Guide to the Marine Zooplankton of south eastern Australia, Tasmanian Aquaculture
& Fisheries Institute
A Cnidaria homepage maintained by University of California, Irvine
Cnidaria page at Tree of Life
Fossil Gallery: Cnidarians
The Hydrozoa Directory
Hexacorallians of the World
[hide]
v
t
e
Eukaryota
Domain : Archaea · Bacteria · Eukaryota
Bikonta
Archaeplastida, or Plantae sensu lato
Viridiplantae/Plantae sensu stricto ·
Rhodophyta · Glaucocystophyceae
Hacrobia, or non-SAR chromalveolata
Haptophyta · Cryptophyta ·
Centroheliozoa
AH/SAR AH
Heterokont ("S") Ochrophyta · Bigyra · Pseudofungi
Halvaria
SAR
Alveolata Ciliates · Myzozoa (Apicomplexa, Dinoflagellata)
Rhizaria Cercozoa · Retaria (Foraminifera, Radiolaria)
Excavata Discoba (Euglenozoa, Percolozoa) · Metamonad · Malawimonas
Apusozoa
Apusomonadida (Apusomonas, Amastigomonas) · Ancyromonadida
(Ancyromonas) · Hemimastigida (Hemimastix, Spironema, Stereonema)
Amoebozoa Lobosea · Conosa · Phalansterium · Breviata
Mesomycetozoea Dermocystida · Ichthyophonida
Filasterea Capsaspora · Ministeria
Choanoflagellate Codonosigidae
Holozoa
Filozoa
Unikonta
Opisthokonta
Holomycota
Eumetazoa (Bilateria,
Metazoa Cnidaria, Ctenophora) ·
or "Animalia" Mesozoa · Parazoa
(Placozoa, Porifera)
Dikarya (Ascomycota, Basidiomycota) ·
Glomeromycota · Zygomycota ·
Fungi Blastocladiomycota ·
Chytridiomycota/Neocallimastigomycota ·
Microsporidia
Nucleariidae
Nuclearia · Micronuclearia · Rabdiophrys ·
Pinaciophora · Pompholyxophrys · Fonticula
[show]
v
t
e
Extant phyla of kingdom Animalia by subkingdom
o
Radiata
Ctenophora
Cnidaria
o Anthozoa
o Hydrozoa
o Scyphozoa
o Cubozoa
o Staurozoa
o Myxozoa
o Polypodiozoa
Scalidophor
a
Kinorhyncha
Loricifera
Priapulida
Nematoida
Nematoda
Nematomorpha
Cycloneuralia
Ecdysozoa
Panarthropod
a
Onychophora
Tardigrada
Arthropoda
Lobopodia
Bilateria Protostomia
Platyhelminthes
Gastrotricha
Platyzoa
Lophotrochoz
oa
Gnathife
ra
Spiralia
Trochoz
oa
Lophophora
Rotifera
Acanthocephala
Gnathostomulid
a
Micrognathozoa
Cycliophora
Sipuncula
Nemertea
Mollusca
Annelida
Phoronida
Brachiopoda
ta
Ambulacraria
Deuterosto
mia
Basal/disput
ed
Bryozoa (?)
Entoprocta (?)
Hemichordata
Echinodermata
Xenoturbellida
Chordata
o Craniata
Vertebrata
Myxini
o Cephalochordata
o Tunicata
Acoelomorpha
o Acoela
o Nemertodermatida
Chaetognatha
Retrieved from "http://en.wikipedia.org/w/index.php?title=Cnidaria&oldid=479630448"
View page ratings
Rate this page
What's this?
Trustworthy
Objective
Complete
Well-written
I am highly knowledgeable about this topic (optional)
Submit ratings
Saved successfully
Your ratings have not been submitted yet
Categories:
Venomous animals
Cnidarians
Hidden categories:
Good articles
Articles with 'species' microformats
Personal tools
Log in / create account
Namespaces
Article
Talk
Variants
Views
Read
Edit
View history
Actions
Search
Search
Special:Search
Navigation
Main page
Contents
Featured content
Current events
Random article
Donate to Wikipedia
Interaction
Help
About Wikipedia
Community portal
Recent changes
Contact Wikipedia
Toolbox
What links here
Related changes
Upload file
Special pages
Permanent link
Cite this page
Rate this page
Print/export
Create a book
Download as PDF
Printable version
Languages
ال عرب ية
Armãneashce
Azərbaycanca
Български
Bosanski
Brezhoneg
Català
Česky
Cymraeg
Dansk
Deutsch
Eesti
Español
Esperanto
Euskara
ف ار سی
Français
Gaelg
Galego
한국어
हिन्दी
Hrvatski
Ido
Bahasa Indonesia
Íslenska
Italiano
עברית
Basa Jawa
Latina
Latviešu
Lëtzebuergesch
Lietuvių
Magyar
Македонски
Nederlands
日本語
Norsk (bokmål)
Norsk (nynorsk)
Occitan
Plattdüütsch
Polski
Português
Runa Simi
Русский
Sicilianu
Simple English
Slovenčina
Slovenščina
Српски / Srpski
Srpskohrvatski / Српскохрватски
Suomi
Svenska
Tagalog
తెలుగు
ไทย
Türkçe
Українська
Zazaki
Zeêuws
中文
This page was last modified on 1 March 2012 at 10:48.
Text is available under the Creative Commons Attribution-ShareAlike License; additional terms
may apply. See Terms of use for details.
Wikipedia® is a registered trademark of the Wikimedia Foundation, Inc., a non-profit
organization.
Contact us
Contact us
Cnidaria
From Wikipedia, the free encyclopedia
(Redirected from Cnideria)
Jump to: navigation, search
Cnidaria
Pacific sea nettles, Chrysaora fuscescens
Scientific classification
Domain:
Eukaryota
Kingdom:
Animalia
Phylum:
Cnidaria
Hatschek, 1888
Subphylum/Classes[3]
Anthozoa—corals and sea anemones
Medusozoa—jellyfish:[1]
Cubozoa—box jellyfish, sea wasps
Hydrozoa—hydroids, hydra-like animals
Scyphozoa—true jellyfish
Staurozoa—stalked jellyfish
Unranked, may not be scyphozoans[2]
Myxozoa—parasites
Polypodiozoa—parasites
Cnidaria ( /naɪˈdɛəriə/ with a silent c) is a phylum containing over 10,000[4] species of
animals found exclusively in aquatic and mostly marine environments. Their distinguishing
feature is cnidocytes, specialized cells that they use mainly for capturing prey. Their bodies
consist of mesoglea, a non-living jelly-like substance, sandwiched between two layers of
epithelium that are mostly one cell thick. They have two basic body forms: swimming medusae
and sessile polyps, both of which are radially symmetrical with mouths surrounded by tentacles
that bear cnidocytes. Both forms have a single orifice and body cavity that are used for digestion
and respiration. Many cnidarian species produce colonies that are single organisms composed of
medusa-like or polyp-like zooids, or both. Cnidarians' activities are coordinated by a
decentralized nerve net and simple receptors. Several free-swimming Cubozoa and Scyphozoa
possess balance-sensing statocysts, and some have simple eyes. Not all cnidarians reproduce
sexually. Many have complex lifecycles with asexual polyp stages and sexual medusae, but some
omit either the polyp or the medusa stage.
Cnidarians were for a long time grouped with Ctenophores in the phylum Coelenterata, but
increasing awareness of their differences caused them to be placed in separate phyla. Cnidarians
are classified into four main groups: the almost wholly sessile Anthozoa (sea anemones, corals,
sea pens); swimming Scyphozoa (jellyfish); Cubozoa (box jellies); and Hydrozoa, a diverse
group that includes all the freshwater cnidarians as well as many marine forms, and has both
sessile members such as Hydra and colonial swimmers such as the Portuguese Man o' War.
Staurozoa have recently been recognised as a class in their own right rather than a sub-group of
Scyphozoa, and there is debate about whether Myxozoa and Polypodiozoa are cnidarians or
closer to bilaterians (more complex animals).
Most cnidarians prey on organisms ranging in size from plankton to animals several times larger
than themselves, but many obtain much of their nutrition from endosymbiotic algae, and a few
are parasites. Many are preyed upon by other animals including starfish, sea slugs, fish and
turtles. Coral reefs, whose polyps are rich in endosymbiotic algae, support some of the world's
most productive ecosystems, and protect vegetation in tidal zones and on shorelines from strong
currents and tides. While corals are almost entirely restricted to warm, shallow marine waters,
other cnidarians live in the depths, in polar seas and in freshwater.
Fossil cnidarians have been found in rocks formed about 580 million years ago, and other fossils
show that corals may have been present shortly before 490 million years ago and diversified a
few million years later. Fossils of cnidarians that do not build mineralized structures are very
rare. Scientists currently think that cnidarians, ctenophores and bilaterians are more closely
related to calcareous sponges than these are to other sponges, and that anthozoans are the
evolutionary "aunts" or "sisters" of other cnidarians, and the most closely related to bilaterians.
Recent analyses have concluded that cnidarians, although considered more "primitive" than
bilaterians, have a wider range of genes.
Jellyfish stings killed several hundred people in the 20th century, and cubozoans are particularly
dangerous. On the other hand, some large jellyfish are considered a delicacy in eastern and
southern Asia. Coral reefs have long been economically important as providers of fishing
grounds, protectors of shore buildings against currents and tides, and more recently as centers of
tourism. However, they are vulnerable to over-fishing, mining for construction materials,
pollution, and damage caused by tourism.
Contents
[hide]
1 Distinguishing features
2 Description
o 2.1 Basic body forms
o 2.2 Colonial forms
o 2.3 Skeletons
o 2.4 Main cell layers
o 2.5 Cnidocytes
o 2.6 Locomotion
o 2.7 Nervous system and senses
o 2.8 Feeding and excretion
o 2.9 Respiration
o 2.10 Regeneration
3 Reproduction
o 3.1 Sexual
o 3.2 Asexual
4 Classification
5 Ecology
6 Evolutionary history
o 6.1 Fossil record
o 6.2 Family tree
7 Interaction with humans
8 Notes
9 Further reading
o
o
9.1 Books
9.2 Journal articles
10 External links
[edit] Distinguishing features
Further information: Sponge, Ctenophore, and Bilateria
Cnidarians form an animal phylum that is more complex than sponges, about as complex as
ctenophores (comb jellies), and less complex than bilaterians, which include almost all other
animals. However, both cnidarians and ctenophores are more complex than sponges as they
have: cells bound by inter-cell connections and carpet-like basement membranes; muscles;
nervous systems; and some have sensory organs. Cnidarians are distinguished from all other
animals by having cnidocytes that fire like harpoons and are used mainly to capture prey but also
as anchors in some species.[5]
Like sponges and ctenophores, cnidarians have two main layers of cells that sandwich a middle
layer of jelly-like material, which is called the mesoglea in cnidarians; more complex animals
have three main cell layers and no intermediate jelly-like layer. Hence, cnidarians and
ctenophores have traditionally been labelled diploblastic, along with sponges.[5][6] However, both
cnidarians and ctenophores have a type of muscle that, in more complex animals, arises from the
middle cell layer.[7] As a result some recent text books classify ctenophores as triploblastic,[8] and
it has been suggested that cnidarians evolved from triploblastic ancestors.[7]
Cnidocytes
Sponges[9][10]
Cnidarians[5][6]
Ctenophores[5][8] Bilateria[5]
No
Yes
No
Colloblasts
No
Digestive and
circulatory organs
Number of main cell
layers
Yes
No
Two, with jelly-like layer between them
No
Yes
Two[5] or
Three[7][8]
Three
Cells in each layer
bound together
No, except that
Homoscleromorpha have
basement membranes.[11]
Yes: inter-cell connections; basement membranes
Sensory organs
No
Yes
Number of cells in
middle "jelly" layer
Many
Few
(Not
applicable)
Cells in outer layers
can move inwards
and change
functions
Yes
No
(Not
applicable)
Nervous system
No
Yes, simple
Simple to
complex
Muscles
None
[edit] Description
[edit] Basic body forms
Aboral end
Oral end
Mouth
Oral end
Aboral end
Exoderm
Gastroderm (Endoderm)
Mesoglea
Digestive cavity
Mostly
epitheliomuscular
Mostly
myoepithelial
Mostly
myocytes
Medusa (left) and polyp (right)[6]
Oral end of actinodiscus polyp, with close-up of the mouth
Adult cnidarians appear as either swimming medusae or sessile polyps. Both are radially
symmetrical, like a wheel and a tube respectively. Since these animals have no heads, their ends
are described as "oral" (nearest the mouth) and "aboral" (furthest from the mouth). Most have
fringes of tentacles equipped with cnidocytes around their edges, and medusae generally have an
inner ring of tentacles around the mouth. The mesoglea of polyps is usually thin and often soft,
but that of medusae is usually thick and springy, so that it returns to its original shape after
muscles around the edge have contracted to squeeze water out, enabling medusae to swim by a
sort of jet propulsion.[6]
[edit] Colonial forms
Tree-like polyp colony[6]
Cnidaria produce a variety of colonial forms, each of which is one organism but consists of
polyp-like zooids. The simplest is a connecting tunnel that runs over the substrate (rock or
seabed) and from which single zooids sprout. In some cases the tunnels form visible webs, and in
others they are enclosed in a fleshy mat. More complex forms are also based on connecting
tunnels but produce "tree-like" groups of zooids. The "trees" may be formed either by a central
zooid that functions as a "trunk" with later zooids growing to the sides as "branches", or in a zigzag shape as a succession of zooids, each of which grows to full size and then produces a single
bud at an angle to itself. In many cases the connecting tunnels and the "stems" are covered in
periderm, a protective layer of chitin.[6] Some colonial forms have other specialized types of
zooid, for example, to pump water through their tunnels.[12]
Siphonophores form complex colonies that consist of: an upside-down polyp that forms a central
stem with a gas-filled float at the top; one or more sets of medusa-like zooids that provide
propulsion; leaf-like bracts that give some protection to other parts; sets of tentacles that bear
nematocytes that capture prey; other tentacles that act as sensors; near the base of each set of
tentacles, a polyp-like zooid that acts as a stomach for the colony; medusa-like zooids that serve
as gonads. Although some of these zooids resemble polyps or medusae in shape, they lack
features that are not relevant to their specific functions, for example the swimming "medusae"
have no digestive, sensory or reproductive cells. The best-known siphonophore is the Portuguese
Man o' War (Physalia physalis).[12][13][14]
[edit] Skeletons
In medusae the only supporting structure is the mesoglea. Hydra and most sea anemones close
their mouths when they are not feeding, and the water in the digestive cavity then acts as a
hydrostatic skeleton, rather like a water-filled balloon. Other polyps such as Tubularia use
columns of water-filled cells for support. Sea pens stiffen the mesoglea with calcium carbonate
spicules and tough fibrous proteins, rather like sponges.[6]
In some colonial polyps a chitinous periderm gives support and some protection to the
connecting sections and to the lower parts of individual polyps. Stony corals secrete massive
calcium carbonate exoskeletons. A few polyps collect materials such as sand grains and shell
fragments, which they attach to their outsides. Some colonial sea anemones stiffen the mesoglea
with sediment particles.[6]
[edit] Main cell layers
Cnidaria are diploblastic animals, in other words they have two main cell layers, while more
complex animals are triploblasts having three main layers. The two main cell layers of cnidarians
form epithelia that are mostly one cell thick, and are attached to a fibrous basement membrane,
which they secrete. They also secrete the jelly-like mesoglea that separates the layers. The layer
that faces outwards, known as the ectoderm ("outside skin"), generally contains the following
types of cells:[5]
Epitheliomuscular cells whose bodies form part of the epithelium but whose bases extend to
form muscle fibers in parallel rows.[15] The fibers of the outward-facing cell layer generally run at
right angles to the fibers of the inward-facing one. In Anthozoa (anemones, corals, etc.) and
Scyphozoa (jellyfish), the mesoglea also contains some muscle cells.[6]
Cnidocytes, the harpoon-like "nettle cells" that give the phylum Cnidaria its name. These appear
between or sometimes on top of the muscle cells.[5]
Nerve cells. Sensory cells appear between or sometimes on top of the muscle cells,[5] and
communicate via synapses (gaps across which chemical signals flow) with motor nerve cells,
which lie mostly between the bases of the muscle cells.[6]
Interstitial cells, which are unspecialized and can replace lost or damaged cells by transforming
into the appropriate types. These are found between the bases of muscle cells.[5]
In addition to epitheliomuscular, nerve and interstitial cells, the inward-facing gastroderm
("stomach skin") contains gland cells that secrete digestive enzymes. In some species it also
contains low concentrations of cnidocytes, which are used to subdue prey that is still
struggling.[5][6]
The mesoglea contains small numbers of amoeba-like cells,[6] and muscle cells in some
species.[5] However the number of middle-layer cells and types are much lower than in
sponges.[6]
[edit] Cnidocytes
A hydra's nematocyst, before firing.
"trigger" cilium[6]
Firing sequence of the cnida in a hydra's nematocyst[6]
Operculum (lid)
"Finger" that turns inside out
/ / / Barbs
Venom
Victim's skin
Victim's tissues
These "nettle cells" function as harpoons, since their payloads remain connected to the bodies of
the cells by threads. Three types of cnidocytes are known:[5][6]
Nematocysts inject venom into prey, and usually have barbs to keep them embedded in the
victims. Most species have nematocysts.[5]
Spirocysts do not penetrate the victim or inject venom, but entangle it by means of small sticky
hairs on the thread.
Ptychocysts are not used for prey capture — instead the threads of discharged ptychocysts are
used for building protective tubes in which their owners live. Ptychocysts are found only in the
order Cerianthria, tube anemones.[6]
The main components of a cnidocyte are:[5][6]
A cilium (fine hair) which projects above the surface and acts as a trigger. Spirocysts do not have
cilia.
A tough capsule, the cnida, which houses the thread, its payload and a mixture of chemicals
which may include venom or adhesives or both. ("cnida" is derived from the Greek word κνίδη,
which means "nettle"[16])
A tube-like extension of the wall of the cnida that points into the cnida, like the finger of a
rubber glove pushed inwards. When a cnidocyte fires, the finger pops out. If the cell is a
venomous nematocyte, the "finger"'s tip reveals a set of barbs that anchor it in the prey.
The thread, which is an extension of the "finger" and coils round it until the cnidocyte fires. The
thread is usually hollow and delivers chemicals from the cnida to the target.
An operculum (lid) over the end of the cnida. The lid may be a single hinged flap or three flaps
arranged like slices of pie.
The cell body which produces all the other parts.
It is difficult to study the firing mechanisms of cnidocytes as these structures are small but very
complex. At least four hypotheses have been proposed:[5]
Rapid contraction of fibers round the cnida may increase its internal pressure.
The thread may be like a coiled spring that extends rapidly when released.
In the case of Chironex (the "sea wasp"), chemical changes in the cnida's contents may cause
them to expand rapidly by polymerization.
