* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download substrates
Radiation damage wikipedia , lookup
Strengthening mechanisms of materials wikipedia , lookup
Nanochemistry wikipedia , lookup
Work hardening wikipedia , lookup
Synthetic setae wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Thermal copper pillar bump wikipedia , lookup
Sol–gel process wikipedia , lookup
Semiconductor wikipedia , lookup
Self-assembled monolayer wikipedia , lookup
Nanoimprint lithography wikipedia , lookup
4. Layers and MEMS technologies
Recent development of MEMS and sensors technology is essentially based on
micromachinig. This technology consists of specific design and fabrication
processes, many of which are borrowed from the integrated circuit industry.
The important feature of micromachining, leading to manufacturing costs
reduction is batch fabrication, i.e. simultaneous manufacturing of hundreds or
thousans of identical structures.
Gas sensors fabricated on 3”
silicon wafer
Schematic process flow in micromachining.
It is repeated until completion of a microstructure.
1. Film Deposition Processes
In technology of MEMS and sensors one of the main steps is deposition of thin
films of materials in interest.
Deposition technology can be classified in two groups:
Depositions with the help of chemical reactions:
• Chemical Vapor Deposition (CVD)
• Electrodeposition
• Epitaxy
• Thermal oxidation
These processes exploit the creation of solid materials directly from chemical
reactions in gas and/or liquid compositions or with the substrate material. The
solid material is usually not the only product formed by the reaction.
Byproducts can include gases, liquids and even other solids.
Depositions that happen because of a physical process:
• Physical Vapor Deposition (PVD)
• Casting
The material deposited is physically moved on to the substrate.
2
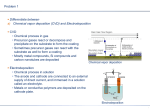
Chemical Vapour Deposition (CVD)
On the substrate placed inside a reactor a solid material condenses due to chemical
reactions between source gases introduced into the reactor.
The two most important CVD technologies are the Low Pressure CVD (LPCVD) and
Plasma Enhanced CVD (PECVD).
The LPCVD process produces layers
with excellent uniformity of
thickness and material
characteristics.
The main problems with the process
are the high deposition
temperature (higher than 600°C)
and the relatively slow
deposition rate.
The material is deposited on both
sides of the substrates (wafers).
Typical hot-wall LPCVD reactor
3
PECVD
The PECVD process can operate at lower temperatures (down to 300° C) thanks to
the extra energy supplied to the gas molecules by the plasma in the reactor.
However, the quality of the films tend to be inferior to processes running at higher
temperatures. The material is deposited on one side of the wafers.
Typical PECVD reactor
• Top electrode RF driven (MHz);
• Substrate sits directly on heated
electrode
• Gas injected into process chamber via
gas inlet in the top electrode
Operating pressure ca. 1 Torr
4
Electrodeposition (electroplating)
Essentially restricted to deposition of electrically conductive materials
(metals: copper, gold, nickel).
The plating process can be also electroless – external electric field and conductive
surface not required – thickness and uniformity of a deposit difficult to control.
Si wafer plated
with Cu using
copper sulphate
Typical setup for electrodeposition.
Example solutions
for electroplating
selected metals
When an electrical potential is applied between a conducting area on the
substrate and a counter electrode (usually platinum) in the liquid, a
chemical redox process takes place resulting in the formation of a layer of
material on the substrate and usually some gas generation at the counter
electrode.
5
Epitaxial growth
If the substrate is an ordered crystal, it is possible to grow on it the material with
the same crystallographic orientation, which is known as epitaxy.
Vapor Phase Epitaxy (VPE) is the most important process in which the conditions
are created to support epitaxial growth.
Scheme of a typical reactor used
in VPE process
In VPE a number of gases are introduced in an induction heated reactor where
only the substrate is heated. The temperature of the substrate typically must be at
least 50% of the melting point of the material to be deposited.
The high growth rate allows obtaining layers exceeding 100 µm in thickness.
6
This is the basic technology of production electronic c-Si and also SOI substrates.
Epitaxial growth
Manufacturing of epitaxial Si using silane:
SiH4 → Si + 2H2
The silane reaction occurs at 650 °C.
