* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Why P. aeruginosa so virulent?
Survey
Document related concepts
Triclocarban wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Human microbiota wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Urinary tract infection wikipedia , lookup
Bacterial morphological plasticity wikipedia , lookup
Quorum sensing wikipedia , lookup
Infection control wikipedia , lookup
Carbapenem-resistant enterobacteriaceae wikipedia , lookup
Transcript
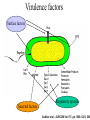
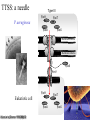
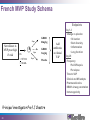
New concept in Antibiotic therapy; Lisboa Sept 22nd, 2008 Why P. aeruginosa so virulent? Jean-François TIMSIT MD Ph D Grenoble, France I have no conflict of interest to declare Pseudomonas aeruginosa :an opportunistic pathogen Gram-negative rod Ubiquitous: soil aquatic habitats Low demanding In the hospital: Water Hospital plumbing, sinks Medical devices Antiseptic solutions Vegetables and fruits Colonization • • • • • Oropharynx Upper digestive tract Trachea Urinary tract 10% adults, 50-60% hospitalized people – Endogeneous infection >60% – Exogeneous 30% Infections • 10% of hospital acquired infection (National prevalence study, 2006) • Immunocompromized host: – neutropenia, HIV+ National ICU database:REA-RAISIN • 2004-2006 (3 years), 56,535 patients • 7808 with at least one NI (UTI, VAP, Bacteremia) 1875 with P. aeruginosa NI (VAP:58%, UTI:17%, BC: 15%, more than one: 15%) 24% of all infected patients 3.3% of patients – Late onset NI: 18 days (2-237d) (vs 10 days for NI due to other organisms) – TIC S: 48%, TIC R/CAZ S: 31%, CAZ R: 21% stable • High SAPS II, DS: 40 days, ICU death 35% From AG Venier - National Meeting REA-RAISIN 2008 Genetic flexibility Large genome (E.coli : 4,6, M.tuberculosis : 4,4, S.aureus : 2,5) 5 500 genes (saccharomyces : 6 200) Function?? 8.4% regulatory genes Hypermutators Transcriptional regulation Adaptability to environment Escape to innate immunity Take advantage to immunity to a concerted attack Host response Inate immunity Alveolar Macrophages Surfactant proteins Defensins Cytokines Chemokines Activation Phagocytosis Alveolar space Lymphocytes Vascular space Neutrophiles Specific immunity (adaptative) Mainly chronic infections 2 strategies in ICUs Rapid and conserted attack Attachment Invisibility resistance Acute infection Prolonged colonization Devices’ attachment Virulence factors Surface factors Secreted factors Regulatory system Sadikot et al - AJRCCM Vol 171. pp 1209–1223, 200 Extra-cellular secretions Lazdunski Ann Fr Anesth Réanim 2003,22,523 TTSS:Type a needle I Type II P. aeruginosa Type III ExoY ExoT P aeruginosa AprA ToxA ExoU ExoS PcsC Membrane interne Membrane externe ExoS PcrV PopD PopB Membrane cytoplasmique ExoY Eukariotic cell Cellule eucaryote Kubori et al. Science 1998,280,602 ExoU ExoT ExoS TTSS • Exo S and T: – – – – ADP rybosyl tranferase and GTPase activity domains Cytosqueletal alterations ( DNA synth.) Cytotoxicity Inh. Of bacterial internalisation by both phagocytic and non phagocytic mamalian cells Invasivness R to phagocytosis bacteremia • Exo Y: – Adenylate cyclase ( intra cellular C-AMP) • Exo U: Cytotoxicity (epith cells) Tissue damage Septic shock – necrotizing toxin with a P lipase activity – Rapid lysis of mamalian cells – caspase 1 driven proinflammatory cytokine production ( innate response) Mortality in excess with TTSS Acute infection SSTT [+] Mortality Chronic Infection 89% 41% TTSS [+] TTSS [-] 21% 3% RR death PcrV alone 7,4 PcrV + toxin (s) 8,7 (Roy Burman et al, J Infect Dis. 2001 ) Anti-PcrV Antibodies Protect Mice Challenged with Lethal Pa Doses Shime et al. J. Immunol 2001;167:5880-5886 Improvement of lung inflammation and damage, hemodynamic parameters of septic shock and mortality KB001 (Humaneered™ Anti-PcrV) • Human Fab’ with V-region sequence close to human germ-line sequence – 91% sequence identity to germ-line – Low likelihood of immunogenicity • High affinity (0.67nM) and potent biological activity • Lacks Fc-mediated effector functions – Unlikely to increase inflammation in the lung • PEGylation – Prolongs half life to approximately 2 weeks – Further reduces potential immunogenicity French MVP Study Schema Endpoints 12 pts Surveillance in MVP pts at high Pa risk R 12 pts Pa > 103 ETA 12 pts KB001 10mg/kg KB001 3mg/kg Placebo 2 > 10 BAL Principal investigator:Prof J Chastre 23 Add antibiotics at clinical VAP Day 1-3 • Change vs placebo • Pa burden • Bact diversity • Inflammation • Lung function Day 28 • Frequency •Pa VAP/sepsis •Pa relapse • Time to VAP • Clinical and MV endpts • Pharmacokinetics • KB001 airway penetration • Immunogenicity T III secretion system and persistence of PA after