* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Model Part of the Presentation
Survey
Document related concepts
Transcript
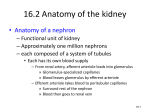
Stochastic Modeling of Coupled Nephrons Saziye Bayram* Bruce E. Pitman** *SUNY-Buffalo State College **SUNY-University at Buffalo Overview Anatomy and Physiology of the Kidney Structural Anatomy and Physiology of Nephrons Tubuloglomerular Feedback (TGF) Mechanism Experimental Findings Earlier Mathematical Models of Nephron’s TGF Mechanism Stochastic Models of Nephron’s TGF Mechanism Goals and Physiological Relevance KIDNEYS Filter waste materials out of the blood & eliminate them as urine from the body. Homeostatic (regulates, balance the state) devices of the body. Single human kidney consists of ~106 nephrons. Filter 180-200 liters of blood daily. Anatomy of Kidney Cortex and Nephrons Figure:www.ams.sunysb.edu/.../ SCICOMP/Kidney.index.html Structural Anatomy of a Nephron Within 24 hrs, kidneys reclaim: ~1,300 g of NaCl (~97% of Cl) ~400 g NaHCO3 (100%) ~180 g glucose (100%) ~almost all of the180 liters of water that entered the tubules (excrete ~0.5 liter only) Each nephron processes a very small fraction of the total blood flow to the kidney, typically 200-300 nl/min for a rat kidney. Figure:anatomy.iupui.edu/.../ urinaryf04/urinaryf04.html TGF System of a Nephron Regulates tubular fluid flow of nephron by monitoring [NaCl] at MD, with a delay. [NaCl] at MD ↑ Diameter of AA ↓ Blood flow ↓ Pressure in capillaries ↓ Rate of filtration ↓ Transit time ↑ [NaCl] at MD ↓ Figure:ccollege.hccs.cc.tx.us/. ../kidneypict.htm Macula Densa EE AA Bowman’s Capsule Glomerulus PT Schematic Diagram of a Nephron Experimental Findings (By Just et al., Cupples et al., Leyssac, Holstein-Rathlou et al., and Casellas et al.) TGF-mediated fluid flow in normotensive rat nephron either approximates a steady state or exhibits limit cycle oscillations (LCO) (20-50 mHz). Irregular and chaotic flow oscillations observed in hypertensive rats. Evidence of interaction between neighboring nephron: 60-70% of nephrons occur in pairs and triples. Sustained oscillations in one nephron can propagate to the coupled nephron. Resultant oscillations are roughly synchronous. Interaction between paired nephrons Types of coupling: A- Vascular Coupling: Electrotonic in Nature B- Hemodynamic Coupling: Pressure related Berne, R.M., and Levy, M.M. (1996), “Principles of Physiology”, Mosby-Year Book, Inc, MO The tubular pressure oscillations of a pair of neighboring nephrons Single Nephron ODE Model: k1 g 3 X 1 , X 2 X k dX 1 1 k11 k9 1 3 1 k8 1 dt k7 g1 X 2 k6 g 2 X 1 , X 2 dX 2 X3 dt dX 3 g 4 X 1 , X 2 g 7 X 2 , X 6 , Z k19 X 3 dt k14 dX 4 3 X 1 k3 X 4 dt 1 k6 dX 5 3 X 4 X 5 dt 1 dX 6 3 X 5 X 6 dt 1 The first mathematical models of the TGF system were deterministic. were complex but still a simplification of the real system. did not capture the irregularities have seen in the experiments with hypertensive rats. Deterministic to Stochastic In reality, there are a variety of factors that change over time, and can neither be controlled nor measured but nevertheless leave their mark on the experimental output. In this regard, we will model certain parameters as random processes of some convenient form (e.g. by adding dynamic noise). In our case: Gain, Delay, and Coupling parameters are of interest because these are the key parameters in understanding the stability of the pressure and flow regulation in renal dynamics these were the main bifurcation parameters and have been considered constant in the former models. computer simulations show that oscillations in the TGF system occur if the feedback gain is above a critical value. Goals and Physiological Relevance To include noise in models of physiological systems, to provide more realistic representation of the process under study, and to contribute to a deeper understanding of the underlying mechanisms. As a stochastic approach, we will hypothesis that gain, delay and/or coupling parameters vary randomly with time. (In fact, the gain magnitude is influenced by a variety of influences, such as arterial blood pressure, which changes over time.) The constructed SDE will be simulated by the Monte Carlo methods and results will be compared with the experimental data. Will estimate one or more of the parameters that determines the dynamics of the TGF mechanism. Will be able to estimate the physiological parameters, and perhaps help to identify the underlying mechanisms of hypertension.
















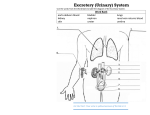
![Urinary System_student handout[1].](http://s1.studyres.com/store/data/008293858_1-b77b303d5bfb3ec35a6e80f57f440bef-150x150.png)