Chemical changes in the liquid in the cnida make it a much more concentrated solution, so that
osmotic pressure forces water in very rapidly to dilute it. This mechanism has been observed in
nematocysts of the class Hydrozoa, sometimes producing pressures as high as 140 atmospheres,
similar to that of scuba air tanks, and fully extending the thread in as little as 2 milliseconds
(0.002 second).[6]
Cnidocytes can only fire once, and about 25% of a hydra's nematocysts are lost from its tentacles
when capturing a brine shrimp. Used cnidocytes have to be replaced, which takes about 48 hours.
To minimise wasteful firing, two types of stimulus are generally required to trigger cnidocytes:
their cilia detect contact, and nearby sensory cells "smell" chemicals in the water. This
combination prevents them from firing at distant or non-living objects. Groups of cnidocytes are
usually connected by nerves and, if one fires, the rest of the group requires a weaker minimum
stimulus than the cells that fire first.[5][6]
[edit] Locomotion
Chrysaora quinquecirrha ("sea nettle") swimming
Medusae swim by a form of jet propulsion: muscles, especially inside the rim of the bell, squeeze
water out of the cavity inside the bell, and the springiness of the mesoglea powers the recovery
stroke. Since the tissue layers are very thin, they provide too little power to swim against currents
and just enough to control movement within currents.[6]
Hydras and some sea anemones can move slowly over rocks and sea or stream beds by various
means: creeping like snails, crawling like inchworms, or by somersaulting. A few can swim
clumsily by waggling their bases.[6]
[edit] Nervous system and senses
Cnidaria have no brains or even central nervous systems. Instead they have decentralized nerve
nets consisting of : sensory neurons that generate signals in response to various types of stimulus,
such as odors; motor neurons that tell muscles to contract; all connected by "cobwebs" of
intermediate neurons. As well as forming the "signal cables", intermediate neurons also form
ganglia that act as local coordination centers. The cilia of the cnidocytes detect physical contact.
Nerves inform cnidocytes when odors from prey or attackers are detected and when
neighbouring cnidocytes fire. Most of the communications between nerve cells are via chemical
synapses, small gaps across which chemicals flow. As this process is too slow to ensure that the
muscles round the rim of a medusa's bell contract simultaneously in swimming the neurons
which control this communicate by much faster electrical signals across gap junctions.[6]
Medusae and complex swimming colonies such as siphonophores and chondrophores sense tilt
and acceleration by means of statocysts, chambers lined with hairs which detect the movements
of internal mineral grains called statoliths. If the body tilts in the wrong direction, the animal
rights itself by increasing the strength of the swimming movements on the side that is too low.
They also have ocelli ("little eyes"), which can detect the direction from which light is coming.
Box jellies have camera eyes, although these probably do not form images, and their lenses
simply produce a clearer indication of the direction from which light is coming.[5]
[edit] Feeding and excretion
Cnidarians feed in several ways: predation, absorbing dissolved organic chemicals, filtering food
particles out of the water, and obtaining nutrients from symbiotic algae within their cells. Most
obtain the majority of their food from predation but some, including the corals Hetroxenia and
Leptogorgia, depend almost completely on their endosymbionts and on absorbing dissolved
nutrients.[5] Cnidaria give their symbiotic algae carbon dioxide, some nutrients and a place in the
sun.[6]
Predatory species use their cnidocytes to poison or entangle prey, and those with venomous
nematocysts may start digestion by injecting digestive enzymes. The "smell" of fluids from
wounded prey makes the tentacles fold inwards and wipe the prey off into the mouth. In medusae
the tentacles round the edge of the bell are often short and most of the prey capture is done by
"oral arms", which are extensions of the edge of the mouth and are often frilled and sometimes
branched to increase their surface area. Medusae often trap prey or suspended food particles by
swimming upwards, spreading their tentacles and oral arms and then sinking. In species for
which suspended food particles are important, the tentacles and oral arms often have rows of
cilia whose beating creates currents that flow towards the mouth, and some produce nets of
mucus to trap particles.[5]
Once the food is in the digestive cavity, gland cells in the gastroderm release enzymes that
reduce the prey to slurry, usually within a few hours. This circulates through the digestive cavity
and, in colonial cnidarians, through the connecting tunnels, so that gastroderm cells can absorb
the nutrients. Absorption may take a few hours, and digestion within the cells may take a few
days. The circulation of nutrients is driven by water currents produced by cilia in the gastroderm
or by muscular movements or both, so that nutrients reach all parts of the digestive cavity.[6]
Nutrients reach the outer cell layer by diffusion or, for animals or zooids such as medusae which
have thick mesogleas, are transported by mobile cells in the mesoglea.[5]
Indigestible remains of prey are expelled through the mouth. The main waste product of cells'
internal processes is ammonia, which is removed by the external and internal water currents.[6]
[edit] Respiration
There are no respiratory organs, and both cell layers absorb oxygen from and expel carbon
dioxide into the surrounding water. When the water in the digestive cavity becomes stale it must
be replaced, and nutrients that have not been absorbed will be expelled with it. Some Anthozoa
have ciliated grooves on their tentacles, allowing them to pump water out of and into the
digestive cavity without opening the mouth. This improves respiration after feeding and allows
these animals, which use the cavity as a hydrostatic skeleton, to control the water pressure in the
cavity without expelling undigested food.[5]
Cnidaria that carry photosynthetic symbionts may have the opposite problem, an excess of
oxygen, which may prove toxic. The animals produce large quantities of antioxidants to
neutralize the excess oxygen.[5]
[edit] Regeneration
All cnidarians can regenerate, allowing them to recover from injury and to reproduce asexually.
Medusae have limited ability to regenerate, but polyps can do so from small pieces or even
collections of separated cells. This enables corals to recover even after apparently being
destroyed by predators.[5]
[edit] Reproduction
1
2
3
4
5
6
7
8
9
10
11
12
13
14
Life cycle of a jellyfish:[5][6]
1–3 Larva searches for site
4–8 Polyp grows
9–11 Polyp strobilates
12–14 Medusa grows
[edit] Sexual
In the Cnidaria sexual reproduction often involves a complex life cycle with both polyp and
medusa stages. For example in Scyphozoa (jellyfish) and Cubozoa (box jellies) a larva swims
until it finds a good site, and then becomes a polyp. This grows normally but then absorbs its
tentacles and splits horizontally into a series of disks that become juvenile medusae, a process
called strobilation. The juveniles swim off and slowly grow to maturity, while the polyp regrows and may continue strobilating periodically. The adults have gonads in the gastroderm, and
these release ova and sperm into the water in the breeding season.[5][6]
Shortened forms of this life cycle are common, for example some oceanic scyphozoans omit the
polyp stage completely, and cubozoan polyps produce only one medusa. Hydrozoa have a
variety of life cycles. Some have no polyp stages and some (e.g. hydra) have no medusae. In
some species the medusae remain attached to the polyp and are responsible for sexual
reproduction; in extreme cases these reproductive zooids may not look much like medusae.
Anthozoa have no medusa stage at all and the polyps are responsible for sexual reproduction.[5]
Spawning is generally driven by environmental factors such as changes in the water temperature,
and their release is triggered by lighting conditions such as sunrise, sunset or the phase of the
moon. Many species of Cnidaria may spawn simultaneously in the same location, so that there
are too many ova and sperm for predators to eat more than a tiny percentage — one famous
example is the Great Barrier Reef, where at least 110 corals and a few non-cnidarian
invertebrates produce enough to turn the water cloudy. These mass spawnings may produce
hybrids, some of which can settle and form polyps, but it is not known how long these can
survive. In some species the ova release chemicals that attract sperm of the same species.[5]
The fertilized eggs develop into larvae by dividing until there are enough cells to form a hollow
sphere (blastula) and then a depression forms at one end (gastrulation) and eventually become
the digestive cavity. However in cnidarians the depression forms at the end further from the yolk
(at the animal pole), while in bilaterians it forms at the other end (vegetal pole).[6] The larvae,
called planulae, swim or crawl by means of cilia.[5] They are cigar-shaped but slightly broader at
the "front" end, which is the aboral, vegetal-pole end and eventually attaches to a substrate if the
species has a polyp stage.[6]
Anthozoan larvae either have large yolks or are capable of feeding on plankton, and some
already have endosymbiotic algae that help to feed them. Since the parents are immobile, these
feeding capabilities extend the larvae's range and avoid overcrowding of sites. Scyphozoan and
hydrozoan larvae have little yolk and most lack endosymbiotic algae, and therefore have to settle
quickly and metamorphose into polyps. Instead these species rely on their medusae to extend
their ranges.[6]
[edit] Asexual
All known cnidaria can reproduce asexually by various means, in addition to regenerating after
being fragmented. Hydrozoan polyps only bud, while the medusae of some hydrozoans can
divide down the middle. Scyphozoan polyps can both bud and split down the middle. In addition
to both of these methods, Anthozoa can split horizontally just above the base.[5][6]
[edit] Classification
Cnidarians were for a long time grouped with Ctenophores in the phylum Coelenterata, but
increasing awareness of their differences caused them to be placed in separate phyla. Cnidarians
are classified into four main groups: sessile Anthozoa (sea anemones, corals, sea pens);
swimming Scyphozoa (jellyfish); Cubozoa (box jellies); and Hydrozoa, a diverse group that
includes all the freshwater cnidarians as well as many marine forms, and has both sessile
members such as Hydra and colonial swimmers such as the Portuguese Man o' War. Staurozoa
have recently been recognised as a class in their own right rather than a sub-group of Scyphozoa,
and there is debate about whether Myxozoa and Polypodiozoa are cnidarians or closer to
bilaterians.
Modern cnidarians are generally classified into four classes:[5]
Hydrozoa
Number of species[4]
Scyphozoa
Cubozoa
Anthozoa
3,600
228
42
6,100
Hydra,
siphonophores
Jellyfish
Box
jellies
Sea anemones,
corals, sea pens
Yes
Yes
Yes
Yes
Yes
Yes
Medusa phase in life
In some species
cycle
Yes, except for Stauromedusae
Yes
if they are scyphozoans
No
Number of medusae
Many
produced per polyp
Many
(not applicable)
Examples
Cells found in mesoglea No
Nematocysts in
exodermis
No
One
Stauromedusae, small sessile cnidarians with stalks and no medusa stage, have traditionally been
classified as members of the Scyphozoa, but recent research suggests they should be regarded as
a separate class, Staurozoa.[17]
The Myxozoa, microscopic parasites, were first classified as protozoans,[18] but recently as
heavily modified cnidarians, and more closely related to Hydrozoa and Scyphozoa than to
Anthozoa.[19] However other recent research suggests that Polypodium hydriforme, a parasite
within the egg cells of sturgeon, is closely related to the Myxozoa and that both Polypodium and
the Myxozoa are intermediate between cnidarians and bilaterian animals.[20]
Some researchers classify the extinct conulariids as cnidarians, while others propose that they
form a completely separate phylum.[21]
[edit] Ecology
Coral reefs support rich ecosystems
Many cnidarians are limited to shallow waters because they depend on endosymbiotic algae for
much of their nutrients. The life cycles of most have polyp stages, which are limited to locations
that offer stable substrates. Nevertheless major cnidarian groups contain species that have
escaped these limitations. Hydrozoans have a worldwide range: some, such as Hydra, live in
freshwater; Obelia appears in the coastal waters of all the oceans; and Liriope can form large
shoals near the surface in mid-ocean. Among anthozoans, a few scleractinian corals, sea pens
and sea fans live in deep, cold waters, and some sea anemones inhabit polar seabeds while others
live near hydrothermal vents over 10 kilometres (6.2 mi) below sea-level. Reef-building corals
are limited to tropical seas between 30°N and 30°S with a maximum depth of 46 metres (151 ft),
temperatures between 20°C and 28°C, high salinity and low carbon dioxide levels.
Stauromedusae, although usually classified as jellyfish, are stalked, sessile animals that live in
cool to Arctic waters.[12] Cnidarians range in size from Hydra, 5–20 millimetres (0.20–0.79 in)
long,[22] to the Lion's mane jellyfish, which may exceed 2 metres (6.6 ft) in diameter and 75
metres (246 ft) in length.[23]
Prey of cnidarians ranges from plankton to animals several times larger than themselves.[12][24]
Some cnidarians are parasites, mainly on jellyfish but a few are major pests of fish.[12] Others
obtain most of their nourishment from endosymbiotic algae or dissolved nutrients.[5] Predators of
cnidarians include: sea slugs, which can incorporate nematocysts into their own bodies for selfdefense;[25] starfish, notably the crown of thorns starfish, which can devastate corals;[12] butterfly
fish and parrot fish, which eat corals;[26] and marine turtles, which eat jellyfish.[23] Some sea
anemones and jellyfish have a symbiotic relationship with some fish; for example clown fish live
among the tentacles of sea anemones, and each partner protects the other against predators.[12]
Coral reefs form some of the world's most productive ecosystems. Common coral reef cnidarians
include both Anthozoans (hard corals, octocorals, anemones) and Hydrozoans (fire corals, lace
corals) The endosymbiotic algae of many cnidarian species are very effective primary producers,
in other words converters of inorganic chemicals into organic ones that other organisms can use,
and their coral hosts use these organic chemicals very efficiently. In addition reefs provide
complex and varied habitats that support a wide range of other organisms.[27] Fringing reefs just
below low-tide level also have a mutually beneficial relationship with mangrove forests at high-
tide level and sea grass meadows in between: the reefs protect the mangroves and seagrass from
strong currents and waves that would damage them or erode the sediments in which they are
rooted, while the mangroves and seagrass protect the coral from large influxes of silt, fresh water
and pollutants. This additional level of variety in the environment is beneficial to many types of
coral reef animals, which for example may feed in the sea grass and use the reefs for protection
or breeding.[28]
[edit] Evolutionary history
[edit] Fossil record
The fossil coral Cladocora from Pliocene rocks in Cyprus
The earliest widely accepted animal fossils are rather modern-looking cnidarians, possibly from
around 580 million years ago, although fossils from the Doushantuo Formation can only be dated
approximately.[29] The identification of some of these as embryos of animals has been contested,
but other fossils from these rocks strongly resemble tubes and other mineralized structures made
by corals.[30] Their presence implies that the cnidarian and bilaterian lineages had already
diverged.[31] Although the Ediacaran fossil Charnia used to be classified as a jellyfish or sea
pen,[32] more recent study of growth patterns in Charnia and modern cnidarians has cast doubt on
this hypothesis,[33][34] and there are now no bona-fide cnidarian body fossils in the Ediacaran.
Few fossils of cnidarians without mineralized skeletons are known from more recent rocks,
except in lagerstätten that preserved soft-bodied animals.[35]
A few mineralized fossils that resemble corals have been found in rocks from the Cambrian
period, and corals diversified in the Early Ordovician.[35] These corals, which were wiped out in
the Permian-Triassic extinction about 251 million years ago,[35] did not dominate reef
construction since sponges and algae also played a major part.[36] During the Mesozoic era rudist
bivalves were the main reef-builders, but they were wiped out in the Cretaceous-Tertiary
extinction 65 million years ago,[37] and since then the main reef-builders have been scleractinian
corals.[35]
[edit] Family tree
Further information: Phylogeny
Metazoa
Glass sponges
Demosponges
Calcareous sponges
Eumetazoa
Ctenophora (comb jellies)
Planulozoa Cnidaria
Anthozoa
(sea anemones and corals)
Medusozoa
Hydrozoa
(Hydra, siphonophores, etc.)
Cubozoa
(box jellies)
Staurozoa
"Scyphozoa"
(jellyfish, excluding
Staurozoa)
Placozoa
Bilateria
Myxozoa
Other Bilateria
(more complex)
Family tree of Cnidaria and the origins of animals[2][38][39][40]
It is difficult to reconstruct the early stages in the evolutionary "family tree" of animals using
only morphology (their shapes and structures), because the large differences between Porifera
(sponges), Cnidaria plus Ctenophora (comb jellies), Placozoa and Bilateria (all the more complex
animals) make comparisons difficult. Hence reconstructions now rely largely or entirely on
molecular phylogenetics, which groups organisms according to similarities and differences in
their biochemistry, usually in their DNA or RNA.[41]
It is now generally thought that the Calcarea (sponges with calcium carbonate spicules) are more
closely related to Cnidaria, Ctenophora (comb jellies) and Bilateria (all the more complex
animals) than they are to the other groups of sponges.[38][42][43] In 1866 it was proposed that
Cnidaria and Ctenophora were more closely related to each other than to Bilateria and formed a
group called Coelenterata ("hollow guts"), because Cnidaria and Ctenophora both rely on the
flow of water in and out of a single cavity for feeding, excretion and respiration. In 1881 it was
proposed that Ctenophora and Bilateria were more closely related to each other, since they
shared features that Cnidaria lack, for example muscles in the middle layer (mesoglea in
Ctenophora, mesoderm in Bilateria). However more recent analyses indicate that these
similarities are rather vague, and the current view, based on molecular phylogenetics, is that
Cnidaria and Bilateria are more closely related to each other than either is to Ctenophora. This
grouping of Cnidaria and Bilateria has been labelled "Planulozoa" because it suggests that the
earliest Bilateria were similar to the planula larvae of Cnidaria.[2][39]
Within the Cnidaria, the Anthozoa (sea anemones and corals) are regarded as the sister-group of
the rest, which suggests that the earliest cnidarians were sessile polyps with no medusa stage.
However it is unclear how the other groups acquired the medusa stage, since Hydrozoa form
medusae by budding from the side of the polyp while the other Medusozoa do so by splitting
them off from the tip of the polyp. The traditional grouping of Scyphozoa included the
Staurozoa, but morphology and molecular phylogenetics indicate that Staurozoa are more closely
related to Cubozoa (box jellies) than to other "Scyphozoa". Similarities in the double body walls
of Staurozoa and the extinct Conulariida suggest that they are closely related. The position of
Anthozoa nearest the beginning of the cnidarian family tree also implies that Anthozoa are the
cnidarians most closely related to Bilateria, and this is supported by the fact that Anthozoa and
Bilateria share some genes that determine the main axes of the body.[2][44]
However in 2005 Katja Seipel and Volker Schmid suggested that cnidarians and ctenophores are
simplified descendants of triploblastic animals, since ctenophores and the medusa stage of some
cnidarians have striated muscle, which in bilaterians arises from the mesoderm. They did not
commit themselves on whether bilaterians evolved from early cnidarians or from the
hypothesized triploblastic ancestors of cnidarians.[7]
In molecular phylogenetics analyses from 2005 onwards, important groups of developmental
genes show the same variety in cnidarians as in chordates.[45] In fact cnidarians, and especially
anthozoans (sea anemones and corals), retain some genes that are present in bacteria, protists,
plants and fungi but not in bilaterians.[46]
The mitochondial genomes in the medusozoan cnidarians unlike that of other animals is linear
with fragmented genes.[47] The reason for this difference is unknown.
[edit] Interaction with humans
Jellyfish stings killed about 1,500 people in the 20th century,[48] and cubozoans are particularly
dangerous. On the other hand, some large jellyfish are considered a delicacy in eastern and
southern Asia. Coral reefs have long been economically important as providers of fishing
grounds, protectors of shore buildings against currents and tides, and more recently as centers of
tourism. However, they are vulnerable to over-fishing, mining for construction materials,
pollution, and damage caused by tourism.