IC grade crystalline silicon
One of the methods of manufacturing SOI (silicon-on-insulator) substrates
7
Thermal oxidation
The substrate can be oxidized in an oxygen rich atmosphere.
In the case of silicon the temperature is raised to 800° C - 1100° which gives high
quality amorphous silicon dioxide.
The final oxide thickness can be controlled by selecting the temperature and
oxidizing conditions.
Thermal oxidation of silicon generates compressive stress in the silicon dioxide
film,as SiO2 molecules take more volume than Si atoms, and there is also a
mismatch between the coefficients of thermal expansion of Si and SiO2 .
As a result, thermally grown oxide films cause bowing of the underlying substrate.
Typical view of a furnace used
for wafers oxidation.
8
Evaporation
Evaporation belongs to PVD processes in which the material is released from a
source and transferred to the substrate.
In evaporation the substrate and evaporation source are placed inside a vacuum
chamber. The source material is then heated to the point where it evaporates and
the vapours condense on the substrate. Two methods of heating the source are the
most popular: e-beam heating and resistive heating.
Nearly any element (e.g.,Al, Si, Ti, Au),
including many high-melting-point
(refractory) metals and compounds (e.g.,
Cr, Mo, Ta, Pd, Pt, Ni/Cr), can be
evaporated.
Deposited films consisting of more than
one element may not have the same
composition as the source material due to
the differences in evaporation rates of
constituting elements.
The compound films may then be
nonstoichiometric.
Schematic view of a thermal
9
evaporation unit with resistive heating
Evaporation
Thermal evaporation using laser beam
(PLD technology)
E-beam thermal evaporation
10
Sputtering
In sputtering, a target made of a material to be deposited is physically
bombarded by a flux of inert-gas ions (usually argon) in a vacuum chamber at a
pressure of 0.1–10 Pa.
Atoms or molecules from the target are ejected and deposited onto the substrate.
There are several kinds of sputtering differing by the ion excitation mechanism.
Deposition of thin films in a typical dc
sputtering unit
Magnetron sputtering
Applying external magnetic field increases the ion density near the target,
thus raising the deposition rates (magnetron sputtering).
Construction of the
magnetron cathode
RF and Ion Beam Sputtering
In RF (radio frequency), the target and the
wafer form two parallel plates with RF
excitation applied to the target.
In ion-beam deposition ions are
generated in a remote plasma, then
accelerated at the target
RF sputtering (left) and ion source sputtering (right)
Casting
In casting the material to be deposited is dissolved in a solvent and then applied
to the substrate by spraying or spinning.
This is particularly useful for polymer materials, which may be easily dissolved in
organic solvents, and it is the common method used to apply photoresist to
substrates (in photolithography).
The thicknesses obtained range from a single monolayer of molecules (adhesion
promotion) to tens of micrometers. In recent years, the casting technology has
also been applied to form films of glass (SOG) materials on substrates.
Thick (5–100 μm) SOG has the ability to uniformly coat surfaces and smooth out
underlying topographical variations, effectively planarizing surface features.
The spin casting process used in deposition
of photoresist in photolithography.
Solution casting and transfer printing
collections of individual single-walled
carbon nanotubes (SWNTs) onto a
14
wide range of substrates
2. Lithography
Lithography involves three sequential steps:
• Application of photoresist, which is a photosensitive emulsion layer;
• Optical exposure to print an image of the mask onto the resist;
• Immersion in an aqueous developer solution to dissolve the exposed resist and
render visible the latent image.
The mask itself consists of a patterned opaque chromium (the most common),
emulsion, or iron oxide layer on a transparent fused-quartz or soda-lime glass
substrate.
The pattern layout is generated using a computer-aided design (CAD) tool
and transferred into the opaque layer at a specialized mask-making facility, often by
electron-beam or laser-beam writing. A complete microfabrication process normally
involves several lithographic operations with different masks.
15
Lithography – positive and negative resist
When resist is exposed to a
radiation source of a specific
wavelength (from UV to blue),
the chemical resistance of the
resist to developer solution
changes.
If the resist is placed in a
developer solution, it will etch
away one of the two regions
(exposed or unexposed).
If the exposed material is etched
away by the developer and the
unexposed region is resilient, the
material is considered to be a
positive resist.