VAP El Sohl et al – AJRCCM 2008; 178:513 25 TTSS + 13 PA at Day 8 Death 68% 9 TTSS - 9 eradication Death 33% 34 VAP MonoABx T III Secretion System and persistence of PA after VAP El Sohl et al – AJRCCM 2008; 178:513 1- 71% PA-VAP TTSS+ 2- VAP-PA-TTSS+: neutrophilic Apoptosis 3- Neutro cytotox correlated with ExoU(ExoS)/Pcrv phenotypes Future prospect for anti-PCRV? • Anti PcrV in P. aeruginosa VAP patients already treated with persistent PA at Day 58 of antimicrobial treatment • End-point – Relapse, recurrence and mortality – Neutrophilic cytotoxicity and elastase Quorum sensing Regulation of >100 genes in a density-dependent manner Homoserine lactones (HSL) 1. Important gene for the life cycle of the bacteria: DNA replication, transcription, cell division, aminoacid synthesis Persistence of the bacteria in the lung, (increase bacterial resistance, quiecent phase) 2. Life in community Promotion of biofilm formations. 3. Virulence factors Pyocyanin, siderophores, rhamnolipids… Quorum sensing system? I-gene R-gene Target-genes Binding and genes activation Auto-inducer synthetase Metabolic, physiologic regulation Transcriptional activator (R-protein) AI/R complex AI (3-oxo-C12-HSL C4-HSL) Freely diffusible AI signals to (from) other bacterias Adapted from Tateda K 2007 Extra cellular product 3 QS system in PA:las, rhl, PQS Regulations of 6-10% of PA genes las rhl PQS (Pseudomonas quinolone signal) PQS synth Rhl system Biofilm production Elastase Lipase Exo A PQS synth Rhamnolipids Elastase Lipase Pyocyanin Exo S Rhl system Rhamnolipids Biofilm formation Elastase Pyocyanin Quorum sensing is more frequent in virulent strains (n=270) (n=50) Van Delden C – Personnal communication – RICAI 2007 QS activity and virulence factors in clinically pathogenic isolates of P aeruginosa – Le Berre et al – CMI 2008; 14:337 Correlation las R=0.7, p=2 10-9 Correlation rhl R=0.3, p=0.02 Synthetic furanones inhibit QS and enhance bacterial clearance in PA lung infection in mice Wu et al – JAC 2004;53:1054 • Semi-synthetic derivates from QS inhibitors from macro alga Delisea Pulchra – In a mouse model: • • • • Supression of bacterial QS in the lung Accelerated lung clearance Reduced the severity of lung pathology In a lethal PA pneumonia mouse model, it prolonged survival time… Inhibition of QS • Macrolides (azythromycin) – QS, inflammation, extracellular virulence factors Tateda et al J infect chemother 2007 – the survival of mouse challenged with PA (Nicolau 1999) – pulmonary function of cystic fibrosis (Jaffe 1998) – 70% the risk of PA infection in HIV patients (Sorvillo 2001) Impact of Macrolides on host defenses (+) TIGHT JUNCTION (-) QS (-) MOTILITY (+) PHOGOCYTOSIS (-) NF K B, AP-1 (-) TNF IL-8 Giamerellos-Bourboulis et al - J. Antimicrob. Agents (2008), doi:10.101 Effect of clarithromycin in patients with sepsis and VAP Giamarellos-Bourboulis CID 2008:1157 Placebo n=100 Clarithro n=100 Age 58 58 PF ratio 218 224 EOP/LOP 44/56 41/59 Septic shock 43 42 PA A. baumannii 12% 43 17% 36 Crude mortality D28 28 Day 7 8 Sepsis related 24 Time until VAP resolution* (*) P=0.006 11.5 31 6 21 7 ANB 006/2001 Phase IIa : Pseudomonas aeruginosa prevention • • • • Multinational multicentric study, P-o-C study Prevention of VAP in PA colonized patient Azithromycin 300 mg daily for 20 days Study stopped after 92 patients/200 85 per protocol analysis – Pa VAP Acquisition and QS markers • Subgroup analysis of QS producing virulence factors strains… Candida-Pseudomonas copathogenicity? • Epidemiologic association between both micro-organisms (Vincent 1995) • PA infection is a risk factor of Candidaemia in burned mice (Neely 1986) • PA forms a dense biofilm on C albicans filaments and kills the fungus (Hogan, Science 2002) • Several virulence factors of PA are involved in killing C albicans filaments (Hogan 2002) • PA HSL is able to inhibit Candida filamentation (Hogan 2004) • Candida Tracheal colonization favors PA pneumonia in Rats (Roux 2006, (abstract)) Candida Colonization of the Respiratory Tract and Subsequent Pseudomonas Ventilator-Associated Pneumonia Azoulay E on behalf of the OUTCOMEREA study group Chest 2006 Impact of an antifungal treatment of tracheal candida colonization on PA VAP risk • Preliminary retrospective data – Case (19)/ Control (38) study – Decrease in the risk of PA VAP or PA colonization: OR=0.68 [0.49-0.9], p=0.046 Nseir et al – ICM 2007 • International interventional study planned Aknowledgments Benoit Guery Benoit Misset Pierre Moine Olivier Epaulard Christian Van Delden Jean Carlet Jean Chastre Kalobios pharma Scanning electron micrograph of a biofilm on a metal surface from an industrial water system • • • • Clinical importance Virulence factors Therapeutic targets Copathogenicity (candida-PA)