Beaches protected from tides and storms by coral reefs are often the best places for housing in
tropical countries. Reefs are an important food source for low-technology fishing, both on the
reefs themselves and in the adjacent seas.[49] However despite their great productivity reefs are
vulnerable to over-fishing, because much of the organic carbon they produce is exhaled as
carbon dioxide by organisms at the middle levels of the food chain and never reaches the larger
species that are of interest to fishermen.[27] Tourism centered on reefs provides much of the
income of some tropical islands, attracting photographers, divers and sports fishermen. However
human activities damage reefs in several ways: mining for construction materials; pollution,
including large influxes of fresh water from storm drains; commercial fishing, including the use
of dynamite to stun fish and the capture of young fish for aquariums; and tourist damage caused
by boat anchors and the cumulative effect of walking on the reefs.[49] Coral, mainly from the
Pacific Ocean has long been used in jewellery, and demand rose sharply in the 1980s.[50]
The dangerous "sea wasp" Chironex fleckeri
Some large jellyfish species have been used in Chinese cuisine at least since 200 AD, and are
now fished in the seas around most of South East Asia. Japan is the largest single consumer of
edible jellyfish, importing at first only from China but now from all of South East Asia as prices
rose in the 1970s. This fishing industry is restricted to daylight hours and calm conditions in two
short seasons, from March to May and August to November.[51] The commercial value of
jellyfish food products depends on the skill with which they are prepared, and "Jellyfish
Masters" guard their trade secrets carefully. Jellyfish is very low in cholesterol and sugars, but
cheap preparation can introduce undesirable amounts of heavy metals.[52]
The "sea wasp" Chironex fleckeri has been described as the world's most venomous animal and
is held responsible for 67 deaths, although it is difficult to identify the animal as it is almost
transparent. Most stingings by C. fleckeri cause only mild symptoms.[53] Seven other box jellies
can cause a set of symptoms called Irukandji syndrome,[54] which takes about 30 minutes to
develop,[55] and from a few hours to two weeks to disappear.[56] Hospital treatment is usually
required, and there have been a few deaths.[54]
[edit] Notes
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
^ Classes in Medusozoa based on "The Taxonomicon - Taxon: Subphylum Medusozoa". Universal
Taxonomic Services. http://www.taxonomy.nl/Taxonomicon/TaxonTree.aspx?id=11582. Retrieved 200901-26.
^ a b c d Collins, A.G. (2002). "Phylogeny of Medusozoa and the Evolution of Cnidarian Life Cycles" (PDF).
Journal of Evolutionary Biology 15 (3): 418–432. doi:10.1046/j.1420-9101.2002.00403.x.
http://cima.uprm.edu/~n_schizas/CMOB_8676/Collins2002.pdf. Retrieved 2008-11-27.
^ Subphyla Anthozoa and Medusozoa based on "The Taxonomicon - Taxon: Phylum Cnidaria". Universal
Taxonomic Services. http://www.taxonomy.nl/Taxonomicon/TaxonTree.aspx?id=11551. Retrieved 200707-10.
^ a b Zhang, Z.-Q. (2011). "Animal biodiversity: An introduction to higher-level classification and taxonomic
richness". Zootaxa 3148: 7–12. http://mapress.com/zootaxa/2011/f/zt03148p012.pdf.
^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af Hinde, R.T., (1998). "The Cnidaria and Ctenophora". In
Anderson, D.T.,. Invertebrate Zoology. Oxford University Press. pp. 28–57. ISBN 0195513681.
^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004).
Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 111–124. ISBN 0030259827.
^ a b c d Seipel, K., and Schmid, V. (June 2005). "Evolution of striated muscle: Jellyfish and the origin of
triploblasty". Developmental Biology 282 (1): 14–26. doi:10.1016/j.ydbio.2005.03.032. PMID 15936326.
^ a b c Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole.
pp. 182–195. ISBN 0030259827.
^ Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 76–97.
ISBN 0030259827.
^ Bergquist, P.R., (1998). "Porifera". In Anderson, D.T.,. Invertebrate Zoology. Oxford University Press.
pp. 10–27. ISBN 0195513681.
^ Exposito, J-Y., Cluzel, C., Garrone, R., and Lethias, C. (2002). "Evolution of collagens". The Anatomical
Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology 268 (3): 302–316.
doi:10.1002/ar.10162. PMID 12382326.
^ a b c d e f g Shostak, S. (2006). "Cnidaria (Coelenterates)". Encyclopedia of Life Sciences. John Wiley & Sons.
doi:10.1038/npg.els.0004117.
^ Bhamrah, H.S., and Juneja, K. (2002). A Textbook of Invertebrates. Anmol Publications. pp. 278–280.
ISBN 8126104163.
http://books.google.com/?id=05So4shx9tIC&pg=PA279&dq=siphonophore+siphonophora. Retrieved
2008-11-17.
^ Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 167–
170. ISBN 0030259827.
^ Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). "Introduction to Metazoa". Invertebrate Zoology (7 ed.).
Brooks / Cole. pp. 103–104. ISBN 0030259827.
^ Trumble, W., and Brown, L. (2002). "Cnida". Shorter Oxford English Dictionary. Oxford University Press.
^ Collins, A.G., Cartwright, P., McFadden, C.S., and Schierwater, B. (2005). "Phylogenetic Context and
Basal Metazoan Model Systems". Integrative and Comparative Biology 45 (4): 585–594.
doi:10.1093/icb/45.4.585.
^ Štolc, A. (1899). "Actinomyxidies, nouveau groupe de Mesozoaires parent des Myxosporidies". Bull. Int.
L'Acad. Sci. Bohème 12: 1–12.
^ E. Jímenez-Guri; Philippe, H; Okamura, B; Holland, PW (July 2007). "Buddenbrockia is a cnidarian worm".
Science 317 (116): 116–118. doi:10.1126/science.1142024. PMID 17615357.
20. ^ Zrzavý, J. and Hypša, V. (2003). "Myxozoa, Polypodium, and the origin of the Bilateria: The phylogenetic
position of "Endocnidozoa" in light of the rediscovery of Buddenbrockia". Cladistics 19 (2): 164–169.
doi:10.1111/j.1096-0031.2003.tb00305.x.
21. ^ "The Conulariida". University of California Museum of Paleontology.
http://www.ucmp.berkeley.edu/cnidaria/conulariida.html. Retrieved 2008-11-27.
22. ^ Blaise, C., and Férard, J-F. (2005). Small-scale Freshwater Toxicity Investigations: Toxicity Test Methods.
Springer. p. 398. ISBN 140203119X. http://books.google.com/?id=Ibew5SLx2oMC&dq=hydra+size+length.
Retrieved 2008-11-21.
23. ^ a b Safina, C. (2007). Voyage of the Turtle: In Pursuit of the Earth's Last Dinosaur. Macmillan. p. 154.
ISBN 0805083189. http://books.google.com/?id=dQD883dAv6YC&pg=PA154&dq=cnidaria+turtle.
Retrieved 2008-11-21.
24. ^ Cowen, R. (2000). History of Life (3 ed.). Blackwell. p. 54. ISBN 0632044446.
http://books.google.com/?id=qvyBS4gwPF4C&pg=PA54&dq=cnidaria+prey. Retrieved 2008-11-21.
25. ^ Frick, K (2003). "Predator Suites and Flabellinid Nudibranch Nematocyst Complements in the Gulf of
Maine.". In: SF Norton (ed). Diving for Science...2003. Proceedings of the American Academy of
Underwater Sciences (22nd Annual Scientific Diving Symposium). http://archive.rubiconfoundation.org/4744. Retrieved 2008-07-03.
26. ^ Choat, J.H. and Bellwood, D.R. (1998). Paxton, J.R. and Eschmeyer, W.N.. ed. Encyclopedia of Fishes. San
Diego: Academic Press. pp. 209–211. ISBN 0-12-547665-5.
27. ^ a b Barnes, R.S.K., and Mann, K.H. (1991). Fundamentals of Aquatic Ecology. Blackwell Publishing.
pp. 217–227. ISBN 0632029838.
http://books.google.com/?id=mOZZlzgdTrwC&pg=PA227&dq=%22Coral+Reef%22+productivity. Retrieved
2008-11-26.
28. ^ Hatcher, B.G. Johannes, R.E., and Robertson, A.J. (1989). "Conservation of Shallow-water Marine
Ecosystems". Oceanography and Marine Biology: An Annual Review: Volume 27. Routledge. p. 320.
ISBN 0080377181.
http://books.google.com/?id=XpmNqFaDZ7cC&pg=PA320&dq=%22Coral+Reef%22+mangrove+%22seagra
ss%22. Retrieved 2008-11-21.
29. ^ Chen, J-Y.; Oliveri, P; Li, CW; Zhou, GQ; Gao, F; Hagadorn, JW; Peterson, KJ; Davidson, EH (2000).
"Putative phosphatized embryos from the Doushantuo Formation of China". Proceedings of the National
Academy of Sciences 97 (9): 4457–4462. doi:10.1073/pnas.97.9.4457. PMC 18256. PMID 10781044.
http://www.pnas.org/content/97/9/4457.full. Retrieved 2009-04-30.
30. ^ Xiao, S., Yuan, X., and Knoll, A.H. (2000). "Eumetazoan fossils in terminal Proterozoic phosphorites?".
Proceedings of the National Academy of Sciences 97 (25): 13684–13689. doi:10.1073/pnas.250491697.
PMC 17636. PMID 11095754.
http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=17636.
31. ^ Chen, J.-Y., Oliveri, P., Gao, F., Dornbos, S.Q., Li, C-W., Bottjer, D.J. and Davidson, E.H. (August 2002).
"Precambrian Animal Life: Probable Developmental and Adult Cnidarian Forms from Southwest China"
(PDF). Developmental Biology 248 (1): 182–196. doi:10.1006/dbio.2002.0714. PMID 12142030.
http://www.uwm.edu/~sdornbos/PDF's/Chen%20et%20al.%202002.pdf. Retrieved 2008-09-03.
32. ^ Donovan, Stephen K., Lewis, David N. (2001). "Fossils explained 35. The Ediacaran biota" (abstract).
Geology Today 17 (3): 115–120. doi:10.1046/j.0266-6979.2001.00285.x.
33. ^ Antcliffe, J.B.; Brasier, M. D. (2007). "Charnia and sea pens are poles apart". Journal of the Geological
Society 164 (1): 49–51. doi:10.1144/0016-76492006-080.
34. ^ Antcliffe, J.B.; Brasier, Martin D. (2007). "Charnia At 50: Developmental Models For Ediacaran Fronds".
Palaeontology 51 (1): 11–26. doi:10.1111/j.1475-4983.2007.00738.x.
35. ^ a b c d "Cnidaria: Fossil Record". University of California Museum of Paleontology.
http://www.ucmp.berkeley.edu/cnidaria/cnidariafr.html. Retrieved 2008-11-27.
36. ^ Copper, P. (January 1994). "Ancient reef ecosystem expansion and collapse". Coral Reefs 13 (1): 3–11.
Bibcode 1994CorRe..13....3C. doi:10.1007/BF00426428.
37. ^ "The Rudists". University of California Museum of Paleontology.
http://www.ucmp.berkeley.edu/taxa/inverts/mollusca/rudists.php. Retrieved 2008-11-27.
38. ^ a b Borchiellini, C., Manuel, M., Alivon, E., Boury-Esnault, N., Vacelet J., and Le Parco, Y. (2001). "Sponge
paraphyly and the origin of Metazoa". Journal of Evolutionary Biology 14 (1): 171–179.
doi:10.1046/j.1420-9101.2001.00244.x.
39. ^ a b Wallberg, A., Thollesson, M., , Farris, J.S., and Jondelius, U. (2004). "The phylogenetic position of the
comb jellies (Ctenophora) and the importance of taxonomic sampling". Cladistics 20 (6): 558–578.
doi:10.1111/j.1096-0031.2004.00041.x.
40. ^ Philippe, H. (April 2009). "Phylogenomics Revives Traditional Views on Deep Animal Relationships".
Current Biology 19: 706–712. doi:10.1016/j.cub.2009.02.052. PMID 19345102.
http://www.cell.com/current-biology/retrieve/pii/S0960982209008057. Retrieved 2011-09-25.
41. ^ Halanych, K.M. (December 2004). "The New View of Animal Phylogeny" (PDF). Annual Review of
Ecology, Evolution, and Systematics 35: 229–256. doi:10.1146/annurev.ecolsys.35.112202.130124.
http://gump.auburn.edu/halanych/lab/Pub.pdfs/Halanych2004.pdf. Retrieved 2008-11-27.
42. ^ Medina, M., Collins, A.G., Silberman, J.D., and Sogin, M.L. (August 2001). "Evaluating hypotheses of
basal animal phylogeny using complete sequences of large and small subunit rRNA". Proceedings of the
National Academy of Sciences 98 (17): 9707–9712. doi:10.1073/pnas.171316998. PMC 55517.
PMID 11504944. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=55517.
43. ^ Müller, W.E.G., Li, J., Schröder, H.C., Qiao, L., and Wang, X. (2007). "The unique skeleton of siliceous
sponges (Porifera; Hexactinellida and Demospongiae) that evolved first from the Urmetazoa during the
Proterozoic: a review". Biogeosciences 4 (2): 219–232. doi:10.5194/bg-4-219-2007.
44. ^ Marques, A.C., and Collins, A.G. (2004). "Cladistic analysis of Medusozoa and cnidarian evolution".
Invertebrate Biology 123 (1): 23–42. doi:10.1111/j.1744-7410.2004.tb00139.x.
http://www.marinespecies.org/aphia.php?p=sourceget&id=38492. Retrieved 2008-11-27.
45. ^ Miller, D.J., Ball, E.E., and Technau, U. (October 2005). "Cnidarians and ancestral genetic complexity in
the animal kingdom". Trends in Genetics 21 (10): 536–539. doi:10.1016/j.tig.2005.08.002.
PMID 16098631.
46. ^ Technau, U., Rudd, S., and Maxwell, P (December 2005). "Maintenance of ancestral complexity and nonmetazoan genes in two basal cnidarians". Trends in Genetics 21 (12): 633–639.
doi:10.1016/j.tig.2005.09.007. PMID 16226338.
47. ^ Smith DR, Kayal E, Yanagihara AA, Collins AG, Pirro S, Keeling PJ (2011) First complete mitochondrial
genome sequence from a box jellyfish reveals a highly fragmented, linear architecture and insights into
telomere evolution. Genome Biol Evol
48. ^ Williamson, J.A., Fenner, P.J., Burnett, J.W., and Rifkin, J. (1996). Venomous and Poisonous Marine
Animals: A Medical and Biological Handbook. UNSW Press. pp. 65–68. ISBN 0868402796.
http://books.google.com/?id=YsZ3GryFIzEC&pg=PA75&lpg=PA75&dq=mollusc+venom+fatal. Retrieved
2008-10-03.
49. ^ a b Clark, J.R. (1998). Coastal Seas: The Conservation Challenge. Blackwell. pp. 8–9. ISBN 0632049553.
http://books.google.com/?id=H82xdtuLxDMC&pg=PA8&dq=%22Coral+Reef%22+productivity. Retrieved
2008-11-28.
50. ^ Cronan, D.S., (1991). Marine Minerals in Exclusive Economic Zones. Springer. pp. 63–65.
ISBN 041229270X. http://books.google.com/?id=4g4nhd8USO8C&pg=PA63&dq=coral+jewellery.
Retrieved 2008-11-28.
51. ^ Omori, M. and Nakano, E. (2001). "Jellyfish fisheries in southeast Asia". In Purcell, J.E.,. Jellyfish Blooms:
Ecological and Societal Importance. Springer. pp. 19–26. ISBN 0792369645.
52. ^ Hsieh, Y-H.P. Leong, F-M., and Rudloe, J. (2001). "Jellyfish as food". In Purcell, J.E.,. Jellyfish Blooms:
Ecological and Societal Importance. Springer. pp. 11–17. ISBN 0792369645.
53. ^ Greenberg, M.I., Hendrickson, R.G., Silverberg, M., Campbell, C., and Morocco, A. (2004). "Box Jellyfish
Envenomation". Greenberg's Text-atlas of Emergency Medicine. Lippincott Williams & Wilkins. p. 875.
ISBN 0781745861.
54. ^ a b Little, M., Pereira, P., Carrette, T., and Seymour, J. (2006). "Jellyfish Responsible for Irukandji
Syndrome". QJM (Quarterly Journal of Medicine) 99 (6): 425–427. doi:10.1093/qjmed/hcl057.
PMID 16687419.
55. ^ Barnes, J. (1964). "Cause and effect in Irukandji stingings". Medical Journal of Australia 1: 897–904.
PMID 14172390.
56. ^ Grady J, Burnett J (2003). "Irukandji-like syndrome in South Florida divers". Annals of Emergency
Medicine 42 (6): 763–6. doi:10.1016/S0196-0644(03)00513-4. PMID 14634600.
[edit] Further reading
[edit] Books
Arai, M.N. (1997). A Functional Biology of Scyphozoa. London: Chapman & Hall [p. 316]. ISBN 0412-45110-7.
Ax, P. (1999). Das System der Metazoa I. Ein Lehrbuch der phylogenetischen Systematik. Gustav
Fischer, Stuttgart-Jena: Gustav Fischer. ISBN 3-437-30803-3.
Barnes, R.S.K., P. Calow, P. J. W. Olive, D. W. Golding & J. I. Spicer (2001). The invertebrates—a
synthesis. Oxford: Blackwell. 3rd edition [chapter 3.4.2, p. 54]. ISBN 0-632-04761-5.
Brusca, R.C., G.J. Brusca (2003). Invertebrates. Sunderland, Mass.: Sinauer Associates. 2nd
edition [chapter 8, p. 219]. ISBN 0-87893-097-3.
Dalby, A. (2003). Food in the Ancient World: from A to Z. London: Routledge.
Moore, J.(2001). An Introduction to the Invertebrates. Cambridge: Cambridge University Press
[chapter 4, p. 30]. ISBN 0-521-77914-6.
Schäfer, W. (1997). Cnidaria, Nesseltiere. In Rieger, W. (ed.) Spezielle Zoologie. Teil 1. Einzeller
und Wirbellose Tiere. Stuttgart-Jena: Gustav Fischer. Spektrum Akademischer Verl., Heidelberg,
2004. ISBN 3-8274-1482-2.
Werner, B. 4. Stamm Cnidaria. In: V. Gruner (ed.) Lehrbuch der speziellen Zoologie. Begr. von
Kaestner. 2 Bde. Stuttgart-Jena: Gustav Fischer, Stuttgart-Jena. 1954, 1980, 1984, Spektrum
Akad. Verl., Heidelberg-Berlin, 1993. 5th edition. ISBN 3-334-60474-8.