The exact opposite process
happens in negative resists.
Transfer of a pattern to a
photosensitive material.
16
Lithography – subtractive and additive processes
A photosensitive layer is often used as a temporary mask when etching an
underlying layer, so that the pattern may be transferred to the underlying layer.
Photoresist may also be used as a template for patterning material deposited after
lithography. The resist is subsequently etched away, and the material deposited on
the resist is "lifted off".
Pattern transfer from patterned
photoresist to underlying layer by
etching (a) and
pattern transfer from patterned
photoresist to overlying layer by
lift-off (b).
17
3. Etching
Differences between anisotropic
and isotropic wet and dry etching.
In order to form a functional MEMS
structure on a substrate, it is
necessary to etch the thin films
previously deposited or the
substrate itself.
In general, there are two classes of
etching processes:
wet etching where the material is
dissolved when immersed in a
chemical solution
dry etching where the material is
sputtered or dissolved using
reactive ions or a vapor phase
etchant.
Anisotropic etching in contrast to isotropic etching means different etch rates in
different directions in the material.
The example is the (111) crystal plane sidewalls that appear when etching a hole in
a (100) silicon wafer in the potassium hydroxide (KOH).
18
Anisotropic etching
Anisotropic wet etching of silicon wafer of
(100) crystallographic orientation.
The etch front begins at the
opening in the mask and
proceeds in the <100>
direction, which is the vertical
direction in (100)-oriented
substrates, creating a cavity
with a flat bottom and slanted
sides.
The sides are {111} planes
making a 54.7º angle with
respect to the horizontal (100)
surface. If left in the etchant
long enough,the etch ultimately
self-limits on four equivalent
but intersecting {111} planes,
forming an inverted pyramid or
V-shaped trench.
Gas sensor on the silicon
membrane
Dry etching
The dry etching technology can split in three separate classes called:
sputter etching, vapor phase (chemical) etching and reactive ion etching (RIE).
In sputter etching the systems used are
very similar in principle to sputtering
deposition systems but the difference is
that substrate is now subjected to the
ion bombardment instead of the target.
Ions Ar, O2
CF4, SF6
In vapor phase etching the material to
be etched is dissolved at the surface in
a chemical reaction with the gas
molecules (mostly isotropic process).
In RIE,under the influence of RF power
the gas molecules break into ions which
are accelerated towards, and react at,
the surface of the material being etched.
The balance of chemical and physical
etching can give sidewalls that have
vertical shapes.
RIE
Illustration of different dry
etching processes
20
Deep Reactive Ion (DRIE) etching
In this process, etch depths of hundreds of microns can be achieved with almost
vertical sidewalls.
The primary technology is based on the so-called "Bosch process", where two
different gas compositions are alternated in the reactor.
The first gas composition creates a polymer on the surface of the substrate, and the
second gas composition etches the substrate. The polymer is immediately
sputtered away by ion bombardment, but only on the horizontal surfaces and not
the sidewalls. Since the polymer only dissolves very slowly in the chemical part of
the etching, it builds up on the sidewalls and protects them from etching.
Profile of a DRIE trench using
the Bosch process.
Etching aspect ratio (ratio of
height to width) of 50 to 1 can
be achieved.
21
4. Screen printing
A wide variety of materials, including metals, chemical compopunds and ceramics,
can be applied using screen printing (e.g. in sensor technology).
Screen printing begins with the production of a stencil, which is a flat, flexible
plate with solid and open areas.
The stencil has a fine-mesh screen as a bottom layer.
Separately, a paste is made of fine particles of the material of interest, along with
an organic binder and a solvent. A mass of paste is applied to the stencil, then
smeared along with a squeegee. A layer of paste is forced though the openings
in the stencil, leaving a pattern on the underlying substrate.
Drying evaporates the solvent. Firing burns off the organic binder and sinters the
remaining metal or ceramic into a solid.
Illustration of the screen
printing process.
22
Screen printing
Image reproduction
Thick film layers on
cofired ceramic
substrate used in gas
sensor technology
layer 1 - top
(interdigital
Au electrodes)
layer 2 - top
(Pt heater)
layer 2 - bottom
(contact pads)
23