[edit] Journal articles
D. Bridge, B. Schierwater, C. W. Cunningham, R. DeSalle R, L. W. Buss: Mitochondrial DNA
structure and the molecular phylogeny of recent cnidaria classes. in: Proceedings of the Academy
of Natural Sciences of Philadelphia. Philadelphia USA 89.1992, p. 8750. ISSN 0097-3157
D. Bridge, C. W. Cunningham, R. DeSalle, L. W. Buss: Class-level relationships in the phylum
Cnidaria—Molecular and morphological evidence. in: Molecular biology and evolution. Oxford
University Press, Oxford 12.1995, p. 679. ISSN 0737-4038
D. G. Fautin: Reproduction of Cnidaria. in: Canadian Journal of Zoology. Ottawa Ont. 80.2002,
p. 1735. (PDF, online) ISSN 0008-4301
G. O. Mackie: What's new in cnidarian biology? in: Canadian Journal of Zoology. Ottawa Ont.
80.2002, p. 1649. (PDF, online) ISSN 0008-4301
P. Schuchert: Phylogenetic analysis of the Cnidaria. in: Zeitschrift für zoologische Systematik und
Evolutionsforschung. Paray, Hamburg-Berlin 31.1993, p. 161. ISSN 0044-3808
G. Kass-Simon, A. A. Scappaticci Jr.: The behavioral and developmental physiology of
nematocysts. in: Canadian Journal of Zoology. Ottawa Ont. 80.2002, p. 1772. (PDF, online)
ISSN 0044-3808
J. Zrzavý (2001). "The interrelationships of metazoan parasites: a review of phylum- and higherlevel hypotheses from recent morphological and molecular phylogenetic analyses" (PDF). Folia
Parasitologica 48 (2): 81–103. PMID 11437135. Archived from the original on 2007-10-25.
http://web.archive.org/web/20071025220832/http://www.paru.cas.cz/folia/pdf/2-01/Zrz.pdf.
Retrieved 2009-01-26.
[edit] External links
Wikispecies has information related to: Cnidaria
The Wikibook Dichotomous Key has a page on the topic of
Cnidaria
Wikimedia Commons has media related to: Cnidaria
Look up Cnidaria in Wiktionary, the free dictionary.
YouTube: Nematocysts Firing
YouTube:My Anemone Eat Meat Defensive and feeding behaviour of sea anemone
Cnidaria - Guide to the Marine Zooplankton of south eastern Australia, Tasmanian Aquaculture
& Fisheries Institute
A Cnidaria homepage maintained by University of California, Irvine
Cnidaria page at Tree of Life
Fossil Gallery: Cnidarians
The Hydrozoa Directory
Hexacorallians of the World
[hide]
v
t
e
Eukaryota
Domain : Archaea · Bacteria · Eukaryota
Bikonta
Archaeplastida, or Plantae sensu lato
Viridiplantae/Plantae sensu stricto ·
Rhodophyta · Glaucocystophyceae
Hacrobia, or non-SAR chromalveolata
Haptophyta · Cryptophyta ·
Centroheliozoa
AH/SAR AH
Heterokont ("S") Ochrophyta · Bigyra · Pseudofungi
Halvaria
SAR
Alveolata Ciliates · Myzozoa (Apicomplexa, Dinoflagellata)
Rhizaria Cercozoa · Retaria (Foraminifera, Radiolaria)
Excavata Discoba (Euglenozoa, Percolozoa) · Metamonad · Malawimonas
Apusozoa
Apusomonadida (Apusomonas, Amastigomonas) · Ancyromonadida
(Ancyromonas) · Hemimastigida (Hemimastix, Spironema, Stereonema)
Amoebozoa Lobosea · Conosa · Phalansterium · Breviata
Mesomycetozoea Dermocystida · Ichthyophonida
Filasterea Capsaspora · Ministeria
Choanoflagellate Codonosigidae
Holozoa
Filozoa
Unikonta
Opisthokonta
Holomycota
Eumetazoa (Bilateria,
Metazoa Cnidaria, Ctenophora) ·
or "Animalia" Mesozoa · Parazoa
(Placozoa, Porifera)
Dikarya (Ascomycota, Basidiomycota) ·
Glomeromycota · Zygomycota ·
Fungi Blastocladiomycota ·
Chytridiomycota/Neocallimastigomycota ·
Microsporidia
Nucleariidae
Nuclearia · Micronuclearia · Rabdiophrys ·
Pinaciophora · Pompholyxophrys · Fonticula
[show]
v
t
e
Extant phyla of kingdom Animalia by subkingdom
o
Radiata
Ctenophora
Cnidaria
o Anthozoa
o Hydrozoa
o Scyphozoa
o Cubozoa
o Staurozoa
o Myxozoa
o Polypodiozoa
Scalidophor
a
Kinorhyncha
Loricifera
Priapulida
Nematoida
Nematoda
Nematomorpha
Cycloneuralia
Ecdysozoa
Panarthropod
a
Onychophora
Tardigrada
Arthropoda
Lobopodia
Bilateria Protostomia
Platyhelminthes
Gastrotricha
Platyzoa
Lophotrochoz
oa
Gnathife
ra
Spiralia
Trochoz
oa
Lophophora
Rotifera
Acanthocephala
Gnathostomulid
a
Micrognathozoa
Cycliophora
Sipuncula
Nemertea
Mollusca
Annelida
Phoronida
Brachiopoda
ta
Ambulacraria
Deuterosto
mia
Basal/disput
ed
Bryozoa (?)
Entoprocta (?)
Hemichordata
Echinodermata
Xenoturbellida
Chordata
o Craniata
Vertebrata
Myxini
o Cephalochordata
o Tunicata
Acoelomorpha
o Acoela
o Nemertodermatida
Chaetognatha
Retrieved from "http://en.wikipedia.org/w/index.php?title=Cnidaria&oldid=479630448"
View page ratings
Rate this page
What's this?
Trustworthy
Objective
Complete
Well-written
I am highly knowledgeable about this topic (optional)
Submit ratings
Saved successfully
Your ratings have not been submitted yet
Categories:
Venomous animals
Cnidarians
Hidden categories:
Good articles
Articles with 'species' microformats
Personal tools
Log in / create account
Namespaces
Article
Talk
Variants
Views
Read
Edit
View history
Actions
Search
Search
Special:Search
Navigation
Main page
Contents
Featured content
Current events
Random article
Donate to Wikipedia
Interaction
Help
About Wikipedia
Community portal
Recent changes
Contact Wikipedia
Toolbox
What links here
Related changes
Upload file
Special pages
Permanent link
Cite this page
Rate this page
Print/export
Create a book
Download as PDF
Printable version
Languages
ال عرب ية
Armãneashce
Azərbaycanca
Български
Bosanski
Brezhoneg
Català
Česky
Cymraeg
Dansk
Deutsch
Eesti
Español
Esperanto
Euskara
ف ار سی
Français
Gaelg
Galego
한국어
हिन्दी
Hrvatski
Ido
Bahasa Indonesia
Íslenska
Italiano
עברית
Basa Jawa
Latina
Latviešu
Lëtzebuergesch
Lietuvių
Magyar
Македонски
Nederlands
日本語
Norsk (bokmål)
Norsk (nynorsk)
Occitan
Plattdüütsch
Polski
Português
Runa Simi
Русский
Sicilianu
Simple English
Slovenčina
Slovenščina
Српски / Srpski
Srpskohrvatski / Српскохрватски
Suomi
Svenska
Tagalog
తెలుగు
ไทย
Türkçe
Українська
Zazaki
Zeêuws
中文
This page was last modified on 1 March 2012 at 10:48.
Text is available under the Creative Commons Attribution-ShareAlike License; additional terms
may apply. See Terms of use for details.
Wikipedia® is a registered trademark of the Wikimedia Foundation, Inc., a non-profit
organization.
Contact us
Cnidaria
From Wikipedia, the free encyclopedia
(Redirected from Cnideria)
Jump to: navigation, search
Cnidaria
Pacific sea nettles, Chrysaora fuscescens
Scientific classification
Domain:
Eukaryota
Kingdom:
Animalia
Phylum:
Cnidaria
Hatschek, 1888
Subphylum/Classes[3]
Anthozoa—corals and sea anemones
Medusozoa—jellyfish:[1]
Cubozoa—box jellyfish, sea wasps
Hydrozoa—hydroids, hydra-like animals
Scyphozoa—true jellyfish
Staurozoa—stalked jellyfish
Unranked, may not be scyphozoans[2]
Myxozoa—parasites
Polypodiozoa—parasites
Cnidaria ( /naɪˈdɛəriə/ with a silent c) is a phylum containing over 10,000[4] species of
animals found exclusively in aquatic and mostly marine environments. Their distinguishing
feature is cnidocytes, specialized cells that they use mainly for capturing prey. Their bodies
consist of mesoglea, a non-living jelly-like substance, sandwiched between two layers of
epithelium that are mostly one cell thick. They have two basic body forms: swimming medusae
and sessile polyps, both of which are radially symmetrical with mouths surrounded by tentacles
that bear cnidocytes. Both forms have a single orifice and body cavity that are used for digestion
and respiration. Many cnidarian species produce colonies that are single organisms composed of
medusa-like or polyp-like zooids, or both. Cnidarians' activities are coordinated by a
decentralized nerve net and simple receptors. Several free-swimming Cubozoa and Scyphozoa
possess balance-sensing statocysts, and some have simple eyes. Not all cnidarians reproduce
sexually. Many have complex lifecycles with asexual polyp stages and sexual medusae, but some
omit either the polyp or the medusa stage.
Cnidarians were for a long time grouped with Ctenophores in the phylum Coelenterata, but
increasing awareness of their differences caused them to be placed in separate phyla. Cnidarians
are classified into four main groups: the almost wholly sessile Anthozoa (sea anemones, corals,
sea pens); swimming Scyphozoa (jellyfish); Cubozoa (box jellies); and Hydrozoa, a diverse
group that includes all the freshwater cnidarians as well as many marine forms, and has both
sessile members such as Hydra and colonial swimmers such as the Portuguese Man o' War.
Staurozoa have recently been recognised as a class in their own right rather than a sub-group of
Scyphozoa, and there is debate about whether Myxozoa and Polypodiozoa are cnidarians or
closer to bilaterians (more complex animals).
Most cnidarians prey on organisms ranging in size from plankton to animals several times larger
than themselves, but many obtain much of their nutrition from endosymbiotic algae, and a few
are parasites. Many are preyed upon by other animals including starfish, sea slugs, fish and
turtles. Coral reefs, whose polyps are rich in endosymbiotic algae, support some of the world's
most productive ecosystems, and protect vegetation in tidal zones and on shorelines from strong
currents and tides. While corals are almost entirely restricted to warm, shallow marine waters,
other cnidarians live in the depths, in polar seas and in freshwater.
Fossil cnidarians have been found in rocks formed about 580 million years ago, and other fossils
show that corals may have been present shortly before 490 million years ago and diversified a
few million years later. Fossils of cnidarians that do not build mineralized structures are very
rare. Scientists currently think that cnidarians, ctenophores and bilaterians are more closely
related to calcareous sponges than these are to other sponges, and that anthozoans are the
evolutionary "aunts" or "sisters" of other cnidarians, and the most closely related to bilaterians.
Recent analyses have concluded that cnidarians, although considered more "primitive" than
bilaterians, have a wider range of genes.
Jellyfish stings killed several hundred people in the 20th century, and cubozoans are particularly
dangerous. On the other hand, some large jellyfish are considered a delicacy in eastern and
southern Asia. Coral reefs have long been economically important as providers of fishing
grounds, protectors of shore buildings against currents and tides, and more recently as centers of
tourism. However, they are vulnerable to over-fishing, mining for construction materials,
pollution, and damage caused by tourism.
Contents
[hide]
1 Distinguishing features
2 Description
o 2.1 Basic body forms
o 2.2 Colonial forms
o 2.3 Skeletons
o 2.4 Main cell layers
o 2.5 Cnidocytes
o 2.6 Locomotion
o 2.7 Nervous system and senses
o 2.8 Feeding and excretion
o 2.9 Respiration
o 2.10 Regeneration
3 Reproduction
o 3.1 Sexual
o 3.2 Asexual
4 Classification
5 Ecology
6 Evolutionary history
o 6.1 Fossil record
o 6.2 Family tree
7 Interaction with humans
8 Notes
9 Further reading
o 9.1 Books
o 9.2 Journal articles
10 External links
[edit] Distinguishing features
Further information: Sponge, Ctenophore, and Bilateria
Cnidarians form an animal phylum that is more complex than sponges, about as complex as
ctenophores (comb jellies), and less complex than bilaterians, which include almost all other
animals. However, both cnidarians and ctenophores are more complex than sponges as they
have: cells bound by inter-cell connections and carpet-like basement membranes; muscles;
nervous systems; and some have sensory organs. Cnidarians are distinguished from all other
animals by having cnidocytes that fire like harpoons and are used mainly to capture prey but also
as anchors in some species.[5]
Like sponges and ctenophores, cnidarians have two main layers of cells that sandwich a middle
layer of jelly-like material, which is called the mesoglea in cnidarians; more complex animals
have three main cell layers and no intermediate jelly-like layer. Hence, cnidarians and
ctenophores have traditionally been labelled diploblastic, along with sponges.[5][6] However, both
cnidarians and ctenophores have a type of muscle that, in more complex animals, arises from the
middle cell layer.[7] As a result some recent text books classify ctenophores as triploblastic,[8] and
it has been suggested that cnidarians evolved from triploblastic ancestors.[7]
Cnidocytes
Sponges[9][10]
Cnidarians[5][6]
Ctenophores[5][8] Bilateria[5]
No
Yes
No
Colloblasts
No
Digestive and
circulatory organs
Number of main cell
layers
Yes
No
No
Yes
Two[5] or
Three[7][8]
Two, with jelly-like layer between them
Three
Cells in each layer
bound together
No, except that
Homoscleromorpha have
basement membranes.[11]
Yes: inter-cell connections; basement membranes
Sensory organs
No
Yes
Number of cells in
middle "jelly" layer
Many
Few
(Not
applicable)
Cells in outer layers
can move inwards
and change
Yes
No
(Not
applicable)
functions
Nervous system
No
Muscles
None
[edit] Description
[edit] Basic body forms
Aboral end
Oral end
Mouth
Oral end
Aboral end
Exoderm
Gastroderm (Endoderm)
Mesoglea
Digestive cavity
Yes, simple
Mostly
epitheliomuscular
Mostly
myoepithelial
Simple to
complex
Mostly
myocytes
Medusa (left) and polyp (right)[6]
Oral end of actinodiscus polyp, with close-up of the mouth
Adult cnidarians appear as either swimming medusae or sessile polyps. Both are radially
symmetrical, like a wheel and a tube respectively. Since these animals have no heads, their ends
are described as "oral" (nearest the mouth) and "aboral" (furthest from the mouth). Most have
fringes of tentacles equipped with cnidocytes around their edges, and medusae generally have an
inner ring of tentacles around the mouth. The mesoglea of polyps is usually thin and often soft,
but that of medusae is usually thick and springy, so that it returns to its original shape after
muscles around the edge have contracted to squeeze water out, enabling medusae to swim by a
sort of jet propulsion.[6]
[edit] Colonial forms
Tree-like polyp colony[6]
Cnidaria produce a variety of colonial forms, each of which is one organism but consists of
polyp-like zooids. The simplest is a connecting tunnel that runs over the substrate (rock or
seabed) and from which single zooids sprout. In some cases the tunnels form visible webs, and in
others they are enclosed in a fleshy mat. More complex forms are also based on connecting
tunnels but produce "tree-like" groups of zooids. The "trees" may be formed either by a central
zooid that functions as a "trunk" with later zooids growing to the sides as "branches", or in a zigzag shape as a succession of zooids, each of which grows to full size and then produces a single
bud at an angle to itself. In many cases the connecting tunnels and the "stems" are covered in
periderm, a protective layer of chitin.[6] Some colonial forms have other specialized types of
zooid, for example, to pump water through their tunnels.[12]
Siphonophores form complex colonies that consist of: an upside-down polyp that forms a central
stem with a gas-filled float at the top; one or more sets of medusa-like zooids that provide
propulsion; leaf-like bracts that give some protection to other parts; sets of tentacles that bear
nematocytes that capture prey; other tentacles that act as sensors; near the base of each set of
tentacles, a polyp-like zooid that acts as a stomach for the colony; medusa-like zooids that serve
as gonads. Although some of these zooids resemble polyps or medusae in shape, they lack
features that are not relevant to their specific functions, for example the swimming "medusae"
have no digestive, sensory or reproductive cells. The best-known siphonophore is the Portuguese
Man o' War (Physalia physalis).[12][13][14]
[edit] Skeletons
In medusae the only supporting structure is the mesoglea. Hydra and most sea anemones close
their mouths when they are not feeding, and the water in the digestive cavity then acts as a
hydrostatic skeleton, rather like a water-filled balloon. Other polyps such as Tubularia use
columns of water-filled cells for support. Sea pens stiffen the mesoglea with calcium carbonate
spicules and tough fibrous proteins, rather like sponges.[6]
In some colonial polyps a chitinous periderm gives support and some protection to the
connecting sections and to the lower parts of individual polyps. Stony corals secrete massive
calcium carbonate exoskeletons. A few polyps collect materials such as sand grains and shell
fragments, which they attach to their outsides. Some colonial sea anemones stiffen the mesoglea
with sediment particles.[6]
[edit] Main cell layers
Cnidaria are diploblastic animals, in other words they have two main cell layers, while more
complex animals are triploblasts having three main layers. The two main cell layers of cnidarians
form epithelia that are mostly one cell thick, and are attached to a fibrous basement membrane,
which they secrete. They also secrete the jelly-like mesoglea that separates the layers. The layer
that faces outwards, known as the ectoderm ("outside skin"), generally contains the following
types of cells:[5]
Epitheliomuscular cells whose bodies form part of the epithelium but whose bases extend to
form muscle fibers in parallel rows.[15] The fibers of the outward-facing cell layer generally run at
right angles to the fibers of the inward-facing one. In Anthozoa (anemones, corals, etc.) and
Scyphozoa (jellyfish), the mesoglea also contains some muscle cells.[6]
Cnidocytes, the harpoon-like "nettle cells" that give the phylum Cnidaria its name. These appear
between or sometimes on top of the muscle cells.[5]
Nerve cells. Sensory cells appear between or sometimes on top of the muscle cells,[5] and
communicate via synapses (gaps across which chemical signals flow) with motor nerve cells,
which lie mostly between the bases of the muscle cells.[6]
Interstitial cells, which are unspecialized and can replace lost or damaged cells by transforming
into the appropriate types. These are found between the bases of muscle cells.[5]
In addition to epitheliomuscular, nerve and interstitial cells, the inward-facing gastroderm
("stomach skin") contains gland cells that secrete digestive enzymes. In some species it also
contains low concentrations of cnidocytes, which are used to subdue prey that is still
struggling.[5][6]
The mesoglea contains small numbers of amoeba-like cells,[6] and muscle cells in some
species.[5] However the number of middle-layer cells and types are much lower than in
sponges.[6]
[edit] Cnidocytes
A hydra's nematocyst, before firing.
"trigger" cilium[6]
Firing sequence of the cnida in a hydra's nematocyst[6]
Operculum (lid)
"Finger" that turns inside out
/ / / Barbs
Venom
Victim's skin
Victim's tissues
These "nettle cells" function as harpoons, since their payloads remain connected to the bodies of
the cells by threads. Three types of cnidocytes are known:[5][6]
Nematocysts inject venom into prey, and usually have barbs to keep them embedded in the
victims. Most species have nematocysts.[5]
Spirocysts do not penetrate the victim or inject venom, but entangle it by means of small sticky
hairs on the thread.
Ptychocysts are not used for prey capture — instead the threads of discharged ptychocysts are
used for building protective tubes in which their owners live. Ptychocysts are found only in the
order Cerianthria, tube anemones.[6]
The main components of a cnidocyte are:[5][6]
A cilium (fine hair) which projects above the surface and acts as a trigger. Spirocysts do not have
cilia.
A tough capsule, the cnida, which houses the thread, its payload and a mixture of chemicals
which may include venom or adhesives or both. ("cnida" is derived from the Greek word κνίδη,
which means "nettle"[16])
A tube-like extension of the wall of the cnida that points into the cnida, like the finger of a
rubber glove pushed inwards. When a cnidocyte fires, the finger pops out. If the cell is a
venomous nematocyte, the "finger"'s tip reveals a set of barbs that anchor it in the prey.
The thread, which is an extension of the "finger" and coils round it until the cnidocyte fires. The
thread is usually hollow and delivers chemicals from the cnida to the target.
An operculum (lid) over the end of the cnida. The lid may be a single hinged flap or three flaps
arranged like slices of pie.
The cell body which produces all the other parts.
It is difficult to study the firing mechanisms of cnidocytes as these structures are small but very
complex. At least four hypotheses have been proposed:[5]
Rapid contraction of fibers round the cnida may increase its internal pressure.
The thread may be like a coiled spring that extends rapidly when released.
In the case of Chironex (the "sea wasp"), chemical changes in the cnida's contents may cause
them to expand rapidly by polymerization.
Chemical changes in the liquid in the cnida make it a much more concentrated solution, so that
osmotic pressure forces water in very rapidly to dilute it. This mechanism has been observed in
nematocysts of the class Hydrozoa, sometimes producing pressures as high as 140 atmospheres,
similar to that of scuba air tanks, and fully extending the thread in as little as 2 milliseconds
(0.002 second).[6]
Cnidocytes can only fire once, and about 25% of a hydra's nematocysts are lost from its tentacles
when capturing a brine shrimp. Used cnidocytes have to be replaced, which takes about 48 hours.
To minimise wasteful firing, two types of stimulus are generally required to trigger cnidocytes:
their cilia detect contact, and nearby sensory cells "smell" chemicals in the water. This
combination prevents them from firing at distant or non-living objects. Groups of cnidocytes are
usually connected by nerves and, if one fires, the rest of the group requires a weaker minimum
stimulus than the cells that fire first.[5][6]
[edit] Locomotion
Chrysaora quinquecirrha ("sea nettle") swimming
Medusae swim by a form of jet propulsion: muscles, especially inside the rim of the bell, squeeze
water out of the cavity inside the bell, and the springiness of the mesoglea powers the recovery
stroke. Since the tissue layers are very thin, they provide too little power to swim against currents
and just enough to control movement within currents.[6]
Hydras and some sea anemones can move slowly over rocks and sea or stream beds by various
means: creeping like snails, crawling like inchworms, or by somersaulting. A few can swim
clumsily by waggling their bases.[6]
[edit] Nervous system and senses
Cnidaria have no brains or even central nervous systems. Instead they have decentralized nerve
nets consisting of : sensory neurons that generate signals in response to various types of stimulus,
such as odors; motor neurons that tell muscles to contract; all connected by "cobwebs" of
intermediate neurons. As well as forming the "signal cables", intermediate neurons also form
ganglia that act as local coordination centers. The cilia of the cnidocytes detect physical contact.
Nerves inform cnidocytes when odors from prey or attackers are detected and when
neighbouring cnidocytes fire. Most of the communications between nerve cells are via chemical
synapses, small gaps across which chemicals flow. As this process is too slow to ensure that the
muscles round the rim of a medusa's bell contract simultaneously in swimming the neurons
which control this communicate by much faster electrical signals across gap junctions.[6]
Medusae and complex swimming colonies such as siphonophores and chondrophores sense tilt
and acceleration by means of statocysts, chambers lined with hairs which detect the movements
of internal mineral grains called statoliths. If the body tilts in the wrong direction, the animal
rights itself by increasing the strength of the swimming movements on the side that is too low.
They also have ocelli ("little eyes"), which can detect the direction from which light is coming.
Box jellies have camera eyes, although these probably do not form images, and their lenses
simply produce a clearer indication of the direction from which light is coming.[5]
[edit] Feeding and excretion
Cnidarians feed in several ways: predation, absorbing dissolved organic chemicals, filtering food
particles out of the water, and obtaining nutrients from symbiotic algae within their cells. Most
obtain the majority of their food from predation but some, including the corals Hetroxenia and
Leptogorgia, depend almost completely on their endosymbionts and on absorbing dissolved
nutrients.[5] Cnidaria give their symbiotic algae carbon dioxide, some nutrients and a place in the
sun.[6]
Predatory species use their cnidocytes to poison or entangle prey, and those with venomous
nematocysts may start digestion by injecting digestive enzymes. The "smell" of fluids from
wounded prey makes the tentacles fold inwards and wipe the prey off into the mouth. In medusae
the tentacles round the edge of the bell are often short and most of the prey capture is done by
"oral arms", which are extensions of the edge of the mouth and are often frilled and sometimes
branched to increase their surface area. Medusae often trap prey or suspended food particles by
swimming upwards, spreading their tentacles and oral arms and then sinking. In species for
which suspended food particles are important, the tentacles and oral arms often have rows of
cilia whose beating creates currents that flow towards the mouth, and some produce nets of
mucus to trap particles.[5]
Once the food is in the digestive cavity, gland cells in the gastroderm release enzymes that
reduce the prey to slurry, usually within a few hours. This circulates through the digestive cavity
and, in colonial cnidarians, through the connecting tunnels, so that gastroderm cells can absorb
the nutrients. Absorption may take a few hours, and digestion within the cells may take a few
days. The circulation of nutrients is driven by water currents produced by cilia in the gastroderm
or by muscular movements or both, so that nutrients reach all parts of the digestive cavity.[6]
Nutrients reach the outer cell layer by diffusion or, for animals or zooids such as medusae which
have thick mesogleas, are transported by mobile cells in the mesoglea.[5]
Indigestible remains of prey are expelled through the mouth. The main waste product of cells'
internal processes is ammonia, which is removed by the external and internal water currents.[6]
[edit] Respiration
There are no respiratory organs, and both cell layers absorb oxygen from and expel carbon
dioxide into the surrounding water. When the water in the digestive cavity becomes stale it must
be replaced, and nutrients that have not been absorbed will be expelled with it. Some Anthozoa
have ciliated grooves on their tentacles, allowing them to pump water out of and into the
digestive cavity without opening the mouth. This improves respiration after feeding and allows
these animals, which use the cavity as a hydrostatic skeleton, to control the water pressure in the
cavity without expelling undigested food.[5]
Cnidaria that carry photosynthetic symbionts may have the opposite problem, an excess of
oxygen, which may prove toxic. The animals produce large quantities of antioxidants to
neutralize the excess oxygen.[5]
[edit] Regeneration
All cnidarians can regenerate, allowing them to recover from injury and to reproduce asexually.
Medusae have limited ability to regenerate, but polyps can do so from small pieces or even
collections of separated cells. This enables corals to recover even after apparently being
destroyed by predators.[5]
[edit] Reproduction
1
2
3
4
5
6
7
8
9
10
11
12
13
14
Life cycle of a jellyfish:[5][6]
1–3 Larva searches for site
4–8 Polyp grows
9–11 Polyp strobilates
12–14 Medusa grows
[edit] Sexual
In the Cnidaria sexual reproduction often involves a complex life cycle with both polyp and
medusa stages. For example in Scyphozoa (jellyfish) and Cubozoa (box jellies) a larva swims
until it finds a good site, and then becomes a polyp. This grows normally but then absorbs its
tentacles and splits horizontally into a series of disks that become juvenile medusae, a process
called strobilation. The juveniles swim off and slowly grow to maturity, while the polyp regrows and may continue strobilating periodically. The adults have gonads in the gastroderm, and
these release ova and sperm into the water in the breeding season.[5][6]
Shortened forms of this life cycle are common, for example some oceanic scyphozoans omit the
polyp stage completely, and cubozoan polyps produce only one medusa. Hydrozoa have a
variety of life cycles. Some have no polyp stages and some (e.g. hydra) have no medusae. In
some species the medusae remain attached to the polyp and are responsible for sexual
reproduction; in extreme cases these reproductive zooids may not look much like medusae.
Anthozoa have no medusa stage at all and the polyps are responsible for sexual reproduction.[5]
Spawning is generally driven by environmental factors such as changes in the water temperature,
and their release is triggered by lighting conditions such as sunrise, sunset or the phase of the
moon. Many species of Cnidaria may spawn simultaneously in the same location, so that there
are too many ova and sperm for predators to eat more than a tiny percentage — one famous
example is the Great Barrier Reef, where at least 110 corals and a few non-cnidarian
invertebrates produce enough to turn the water cloudy. These mass spawnings may produce
hybrids, some of which can settle and form polyps, but it is not known how long these can
survive. In some species the ova release chemicals that attract sperm of the same species.[5]
The fertilized eggs develop into larvae by dividing until there are enough cells to form a hollow
sphere (blastula) and then a depression forms at one end (gastrulation) and eventually become
the digestive cavity. However in cnidarians the depression forms at the end further from the yolk
(at the animal pole), while in bilaterians it forms at the other end (vegetal pole).[6] The larvae,
called planulae, swim or crawl by means of cilia.[5] They are cigar-shaped but slightly broader at
the "front" end, which is the aboral, vegetal-pole end and eventually attaches to a substrate if the
species has a polyp stage.[6]
Anthozoan larvae either have large yolks or are capable of feeding on plankton, and some
already have endosymbiotic algae that help to feed them. Since the parents are immobile, these
feeding capabilities extend the larvae's range and avoid overcrowding of sites. Scyphozoan and
hydrozoan larvae have little yolk and most lack endosymbiotic algae, and therefore have to settle
quickly and metamorphose into polyps. Instead these species rely on their medusae to extend
their ranges.[6]
[edit] Asexual
All known cnidaria can reproduce asexually by various means, in addition to regenerating after
being fragmented. Hydrozoan polyps only bud, while the medusae of some hydrozoans can
divide down the middle. Scyphozoan polyps can both bud and split down the middle. In addition
to both of these methods, Anthozoa can split horizontally just above the base.[5][6]
[edit] Classification
Cnidarians were for a long time grouped with Ctenophores in the phylum Coelenterata, but
increasing awareness of their differences caused them to be placed in separate phyla. Cnidarians
are classified into four main groups: sessile Anthozoa (sea anemones, corals, sea pens);
swimming Scyphozoa (jellyfish); Cubozoa (box jellies); and Hydrozoa, a diverse group that
includes all the freshwater cnidarians as well as many marine forms, and has both sessile
members such as Hydra and colonial swimmers such as the Portuguese Man o' War. Staurozoa
have recently been recognised as a class in their own right rather than a sub-group of Scyphozoa,
and there is debate about whether Myxozoa and Polypodiozoa are cnidarians or closer to
bilaterians.
Modern cnidarians are generally classified into four classes:[5]
Hydrozoa
Number of species[4]
Scyphozoa
Cubozoa
Anthozoa
3,600
228
42
6,100
Hydra,
siphonophores
Jellyfish
Box
jellies
Sea anemones,
corals, sea pens
Yes
Yes
Yes
Yes
Yes
Yes
Medusa phase in life
In some species
cycle
Yes, except for Stauromedusae
Yes
if they are scyphozoans
No
Number of medusae
Many
produced per polyp
Many
(not applicable)
Examples
Cells found in mesoglea No
Nematocysts in
exodermis
No
One
Stauromedusae, small sessile cnidarians with stalks and no medusa stage, have traditionally been
classified as members of the Scyphozoa, but recent research suggests they should be regarded as
a separate class, Staurozoa.[17]
The Myxozoa, microscopic parasites, were first classified as protozoans,[18] but recently as
heavily modified cnidarians, and more closely related to Hydrozoa and Scyphozoa than to
Anthozoa.[19] However other recent research suggests that Polypodium hydriforme, a parasite
within the egg cells of sturgeon, is closely related to the Myxozoa and that both Polypodium and
the Myxozoa are intermediate between cnidarians and bilaterian animals.[20]
Some researchers classify the extinct conulariids as cnidarians, while others propose that they
form a completely separate phylum.[21]
[edit] Ecology
Coral reefs support rich ecosystems
Many cnidarians are limited to shallow waters because they depend on endosymbiotic algae for
much of their nutrients. The life cycles of most have polyp stages, which are limited to locations
that offer stable substrates. Nevertheless major cnidarian groups contain species that have
escaped these limitations. Hydrozoans have a worldwide range: some, such as Hydra, live in
freshwater; Obelia appears in the coastal waters of all the oceans; and Liriope can form large
shoals near the surface in mid-ocean. Among anthozoans, a few scleractinian corals, sea pens
and sea fans live in deep, cold waters, and some sea anemones inhabit polar seabeds while others
live near hydrothermal vents over 10 kilometres (6.2 mi) below sea-level. Reef-building corals
are limited to tropical seas between 30°N and 30°S with a maximum depth of 46 metres (151 ft),
temperatures between 20°C and 28°C, high salinity and low carbon dioxide levels.
Stauromedusae, although usually classified as jellyfish, are stalked, sessile animals that live in
cool to Arctic waters.[12] Cnidarians range in size from Hydra, 5–20 millimetres (0.20–0.79 in)
long,[22] to the Lion's mane jellyfish, which may exceed 2 metres (6.6 ft) in diameter and 75
metres (246 ft) in length.[23]
Prey of cnidarians ranges from plankton to animals several times larger than themselves.[12][24]
Some cnidarians are parasites, mainly on jellyfish but a few are major pests of fish.[12] Others
obtain most of their nourishment from endosymbiotic algae or dissolved nutrients.[5] Predators of
cnidarians include: sea slugs, which can incorporate nematocysts into their own bodies for selfdefense;[25] starfish, notably the crown of thorns starfish, which can devastate corals;[12] butterfly
fish and parrot fish, which eat corals;[26] and marine turtles, which eat jellyfish.[23] Some sea
anemones and jellyfish have a symbiotic relationship with some fish; for example clown fish live
among the tentacles of sea anemones, and each partner protects the other against predators.[12]
Coral reefs form some of the world's most productive ecosystems. Common coral reef cnidarians
include both Anthozoans (hard corals, octocorals, anemones) and Hydrozoans (fire corals, lace
corals) The endosymbiotic algae of many cnidarian species are very effective primary producers,
in other words converters of inorganic chemicals into organic ones that other organisms can use,
and their coral hosts use these organic chemicals very efficiently. In addition reefs provide
complex and varied habitats that support a wide range of other organisms.[27] Fringing reefs just
below low-tide level also have a mutually beneficial relationship with mangrove forests at high-
tide level and sea grass meadows in between: the reefs protect the mangroves and seagrass from
strong currents and waves that would damage them or erode the sediments in which they are
rooted, while the mangroves and seagrass protect the coral from large influxes of silt, fresh water
and pollutants. This additional level of variety in the environment is beneficial to many types of
coral reef animals, which for example may feed in the sea grass and use the reefs for protection
or breeding.[28]
[edit] Evolutionary history
[edit] Fossil record
The fossil coral Cladocora from Pliocene rocks in Cyprus
The earliest widely accepted animal fossils are rather modern-looking cnidarians, possibly from
around 580 million years ago, although fossils from the Doushantuo Formation can only be dated
approximately.[29] The identification of some of these as embryos of animals has been contested,
but other fossils from these rocks strongly resemble tubes and other mineralized structures made
by corals.[30] Their presence implies that the cnidarian and bilaterian lineages had already
diverged.[31] Although the Ediacaran fossil Charnia used to be classified as a jellyfish or sea
pen,[32] more recent study of growth patterns in Charnia and modern cnidarians has cast doubt on
this hypothesis,[33][34] and there are now no bona-fide cnidarian body fossils in the Ediacaran.
Few fossils of cnidarians without mineralized skeletons are known from more recent rocks,
except in lagerstätten that preserved soft-bodied animals.[35]
A few mineralized fossils that resemble corals have been found in rocks from the Cambrian
period, and corals diversified in the Early Ordovician.[35] These corals, which were wiped out in
the Permian-Triassic extinction about 251 million years ago,[35] did not dominate reef
construction since sponges and algae also played a major part.[36] During the Mesozoic era rudist
bivalves were the main reef-builders, but they were wiped out in the Cretaceous-Tertiary
extinction 65 million years ago,[37] and since then the main reef-builders have been scleractinian
corals.[35]
[edit] Family tree
Further information: Phylogeny
Metazoa
Glass sponges
Demosponges
Calcareous sponges
Eumetazoa
Ctenophora (comb jellies)
Planulozoa Cnidaria
Anthozoa
(sea anemones and corals)
Medusozoa
Hydrozoa
(Hydra, siphonophores, etc.)
Cubozoa
(box jellies)
Staurozoa
"Scyphozoa"
(jellyfish, excluding
Staurozoa)
Placozoa
Bilateria
Myxozoa
Other Bilateria
(more complex)
Family tree of Cnidaria and the origins of animals[2][38][39][40]
It is difficult to reconstruct the early stages in the evolutionary "family tree" of animals using
only morphology (their shapes and structures), because the large differences between Porifera
(sponges), Cnidaria plus Ctenophora (comb jellies), Placozoa and Bilateria (all the more complex
animals) make comparisons difficult. Hence reconstructions now rely largely or entirely on
molecular phylogenetics, which groups organisms according to similarities and differences in
their biochemistry, usually in their DNA or RNA.[41]
It is now generally thought that the Calcarea (sponges with calcium carbonate spicules) are more
closely related to Cnidaria, Ctenophora (comb jellies) and Bilateria (all the more complex
animals) than they are to the other groups of sponges.[38][42][43] In 1866 it was proposed that
Cnidaria and Ctenophora were more closely related to each other than to Bilateria and formed a
group called Coelenterata ("hollow guts"), because Cnidaria and Ctenophora both rely on the
flow of water in and out of a single cavity for feeding, excretion and respiration. In 1881 it was
proposed that Ctenophora and Bilateria were more closely related to each other, since they
shared features that Cnidaria lack, for example muscles in the middle layer (mesoglea in
Ctenophora, mesoderm in Bilateria). However more recent analyses indicate that these
similarities are rather vague, and the current view, based on molecular phylogenetics, is that
Cnidaria and Bilateria are more closely related to each other than either is to Ctenophora. This
grouping of Cnidaria and Bilateria has been labelled "Planulozoa" because it suggests that the
earliest Bilateria were similar to the planula larvae of Cnidaria.[2][39]
Within the Cnidaria, the Anthozoa (sea anemones and corals) are regarded as the sister-group of
the rest, which suggests that the earliest cnidarians were sessile polyps with no medusa stage.
However it is unclear how the other groups acquired the medusa stage, since Hydrozoa form
medusae by budding from the side of the polyp while the other Medusozoa do so by splitting
them off from the tip of the polyp. The traditional grouping of Scyphozoa included the
Staurozoa, but morphology and molecular phylogenetics indicate that Staurozoa are more closely
related to Cubozoa (box jellies) than to other "Scyphozoa". Similarities in the double body walls
of Staurozoa and the extinct Conulariida suggest that they are closely related. The position of
Anthozoa nearest the beginning of the cnidarian family tree also implies that Anthozoa are the
cnidarians most closely related to Bilateria, and this is supported by the fact that Anthozoa and
Bilateria share some genes that determine the main axes of the body.[2][44]
However in 2005 Katja Seipel and Volker Schmid suggested that cnidarians and ctenophores are
simplified descendants of triploblastic animals, since ctenophores and the medusa stage of some
cnidarians have striated muscle, which in bilaterians arises from the mesoderm. They did not
commit themselves on whether bilaterians evolved from early cnidarians or from the
hypothesized triploblastic ancestors of cnidarians.[7]
In molecular phylogenetics analyses from 2005 onwards, important groups of developmental
genes show the same variety in cnidarians as in chordates.[45] In fact cnidarians, and especially
anthozoans (sea anemones and corals), retain some genes that are present in bacteria, protists,
plants and fungi but not in bilaterians.[46]
The mitochondial genomes in the medusozoan cnidarians unlike that of other animals is linear
with fragmented genes.[47] The reason for this difference is unknown.
[edit] Interaction with humans
Jellyfish stings killed about 1,500 people in the 20th century,[48] and cubozoans are particularly
dangerous. On the other hand, some large jellyfish are considered a delicacy in eastern and
southern Asia. Coral reefs have long been economically important as providers of fishing
grounds, protectors of shore buildings against currents and tides, and more recently as centers of
tourism. However, they are vulnerable to over-fishing, mining for construction materials,
pollution, and damage caused by tourism.
Beaches protected from tides and storms by coral reefs are often the best places for housing in
tropical countries. Reefs are an important food source for low-technology fishing, both on the
reefs themselves and in the adjacent seas.[49] However despite their great productivity reefs are
vulnerable to over-fishing, because much of the organic carbon they produce is exhaled as
carbon dioxide by organisms at the middle levels of the food chain and never reaches the larger
species that are of interest to fishermen.[27] Tourism centered on reefs provides much of the
income of some tropical islands, attracting photographers, divers and sports fishermen. However
human activities damage reefs in several ways: mining for construction materials; pollution,
including large influxes of fresh water from storm drains; commercial fishing, including the use
of dynamite to stun fish and the capture of young fish for aquariums; and tourist damage caused
by boat anchors and the cumulative effect of walking on the reefs.[49] Coral, mainly from the
Pacific Ocean has long been used in jewellery, and demand rose sharply in the 1980s.[50]
The dangerous "sea wasp" Chironex fleckeri
Some large jellyfish species have been used in Chinese cuisine at least since 200 AD, and are
now fished in the seas around most of South East Asia. Japan is the largest single consumer of
edible jellyfish, importing at first only from China but now from all of South East Asia as prices
rose in the 1970s. This fishing industry is restricted to daylight hours and calm conditions in two
short seasons, from March to May and August to November.[51] The commercial value of
jellyfish food products depends on the skill with which they are prepared, and "Jellyfish
Masters" guard their trade secrets carefully. Jellyfish is very low in cholesterol and sugars, but
cheap preparation can introduce undesirable amounts of heavy metals.[52]
The "sea wasp" Chironex fleckeri has been described as the world's most venomous animal and
is held responsible for 67 deaths, although it is difficult to identify the animal as it is almost
transparent. Most stingings by C. fleckeri cause only mild symptoms.[53] Seven other box jellies
can cause a set of symptoms called Irukandji syndrome,[54] which takes about 30 minutes to
develop,[55] and from a few hours to two weeks to disappear.[56] Hospital treatment is usually
required, and there have been a few deaths.[54]
[edit] Notes
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
^ Classes in Medusozoa based on "The Taxonomicon - Taxon: Subphylum Medusozoa". Universal
Taxonomic Services. http://www.taxonomy.nl/Taxonomicon/TaxonTree.aspx?id=11582. Retrieved 200901-26.
^ a b c d Collins, A.G. (2002). "Phylogeny of Medusozoa and the Evolution of Cnidarian Life Cycles" (PDF).
Journal of Evolutionary Biology 15 (3): 418–432. doi:10.1046/j.1420-9101.2002.00403.x.
http://cima.uprm.edu/~n_schizas/CMOB_8676/Collins2002.pdf. Retrieved 2008-11-27.
^ Subphyla Anthozoa and Medusozoa based on "The Taxonomicon - Taxon: Phylum Cnidaria". Universal
Taxonomic Services. http://www.taxonomy.nl/Taxonomicon/TaxonTree.aspx?id=11551. Retrieved 200707-10.
^ a b Zhang, Z.-Q. (2011). "Animal biodiversity: An introduction to higher-level classification and taxonomic
richness". Zootaxa 3148: 7–12. http://mapress.com/zootaxa/2011/f/zt03148p012.pdf.
^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af Hinde, R.T., (1998). "The Cnidaria and Ctenophora". In
Anderson, D.T.,. Invertebrate Zoology. Oxford University Press. pp. 28–57. ISBN 0195513681.
^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004).
Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 111–124. ISBN 0030259827.
^ a b c d Seipel, K., and Schmid, V. (June 2005). "Evolution of striated muscle: Jellyfish and the origin of
triploblasty". Developmental Biology 282 (1): 14–26. doi:10.1016/j.ydbio.2005.03.032. PMID 15936326.
^ a b c Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole.
pp. 182–195. ISBN 0030259827.
^ Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 76–97.
ISBN 0030259827.
^ Bergquist, P.R., (1998). "Porifera". In Anderson, D.T.,. Invertebrate Zoology. Oxford University Press.
pp. 10–27. ISBN 0195513681.
^ Exposito, J-Y., Cluzel, C., Garrone, R., and Lethias, C. (2002). "Evolution of collagens". The Anatomical
Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology 268 (3): 302–316.
doi:10.1002/ar.10162. PMID 12382326.
^ a b c d e f g Shostak, S. (2006). "Cnidaria (Coelenterates)". Encyclopedia of Life Sciences. John Wiley & Sons.
doi:10.1038/npg.els.0004117.
^ Bhamrah, H.S., and Juneja, K. (2002). A Textbook of Invertebrates. Anmol Publications. pp. 278–280.
ISBN 8126104163.
http://books.google.com/?id=05So4shx9tIC&pg=PA279&dq=siphonophore+siphonophora. Retrieved
2008-11-17.
^ Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 167–
170. ISBN 0030259827.
^ Ruppert, E.E., Fox, R.S., and Barnes, R.D. (2004). "Introduction to Metazoa". Invertebrate Zoology (7 ed.).
Brooks / Cole. pp. 103–104. ISBN 0030259827.
^ Trumble, W., and Brown, L. (2002). "Cnida". Shorter Oxford English Dictionary. Oxford University Press.
^ Collins, A.G., Cartwright, P., McFadden, C.S., and Schierwater, B. (2005). "Phylogenetic Context and
Basal Metazoan Model Systems". Integrative and Comparative Biology 45 (4): 585–594.
doi:10.1093/icb/45.4.585.
^ Štolc, A. (1899). "Actinomyxidies, nouveau groupe de Mesozoaires parent des Myxosporidies". Bull. Int.
L'Acad. Sci. Bohème 12: 1–12.
^ E. Jímenez-Guri; Philippe, H; Okamura, B; Holland, PW (July 2007). "Buddenbrockia is a cnidarian worm".
Science 317 (116): 116–118. doi:10.1126/science.1142024. PMID 17615357.
20. ^ Zrzavý, J. and Hypša, V. (2003). "Myxozoa, Polypodium, and the origin of the Bilateria: The phylogenetic
position of "Endocnidozoa" in light of the rediscovery of Buddenbrockia". Cladistics 19 (2): 164–169.
doi:10.1111/j.1096-0031.2003.tb00305.x.
21. ^ "The Conulariida". University of California Museum of Paleontology.
http://www.ucmp.berkeley.edu/cnidaria/conulariida.html. Retrieved 2008-11-27.
22. ^ Blaise, C., and Férard, J-F. (2005). Small-scale Freshwater Toxicity Investigations: Toxicity Test Methods.
Springer. p. 398. ISBN 140203119X. http://books.google.com/?id=Ibew5SLx2oMC&dq=hydra+size+length.
Retrieved 2008-11-21.
23. ^ a b Safina, C. (2007). Voyage of the Turtle: In Pursuit of the Earth's Last Dinosaur. Macmillan. p. 154.
ISBN 0805083189. http://books.google.com/?id=dQD883dAv6YC&pg=PA154&dq=cnidaria+turtle.
Retrieved 2008-11-21.
24. ^ Cowen, R. (2000). History of Life (3 ed.). Blackwell. p. 54. ISBN 0632044446.
http://books.google.com/?id=qvyBS4gwPF4C&pg=PA54&dq=cnidaria+prey. Retrieved 2008-11-21.
25. ^ Frick, K (2003). "Predator Suites and Flabellinid Nudibranch Nematocyst Complements in the Gulf of
Maine.". In: SF Norton (ed). Diving for Science...2003. Proceedings of the American Academy of
Underwater Sciences (22nd Annual Scientific Diving Symposium). http://archive.rubiconfoundation.org/4744. Retrieved 2008-07-03.
26. ^ Choat, J.H. and Bellwood, D.R. (1998). Paxton, J.R. and Eschmeyer, W.N.. ed. Encyclopedia of Fishes. San
Diego: Academic Press. pp. 209–211. ISBN 0-12-547665-5.
27. ^ a b Barnes, R.S.K., and Mann, K.H. (1991). Fundamentals of Aquatic Ecology. Blackwell Publishing.
pp. 217–227. ISBN 0632029838.
http://books.google.com/?id=mOZZlzgdTrwC&pg=PA227&dq=%22Coral+Reef%22+productivity. Retrieved
2008-11-26.
28. ^ Hatcher, B.G. Johannes, R.E., and Robertson, A.J. (1989). "Conservation of Shallow-water Marine
Ecosystems". Oceanography and Marine Biology: An Annual Review: Volume 27. Routledge. p. 320.
ISBN 0080377181.
http://books.google.com/?id=XpmNqFaDZ7cC&pg=PA320&dq=%22Coral+Reef%22+mangrove+%22seagra
ss%22. Retrieved 2008-11-21.
29. ^ Chen, J-Y.; Oliveri, P; Li, CW; Zhou, GQ; Gao, F; Hagadorn, JW; Peterson, KJ; Davidson, EH (2000).
"Putative phosphatized embryos from the Doushantuo Formation of China". Proceedings of the National
Academy of Sciences 97 (9): 4457–4462. doi:10.1073/pnas.97.9.4457. PMC 18256. PMID 10781044.
http://www.pnas.org/content/97/9/4457.full. Retrieved 2009-04-30.
30. ^ Xiao, S., Yuan, X., and Knoll, A.H. (2000). "Eumetazoan fossils in terminal Proterozoic phosphorites?".
Proceedings of the National Academy of Sciences 97 (25): 13684–13689. doi:10.1073/pnas.250491697.
PMC 17636. PMID 11095754.
http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=17636.
31. ^ Chen, J.-Y., Oliveri, P., Gao, F., Dornbos, S.Q., Li, C-W., Bottjer, D.J. and Davidson, E.H. (August 2002).
"Precambrian Animal Life: Probable Developmental and Adult Cnidarian Forms from Southwest China"
(PDF). Developmental Biology 248 (1): 182–196. doi:10.1006/dbio.2002.0714. PMID 12142030.
http://www.uwm.edu/~sdornbos/PDF's/Chen%20et%20al.%202002.pdf. Retrieved 2008-09-03.
32. ^ Donovan, Stephen K., Lewis, David N. (2001). "Fossils explained 35. The Ediacaran biota" (abstract).
Geology Today 17 (3): 115–120. doi:10.1046/j.0266-6979.2001.00285.x.
33. ^ Antcliffe, J.B.; Brasier, M. D. (2007). "Charnia and sea pens are poles apart". Journal of the Geological
Society 164 (1): 49–51. doi:10.1144/0016-76492006-080.
34. ^ Antcliffe, J.B.; Brasier, Martin D. (2007). "Charnia At 50: Developmental Models For Ediacaran Fronds".
Palaeontology 51 (1): 11–26. doi:10.1111/j.1475-4983.2007.00738.x.
35. ^ a b c d "Cnidaria: Fossil Record". University of California Museum of Paleontology.
http://www.ucmp.berkeley.edu/cnidaria/cnidariafr.html. Retrieved 2008-11-27.
36. ^ Copper, P. (January 1994). "Ancient reef ecosystem expansion and collapse". Coral Reefs 13 (1): 3–11.
Bibcode 1994CorRe..13....3C. doi:10.1007/BF00426428.
37. ^ "The Rudists". University of California Museum of Paleontology.
http://www.ucmp.berkeley.edu/taxa/inverts/mollusca/rudists.php. Retrieved 2008-11-27.
38. ^ a b Borchiellini, C., Manuel, M., Alivon, E., Boury-Esnault, N., Vacelet J., and Le Parco, Y. (2001). "Sponge
paraphyly and the origin of Metazoa". Journal of Evolutionary Biology 14 (1): 171–179.
doi:10.1046/j.1420-9101.2001.00244.x.
39. ^ a b Wallberg, A., Thollesson, M., , Farris, J.S., and Jondelius, U. (2004). "The phylogenetic position of the
comb jellies (Ctenophora) and the importance of taxonomic sampling". Cladistics 20 (6): 558–578.
doi:10.1111/j.1096-0031.2004.00041.x.
40. ^ Philippe, H. (April 2009). "Phylogenomics Revives Traditional Views on Deep Animal Relationships".
Current Biology 19: 706–712. doi:10.1016/j.cub.2009.02.052. PMID 19345102.
http://www.cell.com/current-biology/retrieve/pii/S0960982209008057. Retrieved 2011-09-25.
41. ^ Halanych, K.M. (December 2004). "The New View of Animal Phylogeny" (PDF). Annual Review of
Ecology, Evolution, and Systematics 35: 229–256. doi:10.1146/annurev.ecolsys.35.112202.130124.
http://gump.auburn.edu/halanych/lab/Pub.pdfs/Halanych2004.pdf. Retrieved 2008-11-27.
42. ^ Medina, M., Collins, A.G., Silberman, J.D., and Sogin, M.L. (August 2001). "Evaluating hypotheses of
basal animal phylogeny using complete sequences of large and small subunit rRNA". Proceedings of the
National Academy of Sciences 98 (17): 9707–9712. doi:10.1073/pnas.171316998. PMC 55517.
PMID 11504944. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=55517.
43. ^ Müller, W.E.G., Li, J., Schröder, H.C., Qiao, L., and Wang, X. (2007). "The unique skeleton of siliceous
sponges (Porifera; Hexactinellida and Demospongiae) that evolved first from the Urmetazoa during the
Proterozoic: a review". Biogeosciences 4 (2): 219–232. doi:10.5194/bg-4-219-2007.
44. ^ Marques, A.C., and Collins, A.G. (2004). "Cladistic analysis of Medusozoa and cnidarian evolution".
Invertebrate Biology 123 (1): 23–42. doi:10.1111/j.1744-7410.2004.tb00139.x.
http://www.marinespecies.org/aphia.php?p=sourceget&id=38492. Retrieved 2008-11-27.
45. ^ Miller, D.J., Ball, E.E., and Technau, U. (October 2005). "Cnidarians and ancestral genetic complexity in
the animal kingdom". Trends in Genetics 21 (10): 536–539. doi:10.1016/j.tig.2005.08.002.
PMID 16098631.
46. ^ Technau, U., Rudd, S., and Maxwell, P (December 2005). "Maintenance of ancestral complexity and nonmetazoan genes in two basal cnidarians". Trends in Genetics 21 (12): 633–639.
doi:10.1016/j.tig.2005.09.007. PMID 16226338.
47. ^ Smith DR, Kayal E, Yanagihara AA, Collins AG, Pirro S, Keeling PJ (2011) First complete mitochondrial
genome sequence from a box jellyfish reveals a highly fragmented, linear architecture and insights into
telomere evolution. Genome Biol Evol
48. ^ Williamson, J.A., Fenner, P.J., Burnett, J.W., and Rifkin, J. (1996). Venomous and Poisonous Marine
Animals: A Medical and Biological Handbook. UNSW Press. pp. 65–68. ISBN 0868402796.
http://books.google.com/?id=YsZ3GryFIzEC&pg=PA75&lpg=PA75&dq=mollusc+venom+fatal. Retrieved
2008-10-03.
49. ^ a b Clark, J.R. (1998). Coastal Seas: The Conservation Challenge. Blackwell. pp. 8–9. ISBN 0632049553.
http://books.google.com/?id=H82xdtuLxDMC&pg=PA8&dq=%22Coral+Reef%22+productivity. Retrieved
2008-11-28.
50. ^ Cronan, D.S., (1991). Marine Minerals in Exclusive Economic Zones. Springer. pp. 63–65.
ISBN 041229270X. http://books.google.com/?id=4g4nhd8USO8C&pg=PA63&dq=coral+jewellery.
Retrieved 2008-11-28.
51. ^ Omori, M. and Nakano, E. (2001). "Jellyfish fisheries in southeast Asia". In Purcell, J.E.,. Jellyfish Blooms:
Ecological and Societal Importance. Springer. pp. 19–26. ISBN 0792369645.
52. ^ Hsieh, Y-H.P. Leong, F-M., and Rudloe, J. (2001). "Jellyfish as food". In Purcell, J.E.,. Jellyfish Blooms:
Ecological and Societal Importance. Springer. pp. 11–17. ISBN 0792369645.
53. ^ Greenberg, M.I., Hendrickson, R.G., Silverberg, M., Campbell, C., and Morocco, A. (2004). "Box Jellyfish
Envenomation". Greenberg's Text-atlas of Emergency Medicine. Lippincott Williams & Wilkins. p. 875.
ISBN 0781745861.
54. ^ a b Little, M., Pereira, P., Carrette, T., and Seymour, J. (2006). "Jellyfish Responsible for Irukandji
Syndrome". QJM (Quarterly Journal of Medicine) 99 (6): 425–427. doi:10.1093/qjmed/hcl057.
PMID 16687419.
55. ^ Barnes, J. (1964). "Cause and effect in Irukandji stingings". Medical Journal of Australia 1: 897–904.
PMID 14172390.
56. ^ Grady J, Burnett J (2003). "Irukandji-like syndrome in South Florida divers". Annals of Emergency
Medicine 42 (6): 763–6. doi:10.1016/S0196-0644(03)00513-4. PMID 14634600.
[edit] Further reading
[edit] Books
Arai, M.N. (1997). A Functional Biology of Scyphozoa. London: Chapman & Hall [p. 316]. ISBN 0412-45110-7.
Ax, P. (1999). Das System der Metazoa I. Ein Lehrbuch der phylogenetischen Systematik. Gustav
Fischer, Stuttgart-Jena: Gustav Fischer. ISBN 3-437-30803-3.
Barnes, R.S.K., P. Calow, P. J. W. Olive, D. W. Golding & J. I. Spicer (2001). The invertebrates—a
synthesis. Oxford: Blackwell. 3rd edition [chapter 3.4.2, p. 54]. ISBN 0-632-04761-5.
Brusca, R.C., G.J. Brusca (2003). Invertebrates. Sunderland, Mass.: Sinauer Associates. 2nd
edition [chapter 8, p. 219]. ISBN 0-87893-097-3.
Dalby, A. (2003). Food in the Ancient World: from A to Z. London: Routledge.
Moore, J.(2001). An Introduction to the Invertebrates. Cambridge: Cambridge University Press
[chapter 4, p. 30]. ISBN 0-521-77914-6.
Schäfer, W. (1997). Cnidaria, Nesseltiere. In Rieger, W. (ed.) Spezielle Zoologie. Teil 1. Einzeller
und Wirbellose Tiere. Stuttgart-Jena: Gustav Fischer. Spektrum Akademischer Verl., Heidelberg,
2004. ISBN 3-8274-1482-2.
Werner, B. 4. Stamm Cnidaria. In: V. Gruner (ed.) Lehrbuch der speziellen Zoologie. Begr. von
Kaestner. 2 Bde. Stuttgart-Jena: Gustav Fischer, Stuttgart-Jena. 1954, 1980, 1984, Spektrum
Akad. Verl., Heidelberg-Berlin, 1993. 5th edition. ISBN 3-334-60474-8.
[edit] Journal articles
D. Bridge, B. Schierwater, C. W. Cunningham, R. DeSalle R, L. W. Buss: Mitochondrial DNA
structure and the molecular phylogeny of recent cnidaria classes. in: Proceedings of the Academy
of Natural Sciences of Philadelphia. Philadelphia USA 89.1992, p. 8750. ISSN 0097-3157
D. Bridge, C. W. Cunningham, R. DeSalle, L. W. Buss: Class-level relationships in the phylum
Cnidaria—Molecular and morphological evidence. in: Molecular biology and evolution. Oxford
University Press, Oxford 12.1995, p. 679. ISSN 0737-4038
D. G. Fautin: Reproduction of Cnidaria. in: Canadian Journal of Zoology. Ottawa Ont. 80.2002,
p. 1735. (PDF, online) ISSN 0008-4301
G. O. Mackie: What's new in cnidarian biology? in: Canadian Journal of Zoology. Ottawa Ont.
80.2002, p. 1649. (PDF, online) ISSN 0008-4301
P. Schuchert: Phylogenetic analysis of the Cnidaria. in: Zeitschrift für zoologische Systematik und
Evolutionsforschung. Paray, Hamburg-Berlin 31.1993, p. 161. ISSN 0044-3808
G. Kass-Simon, A. A. Scappaticci Jr.: The behavioral and developmental physiology of
nematocysts. in: Canadian Journal of Zoology. Ottawa Ont. 80.2002, p. 1772. (PDF, online)
ISSN 0044-3808
J. Zrzavý (2001). "The interrelationships of metazoan parasites: a review of phylum- and higherlevel hypotheses from recent morphological and molecular phylogenetic analyses" (PDF). Folia
Parasitologica 48 (2): 81–103. PMID 11437135. Archived from the original on 2007-10-25.
http://web.archive.org/web/20071025220832/http://www.paru.cas.cz/folia/pdf/2-01/Zrz.pdf.
Retrieved 2009-01-26.
[edit] External links
Wikispecies has information related to: Cnidaria
The Wikibook Dichotomous Key has a page on the topic of
Cnidaria
Wikimedia Commons has media related to: Cnidaria
Look up Cnidaria in Wiktionary, the free dictionary.
YouTube: Nematocysts Firing
YouTube:My Anemone Eat Meat Defensive and feeding behaviour of sea anemone
Cnidaria - Guide to the Marine Zooplankton of south eastern Australia, Tasmanian Aquaculture
& Fisheries Institute
A Cnidaria homepage maintained by University of California, Irvine
Cnidaria page at Tree of Life
Fossil Gallery: Cnidarians
The Hydrozoa Directory
Hexacorallians of the World
[hide]
v
t
e
Eukaryota
Domain : Archaea · Bacteria · Eukaryota
Bikonta
Archaeplastida, or Plantae sensu lato
Viridiplantae/Plantae sensu stricto ·
Rhodophyta · Glaucocystophyceae
Hacrobia, or non-SAR chromalveolata
Haptophyta · Cryptophyta ·
Centroheliozoa
AH/SAR AH
Heterokont ("S") Ochrophyta · Bigyra · Pseudofungi
Halvaria
SAR
Alveolata Ciliates · Myzozoa (Apicomplexa, Dinoflagellata)
Rhizaria Cercozoa · Retaria (Foraminifera, Radiolaria)
Excavata Discoba (Euglenozoa, Percolozoa) · Metamonad · Malawimonas
Apusozoa
Apusomonadida (Apusomonas, Amastigomonas) · Ancyromonadida
(Ancyromonas) · Hemimastigida (Hemimastix, Spironema, Stereonema)
Amoebozoa Lobosea · Conosa · Phalansterium · Breviata
Mesomycetozoea Dermocystida · Ichthyophonida
Filasterea Capsaspora · Ministeria
Choanoflagellate Codonosigidae
Holozoa
Filozoa
Unikonta
Opisthokonta
Holomycota
Eumetazoa (Bilateria,
Metazoa Cnidaria, Ctenophora) ·
or "Animalia" Mesozoa · Parazoa
(Placozoa, Porifera)
Dikarya (Ascomycota, Basidiomycota) ·
Glomeromycota · Zygomycota ·
Fungi Blastocladiomycota ·
Chytridiomycota/Neocallimastigomycota ·
Microsporidia
Nucleariidae
Nuclearia · Micronuclearia · Rabdiophrys ·
Pinaciophora · Pompholyxophrys · Fonticula
[show]
v
t
e
Extant phyla of kingdom Animalia by subkingdom
o
Radiata
Ctenophora
Cnidaria
o Anthozoa
o Hydrozoa
o Scyphozoa
o Cubozoa
o Staurozoa
o Myxozoa
o Polypodiozoa
Scalidophor
a
Kinorhyncha
Loricifera
Priapulida
Nematoida
Nematoda
Nematomorpha
Cycloneuralia
Ecdysozoa
Panarthropod
a
Onychophora
Tardigrada
Arthropoda
Lobopodia
Bilateria Protostomia
Platyhelminthes
Gastrotricha
Platyzoa
Lophotrochoz
oa
Gnathife
ra
Spiralia
Trochoz
oa
Lophophora
Rotifera
Acanthocephala
Gnathostomulid
a
Micrognathozoa
Cycliophora
Sipuncula
Nemertea
Mollusca
Annelida
Phoronida
Brachiopoda
ta
Ambulacraria
Deuterosto
mia
Basal/disput
ed
Bryozoa (?)
Entoprocta (?)
Hemichordata
Echinodermata
Xenoturbellida
Chordata
o Craniata
Vertebrata
Myxini
o Cephalochordata
o Tunicata
Acoelomorpha
o Acoela
o Nemertodermatida
Chaetognatha
Retrieved from "http://en.wikipedia.org/w/index.php?title=Cnidaria&oldid=479630448"
View page ratings
Rate this page
What's this?
Trustworthy
Objective
Complete
Well-written
I am highly knowledgeable about this topic (optional)
Submit ratings
Saved successfully
Your ratings have not been submitted yet
Categories:
Venomous animals
Cnidarians
Hidden categories:
Good articles
Articles with 'species' microformats
Personal tools
Log in / create account
Namespaces
Article
Talk
Variants
Views
Read
Edit
View history
Actions
Search
Search
Special:Search
Navigation
Main page
Contents
Featured content
Current events
Random article
Donate to Wikipedia
Interaction
Help
About Wikipedia
Community portal
Recent changes
Contact Wikipedia
Toolbox
What links here
Related changes
Upload file
Special pages
Permanent link
Cite this page
Rate this page
Print/export
Create a book
Download as PDF
Printable version
Languages
ال عرب ية
Armãneashce
Azərbaycanca
Български
Bosanski
Brezhoneg
Català
Česky
Cymraeg
Dansk
Deutsch
Eesti
Español
Esperanto
Euskara
ف ار سی
Français
Gaelg
Galego
한국어
हिन्दी
Hrvatski
Ido
Bahasa Indonesia
Íslenska
Italiano
עברית
Basa Jawa
Latina
Latviešu
Lëtzebuergesch
Lietuvių
Magyar
Македонски
Nederlands
日本語
Norsk (bokmål)
Norsk (nynorsk)
Occitan
Plattdüütsch
Polski
Português
Runa Simi
Русский
Sicilianu
Simple English
Slovenčina
Slovenščina
Српски / Srpski
Srpskohrvatski / Српскохрватски
Suomi
Svenska
Tagalog
తెలుగు
ไทย
Türkçe
Українська
Zazaki
Zeêuws
中文
This page was last modified on 1 March 2012 at 10:48.
Text is available under the Creative Commons Attribution-ShareAlike License; additional terms
may apply. See Terms of use for details.
Wikipedia® is a registered trademark of the Wikimedia Foundation, Inc., a non-profit
organization.
Contact us
From Wikipedia, the free encyclopedia
(Redirected from Ediacarian)
Jump to: navigation, search
Ediacaran Period
630–542 million years ago
PreЄ
Є
O
S
D
C
P
T
J
K
Pg
N
Mean atmospheric O2 content over
period duration
ca. 8 Vol %[1]
(40 % of modern
level)
ca. 4500 ppm[2]
Mean atmospheric CO2 content over (16 times preindustrial level)
period duration
Events of the Ediacaran Period
view • discuss • edit
-640 —
–
-630 —
–
-620 —
–
-610 —
–
-600 —
–
-590 —
–
-580 —
–
-570 —
–
-560 —
–
-550 —
–
-540 —
–
-530 —
–
-520 —
–
-510 —
–
-500 —
Cryogenian
Ediacaran
Cambrian
←
Last Ediacaran communities
←
Last putative Ediacaran
←
Embryos?
←
Last extensive glaciation
←
First Ediacaran megafossil
←
Gaskiers
Glaciation
Aspidella
discs
The Ediacaran Period /ˌiːdiˈækərən/,
named after the Ediacara Hills of South
Australia, is the last geological period of the
Neoproterozoic Era and of the Proterozoic
Eon, immediately preceding the Cambrian
Period, the first period of the Paleozoic Era
and of the Phanerozoic Eon. Its status as an
official geological period was ratified in
2004 by the International Union of
Geological Sciences (IUGS), making it the
first new geological period declared in 120
years.[5][6][7]
Rangeomorphs
Neoproterozoic
(last era of the Precambrian)
Palæozoic
(first era of the Phanerozoic)
Axis scale: millions of years ago.
References: Waggoner 1998,[3] Hofmann 1990[4]
Although the Period takes its name from the Ediacara Hills where geologist Reg Sprigg first
discovered fossils of the eponymous biota in 1946, the type section is located in the bed of the
Enorama Creek[8] within Brachina Gorge[9] in the Flinders Ranges of South Australia, at
31°19′53.8″S 138°38′0.1″E31.331611°S 138.633361°E.
Contents
[hide]
1 Ediacaran and Vendian
2 Borders of the Ediacaran
3 Dating
4 Biota
5 See also
6 References
7 External links
[edit] Ediacaran and Vendian
The Ediacaran Period overlaps, but is shorter than the Vendian Period, a name that was earlier,
in 1952, proposed by Russian geologist and paleontologist Boris Sokolov. The Vendian concept
was formed stratigraphically top-down, and the lower boundary of the Cambrian became the
upper boundary of the Vendian.[10][11] Paleontological substantiation of this boundary was
worked out separately for the siliciclastic basin (base of the Baltic Stage of the Eastern European
Platform[12]) and for the carbonate basin (base of the Tommotian Stage of the Siberian
Platform).[13] The lower boundary of the Vendian was suggested to be defined at the base of the
Varanger (Laplandian) tillites.[11][14]
The Vendian in its type area consists of large subdivisions such as Laplandian, Redkino, Kotlin
and Rovno Regional stages with the globally traceable subdivisions and their boundaries,
including its lower one. The Redkino, Kotlin and Rovno regional stages have been substantiated
in the type area of the Vendian on the basis of the abundant organic-walled microfossils,
megascopic algae, metazoan body fossils and ichnofossils.[11][15] The lower boundary of the
Vendian could have a biostratigraphic substantiation as well taking into consideration the
worldwide occurrence of the Pertatataka assemblage of giant acanthomorph acritarchs.[14]
[edit] Borders of the Ediacaran
The 'golden spike' (bronze disk in the lower section of the image) or 'type section' of the Global
Boundary Stratotype Section and Point (GSSP) for the base of Ediacaran period. To read the disk, click
through to the full image.
The Ediacaran Period (ca. 635-542 Mya) represents the time from the end of global Marinoan
glaciation to the first appearance worldwide of somewhat complicated trace fossils (Treptichnus
pedum).[5]
Although the Ediacaran Period does contain soft-bodied fossils, it is unusual in comparison to
later periods because its beginning is not defined by a change in the fossil record. Rather, the
beginning is defined at the base of a chemically distinctive carbonate layer (this bed is
characterized by an unusual depletion of 13C), referred to as a "cap carbonate", because it caps
glacial deposits and indicates a sudden climatic change at the end of the Marinoan ice age. The
lower boundary GSSP of the Ediacaran is at the base of the cap carbonate (Nuccaleena
Formation), immediately above the Elatina diamictite in the Enorama Creek section, Brachina
Gorge, Flinders Ranges, South Australia.
The GSSP of the upper boundary of the Ediacaran is the lower boundary of the Cambrian on the
SE coast of Newfoundland approved by the International Commission on Stratigraphy as a
preferred alternative to the base of the Tommotian Stage in Siberia which was selected on the
basis of the ichnofossils Treptichnus pedum. In the history of stratigraphy it was the first case of
usage of bioturbations for the System boundary definition.
Nevertheless, the definitions of the lower and upper boundaries of the Ediacaran on the basis of
chemostratigraphy and ichnofossils are disputable.[14][16]
Cap carbonates generally have a restricted geographic distribution (due to specific conditions of
their precipitation) and usually siliciclastic sediments replace laterally the cap carbonates in a
rather short distance and cap carbonates do not occur above every tillite elsewhere in the world.
The C-isotope chemostratigraphic characteristics obtained for contemporaneous cap carbonates
in different parts of the world may be variable in a wide range owing to different degrees of
secondary alteration of carbonates, dissimilar criteria used for selection of the least altered
samples, and, as far as the C-isotope data are concerned, due to primary lateral variations of δ
l3
Ccarb in the upper layer of the ocean.[14][17] Furthermore, Oman presents in its stratigraphic
record a large negative carbon isotope excursion, within the Shuram[18] Formation that is clearly
away from any glacial evidence[19] strongly questioning systematic association of negative δ
l3
Ccarb excursion and glacial events.[20] As to the Treptichnus pedum, a reference ichnofossil for
the lower boundary of the Cambrian, its usage for the stratigraphic detection of this boundary is
always risky because of the occurrence of very similar trace fossils belonging to the Treptichnids
group well below the T. pedum in Namibia, Spain and Newfoundland, and possibly, in the
western United States. The stratigraphic range of T. pedum overlaps the range of the Ediacaran
fossils in Namibia, and probably in Spain.[14][21]
[edit] Dating
No dating has been possible at the type section of the Ediacaran Period in South Australia.
Therefore the age range of 635 to 542 million years before the present is based on correlations to
other countries where dating has been possible. The base age of approximately 635 million years
ago is based on U-Pb (uranium-lead) isochron dating from Namibia[22] and China.[23] Applying
this age to the base of the Ediacaran assumes that individual cap carbonates are synchronous
around the world and that the correct cap carbonate layers have been correlated between
Australian and Namibia. This is controversial because an age of about 580 million years has been
obtained in association with glacial rocks in Tasmania which some scientists tentatively correlate
with those just beneath the Ediacaran rocks of the Flinders Ranges.[24] The age of the top is the
same as the widely recognised age for the base of the Cambrian Period[25] 542± 0.3 Mya (million
years ago).[26]
[edit] Biota
Main article: Ediacara biota
The animal fossil record from this period is sparse, possibly because animals had yet to evolve
hard shells, which make for easier fossilization. The Ediacaran biota include the oldest definite
multicellular organisms with tissues, and the most common types resemble segmented worms,
fronds, disks, or immobile bags. They bear little resemblance to modern lifeforms, and their
relationship even with the later lifeforms of the Cambrian explosion is difficult to interpret. More
than 100 genera have been described, and well known forms include Arkarua, Charnia,
Dickinsonia, Ediacaria, Marywadea, Onega, Pteridinium, and Yorgia.
In addition, there are unverified claims of "footprints" - tracks made by legged organisms
thought to resemble arthropods or legged worms. The significance of these finds awaits their
publication.[27]
[edit] See also
List of fossil sites (with link directory)
[edit] References
1. ^ Image:Sauerstoffgehalt-1000mj.svg
2. ^ Image:Phanerozoic Carbon Dioxide.png
3. ^ Waggoner, Ben (1998). "Interpreting the Earliest Metazoan Fossils: What Can We Learn?".
Integrative and Comparative Biology 38 (6): 975–982. doi:10.1093/icb/38.6.975. ISSN 15407063. http://intl-icb.oxfordjournals.org/cgi/content/abstract/38/6/975. Retrieved 2007-03-08.
4. ^ Hofmann, H.J.; Narbonne, G.M., Aitken, J.D. (1990). "Ediacaran remains from intertillite beds
in northwestern Canada". Geology 18 (12): 1199–1202. doi:10.1130/00917613(1990)018<1199:ERFIBI>2.3.CO;2.
http://geology.geoscienceworld.org/cgi/content/abstract/18/12/1199.
5. ^ a b A. Knoll, M. Walter, G. Narbonne, and N. Christie-Blick (2004) "The Ediacaran Period: A New
Addition to the Geologic Time Scale." Submitted on Behalf of the Terminal Proterozoic
Subcommission of the International Commission on Stratigraphy.
6. ^ Knoll, A. H.; Walter, MR; Narbonne, G. M; Christie-Blick, N (2004). "A new period for the
geologic time scale". Science 305 (5684): 621–622. doi:10.1126/science.1098803.
PMID 15286353. http://www.stratigraphy.org/bak/ediacaran/Knoll_et_al_2004b.pdf.
7. ^ Knoll, A. H., Walter, M. R., Narbonne, G. M., and Christie-Blick, N. (2006). "The Ediacaran
Period: A new addition to the geologic time scale". Lethaia 39: 13–30.
doi:10.1080/00241160500409223.
http://geol.queensu.ca/people/narbonne/KnollWalterNarbonneChristieBlick_Lethaia_2006.pdf.
8. ^ "Geological time gets a new period: Geologists have added a new period to their official
calendar of Earth's history—the first in 120 years". London: BBC. 2004-05-17.
http://news.bbc.co.uk/1/hi/sci/tech/3721481.stm. Accessed 27 December 2010.
9. ^ South Australian Museum Newsletter April 2005 Accessed 9 August 2010.
10. ^ B. M. Sokolov (1952). "On the age of the old sedimentary cover of the Russian Platform".
Izvestiya Akademii Nauk SSSR, Seriya eologicheskaya 5: 21–31.
11. ^ a b c Sokolov, B.S. (1997). "Essays on the Advent of the Vendian System." 153 pp. KMK Scientific
Press, Moscow. (in Russian)
12. ^ Sokolov B. S. (1965) "Abstracts of All-Union Symposium on Paleontology of the Precambrian
and Early Cambrian." Nauka, Novosibirsk.
13. ^ Rozanov, A.Y., Missarzhevskij, V.V., Volkova, N.A., Voronova, L.G., Krylov, I.N., Keller, B.M.,
Korolyuk, I.K., Lendzion, K., Michniak, R., Pykhova, N.G., and Sidorov, A.D. (1969). "The
Tommotian Stage and the problem of the lower boundary of the Cambrian". Trudy
Geologičeskogo Instituta AN SSSR 206: 1–380.
14. ^ a b c d e M. A. Fedonkin, B. S. Sokolov, M. A. Semikhatov, N. M. Chumakov (2007). "Vendian
versus Ediacaran: priorities, contents, prospectives".
http://vendian.net76.net/Vendian_vs_Ediacaran.htm. In: "The Rise and Fall of the Vendian
(Ediacaran) Biota". Origin of the Modern Biosphere. Transactions
Com plet e
Animals Eukaryotes Life on Earth
Bilateria Myxozoa Ctenophora Placozoa Porifera
Hydrozoa Anthozoa Scyphozoa
Cnidaria
Sea anemones, corals, jellyfish, sea pens, hydra
Daphne G. Fautin and Sandra L. Romano
close boxTree following Werner (1973) and Bridge et al. (1995).
Containing group: Animals
Introduction
The exclusively aquatic phylum Cnidaria is represented by polyps such as
sea anemones and corals, and by medusae such as jellyfish. A polypoid or a
medusoid cnidarian is a radially or biradially symmetrical, uncephalized
animal with a single body opening, the mouth. The mouth is surrounded by
tentacles studded with microscopic stinging capsules known as nematocysts
that are the agents of offense and defense. The possession of intrinsic
nematocysts is the defining characteristic of the phylum (Hessinger and
Lenhoff 1988); nematocysts are the most diverse and widespread of three
types of cnidae (cnidos = thread) -- hence the preferred name of the
phylum.
Cnidarians are diploblastic -- that is, the body and tentacles consist of two
cell layers, the endoderm (sometimes referred to as the gastrodermis) and
the ectoderm (the epidermis). Between the two cell layers is the mesoglea,
which ranges from little more than a glue to bind the layers (for example, in
Hydra) to the vast bulk of the animal (for example, in jellyfish of Class
Scyphozoa). The body encompasses a single sac-like body space, the
coelenteron (koilos = cavity; enteron = intestine), which communicates with
the surrounding medium through the mouth. The less preferred name of the
phylum, Coelenterata, is based on this attribute. The coelenteron (also
termed the gastrovascular cavity) serves for gas exchange and digestion.
All cnidarians are carnivorous, with cnidae and tentacles active in prey
capture. Because polyps are typically sessile, and only some medusae
possess sensory structures (the most sophisticated occur in the Cubozoa;
Pearse and Pearse 1978), cnidarians are generally believed to be passive
predators, feeding on prey items that blunder into their tentacles. Some
cnidarians can absorb dissolved organic matter directly from seawater (e.g.
Schlichter 1975), but it is not known how widespread this ability is. Living
within the tissues of anthozoans of many species and hydrozoans and
scyphozoans of a small number of species are unicellular algae from which
the animals derive reduced carbon (Shick 1991). Dinoflagellate symbionts,
termed zooxanthellae, are by far the most common algal symbionts; they
are exclusively marine. Green algal symbionts, termed zoochlorellae, occur
in both marine and freshwater cnidarians.
The text-book depiction of the typical cnidarian life cycle is an alternation
between a medusa and a polyp (termed metagenesis), the former the
sexually reproductive stage and the latter the asexual stage. In fact, an
attribute of the entire class Anthozoa is the absence of a medusa. At least
some individuals of all anthozoan species form gametes; those of some
species may reproduce vegetatively as well. The other three classes --
Cubozoa, Hydrozoa, and Scyphozoa -- are often grouped as the "Medusozoa"
because the medusa phase is present in them all. Indeed, the medusa
dominates the life cycle of members of the classes Cubozoa and Scyphozoa
(Cubozoa was formerly considered an order of Scyphozoa, and some
specialists still consider it as such). Life cycles of the Hydrozoa are the most
diverse in the phylum: although the polyp is the more conspicuous and
persistent stage in most taxa, some lack the medusa phase, whereas others
lack the polyp phase. Hydra, which is used in many textbooks to illustrate
the phylum, is utterly atypical: a hydrozoan, it lacks a medusa, it has
aggregations of gametogenic tissue that function as gonads, and it is among
only a handful of freshwater cnidarian species.
The cnidarian larva is the planula, a pear-shaped, entirely ciliated animal. In
the "typical" cnidarian life cycle, male and female medusae spawn freely into
the sea, where fertilization occurs and a planula develops. At
metamorphosis, the planula settles on and attaches to the substratum,
where it metamorphoses into a polyp. The primary polyp produces additional
polyps asexually, by budding, stolonic outgrowth, or some other process, to
form a clone or a colony. At the appropriate time, determined perhaps by
size of the colony or environmental conditions, rather than or in addition to
polyps, medusae are produced asexually (in Cubozoa, each polyp
metamorphoses into a medusa). They are released to take up a pelagic
existence and the cycle begins anew.
Idealized lifecycle of the Cnidaria.
Characteristics
The cnida, or nematocyst, which is the sine qua non of the phylum, is
secreted by the Golgi apparatus of a cell termed a cnidoblast (Watson 1988).
A cnida therefore is technically not an organelle, but, rather, the most
complex secretory product known. Upon receiving the appropriate physical
and/or chemical stimulus, a cnida fires, everting a tubule many times the
length of the capsule. The tubule may deliver a toxin, may stick to a prey
item, or may entangle an object, depending on the type of cnida. A cnida
can fire but once. There are three major types of cnidae: nematocysts,
spirocysts, and ptychocysts. Nematocysts occur in all classes of Cnidaria, but
some of the 30-plus varieties of nematocysts are restricted to members of
certain classes (Fautin and Mariscal 1991). Spirocysts are found only in
Anthozoa; they are adhesive in nature. Ptychocysts are the most
taxonomically restricted in distribution, occurring only in the anthozoan
order Ceriantharia; their function is to entangle bits of mud among their
robust tubules to form the feltwork that constitutes the tube of these
burrowing animals.
Left: Fired "basitrich" (basitrichous isorhiza) from a sea anemone. The now empty capsule is in the lower right of
the photo; the spiny basal part of the fired tubule extends to the upper left; beyond the frame of the photo is the
non-spiny, distal part of the tubule, which is many times longer than the capsule. Middle: "Holotrich"
(holotrichous isorhiza) from a corallimorpharian. Right: Unfired "basitrichs" (basitrichous isorhizas) from a sea
anemone. The longitudinal line inside each capsule is the spiny basal part of the unfired tubule.
Two body forms are characteristic of cnidarians -- the polyp and the
medusa. With a few exceptions, a columnar polyp is sedentary, being
attached to or burrowed into the substratum by the end opposite the mouth.
Thus its tentacles are typically considered to point upward and outward.
Polyps of some species propagate vegetatively, forming colonies (if the
progeny remain attached to one another) or clones (if the progeny
separate). Polymorphism occurs in colonies of some species of hydrozoans
and anthozoans, the polyps being specialized for functions such as feeding,
defense, and sexual reproduction. Polyps of some taxa form a skeleton
within or external to their tissues; some skeletons are mineralic (of calcium
carbonate), others are organic (of chitin or another carbohydrate), and some
are both. The spheroidal or discoidal medusae are solitary, and those of
most species are pelagic. Although typically depicted as living with mouth
and tentacles pointing down, medusae assume all orientations in the water.
Medusae of few species possess the ability to propagate vegetatively. The
common name of medusae, jellyfish, alludes to the massive amount of
mesoglea that contributes to their buoyancy.
All cnidarians have hydrostatic skeletons, regardless of whether they also
have mineralic and/or organic exoskeletons or endoskeletons. The muscles
of the body wall operate against the fluid in the coelenteron to extend
individual polyps and to effect the swimming of medusae, for example. The
hollow tentacles of anthozoans are extended through hydrostatic action as
well.
Discussion of Phylogenetic Relationships
Cnidaria is thought to have one of the longest fossil histories of metazoan
phlya with representatives in the Ediacaran fauna of the late Precambrian
(Scrutton 1979). These earliest fossils are both medusoid and polypoid, and
thought to represent all cnidarian classes (Scrutton 1979).
The four extant cnidarian classes are identifiable as early as the Ordovician
(Robson 1985), but evolutionary relationships among them have been the
subject of much debate (e.g. Brooks 1886, Hyman 1940, Jagersten 1955,
Hand 1959, Pantin 1960, Werner 1973, Petersen 1979, Barnes 1987, Ax
1989). Anthozoa is alternatively considered the most basal or the most
derived group. The former hypothesis posits that the polyp is the original
body form, with the medusa (and metagenesis) being derived (Fig. 1A). The
latter perspective is that, in the "typical" life cycle, the medusa is
gametogenic, and so constitutes the definitive, or adult, stage, with the
polyp being a persistent larva. Thus, it is reasoned, the polyp evolved
secondarily, and loss of the original body form, the medusa, places Anthozoa
as the most derived taxon (Fig. 1B). A comprehensive morphological
cladistic analysis by Schuchert (1993) supports the basal position of
Anthozoa with the Scyphozoa and Cubozoa being more closely related to
each other than to Hydrozoa. Morphological, mtDNA, and 18S rDNA data
separately and together also support the basal position of Anthozoa but do
not resolve the relationships among Scyphozoa, Cubozoa and Hydrozoa
(Bridge et al. 1995). The phylogenetic tree at the beginning of this page is
that of Bridge et al. (1995). Neither of these treatments attempts to include
the extinct class Conulata, which has been considered by most
paleontologists to be related to the Scyphozoa.
Alternative views of cnidarian life-cycle evolution and systematic relationships. (After Bridge et al. 1995.)
Their diploblastic structure and their single body opening and cavity had
been thought to ally cnidarians with ctenophores. Indeed, until relatively
recently the phylum Coelenterata was considered to include animals now
placed in Cnidaria and Ctenophora. However, ctenophores lack a
metagenetic life cycle and cnidae. Cnidae have been found in one
ctenophore, but it is now known that the ctenophore acquires those cnidae
from the hydromedusae upon which it preys (Mills and Miller 1984). Thus, it
is generally agreed that the similarity in body form between pelagic
ctenophores and pelagic cnidarians is convergent; benthic ctenophores do
not resemble cnidarians at all. Cnidaria, therefore, is a well circumscribed
taxon; it is considered by many to be a sister group of all metazoans other
than sponges.
References
Ax, P. 1989. Basic phylogenetic systematization of Metazoa. Pp. 453-470 in K. B. B. Fernholm and H. Jornvall (eds.). The
Hierarchy of Life. Elsevier, Amsterdam.
Bridge, D., C. W. Cunningham, R. DeSalle, and L. W. Buss. 1995. Class-level relationships in the phylum Cnidaria: Molecular
and morphological evidence. Molec. Biol. Evol. 12:679-689.
Bridge, D., C. W. Cunningham, B. Schierwater, R. DeSalle, and L. W.
Buss. 1992. Class-level relationships in the phylum Cnidaria: Evidence from mitochondrial genome structure. Proc. Nat. Acad.
Sci. USA 89:8750-8753.
Brusca, C. B. and G. J. Brusca. 1990. Invertebrates. Sinauer Associates, Sunderland MA.
Dunn, D. F. 1982. Cnidaria. Pp. 669-705 in S. P. Parker (ed.) Synopsis and Classification of Living organisms. McGraw-Hill,
New York.
Fautin, D. G. and R. N. Mariscal. 1991. Cnidaria: Anthozoa. Pp. 267-358 in F. W. Harrison and J. A. Westfall (eds.) Microscopic
Anatomy of Invertebrates, volume 2: Placozoa, Porifera, Cnidaria, and Ctenophora. Wiley-Liss, New York and other cities.
Hand, C. 1959. On the origin and phylogeny of the Coelenterata. Syst. Zool. 8:192-202.
Hessinger, D. A. and H. M. Lenhoff. 1988. Preface. Pp. xi-xii in D. A. Hessinger and H. M. Lenhoff (eds.) The Biology of
Nematocysts. Academic Press, San Diego and other cities.
Hill, D. and J. W. Wells. 1956. Cnidaria -- general features. Pp. F5-F9 in R. C. Moore (ed.) Treatise on Invertebrate
Paleontology: Part F, Coelenterata. Geological Society of America and University of Kansas Press.
Hyman, L. H. 1940. The Invertebrates: Protozoa through Ctenophora. McGraw-Hill, New York. 726 pp.
Hyman, L. H. 1956. Morphology of living coelenterates. Pp. F10-F20 in R. C. Moore (ed.) Treatise on Invertebrate
Paleontology: Part F, Coelenterata. Geological Society of America and University of Kansas Press.
Jagersten, G. 1955. On the early phylogeny of the Metazoa: the bilaterogastraea theory. Zool. Bidr. Uppsala 30: 321-354.
Mackie, G. O. (ed.). 1976. Coelenterate Ecology and Behavior (Selected papers from the Third International Symposium on
Coelenterate Biology, held at the University of Victoria, Victoria, British Columbia, May 10-13, 1976). Plenum Press, New
York and London. 744 pp.
Meglitsch, P. A. and F. R. Schram. 1991. Invertebrate Zoology (3rd ed.). Oxford University Press, New York and Oxford. 623
pp.
Mills, C. E. and R. L. Miller. 1984. Ingestion of a medusa (Aegina citrea) by the nematocyst-containing ctenophore Haeckelia
rubra (formerly Euchlora rubra): phylogenetic implications. Mar. Biol. 78:215-221.
Muscatine, L. and H. M. Lenhoff (eds.). 1974. Coelenterate Biology: Reviews and New Perspectives. Academic Press, New York
and other cities. 501 pp.
Nielsen, C. 1995. Animal Evolution: Interrelationships of the Living Phyla. Oxford University Press, Oxford. 467 pp.
Pantin, C. 1960. Diploblastic animals. Proc. Linn. Soc.171:1-14.
Pearse, J. S. and V. B. Pearse. 1978. Vision in cubomedusan jellyfishes. Science 199: 458.
Pearse, V., J. Pearse, M. Buchsbaum, and R. Buchsbaum. 1986. Living Invertebrates. Blackwell Scientific Publications, Palo
Alto, CA. 848 pp.
Petersen, K. W. 1979. Development of coloniality in Hydrozoa. Pp. 105-139 in G. Larwood and B. R. Rosen (eds.). Biology and
Systematics of Colonial Organisms. Academic Press, New York.
Rees, W. J. (ed.). 1966. The Cnidaria and their Evolution (The Proceedings of a Symposium held at The Zoological Society of
London on 3 and 4 March 1965). Zoological Society of London and Academic Press, London and other cities. 449 pp.
Robson, E. A. 1985. Speculations on coelenterates. Pp. 60-77 in S. Conway Morris, J. D. George, R. Gibson, and H. M. Platt
(eds). The Origins and Relationships of Lower Invertebrates. Clarendon Press, Oxford.
Ruppert, E. E. and R. D. Barnes. 1994. Invertebrate Zoology (6th ed.). Harcourt Brace College Publishers, Fort Worth. 1056
pp.
Schlichter, D. 1975. The importance of dissolved organic compounds in sea water for the nutrition of Anemonia sulcata
Pennant (Coelenterata). Pp. 395-405 in H. Barnes (ed.). Proceedings of the 9th European Marine Biolological Symposium.
Schuchert, P. 1993. Phylogenetic analysis of the Cnidaria. Z. Zool. Syst. Evolut.-forsch. 31:161-173.
Scrutton, C. T. 1979. Early Fossil Cnidarians. Pp. 161-207 in M. R. House (ed.) The Origin of Major Invertebrate Groups.
Academic Press, London.
Shick, J. M. 1991. A Functional Biology of Sea Anemones. Chapman and Hall, London and other cities. 395 pp.
Tardent, P. and R. Tardent (eds.). 1980. Developmental and Cellular Biology of Coelenterates (Proceedings of the 4th
International Coelenterate Conference held in Interlaken, Switzerland, 4-8 September, 1979). Elsevier/North-Holland
Biomedical Press, Amsterdam and other cities. 499 pp.
Tokioka, T. and S. Nishimura (eds). 1973. Proceedings of the Second International Symposium on Cnidaria. Publ. Seto Mar.
Biol. Lab. 20:1-793.
Watson, G. M. 1988. Ultrastructure and cytochemistry of developing nematocysts. Pp. 143-164 in D. A. Hessinger and H. M.
Lenhoff (eds.) The Biology of Nematocysts. Academic Press, San Diego and other cities.
Werner, B. 1973. New investigations on systematics and evolution of the class Scyphozoa and the phylum Cnidaria. Publ. Seto
Mar. Biol. Lab. (Proceedings of the Second International Symposium on Cnidaria) 20:35-61.
Williams, R. B., P. F. S. Cornelius, R. G. Hughes, and E. A. Robson (eds.). 1991. Coelenterate Biology: Recent Research on
Cnidaria and Ctenophora (Proceedings of the Fifth International Conference on Coelenterate Biology, 1989). Kluwer
Academic Publishers, Dordrecht and other cities. 742 pp.
Information on the Internet
Cnidaria Home Page. University of California, Irvine.
Title Illustrations
Scientific Name Heteractis malu
Comments A sea anemone (Anthozoa)
Reference From D. G. Fautin and G. R. Allen. 1992. Field Guide to Anemonefishes and their Host Sea
Anemones. Western Australia Museum.
Creator photographed by Art Reed
Specimen Condition Live Specimen
Copyright © 1992 Western Australia Museum
Scientific Name Aglantha digitale
Comments A direct-developing holoplanktonic hydromedusa (Hydrozoa) that has no polyp. The gonads are
visible through the transparent bell.
Copyright