* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Enzymes HW Key
Survey
Document related concepts
Transcript
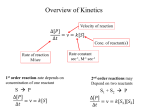
Chemistry 160 Protein Function and Enzymes Homework 1. What is the difference between the lock and key hypothesis and the induced fit model? Lock and key is that the enzyme and substrate are rigid and have complementary shapes and fit together like a lock and a key. The induced fit hypothesis is more like a hand in a glove. Both enzyme and substrate change shape upon binding. 2. Explain how a single amino acid substitution in a protein can affect function. Sickle cell anemia is a classic example. A single amino acid, glutamate, which is charged, is replaced by a valine, which is nonpolar. They have very different characteristics. In this case, the valine creates a “sticky spot” causing cells to sickle. 3. Describe how the structure of hemoglobin changes when oxygen is bound. How does that allow other oxygens to bind? In hemoglobin, when oxygen , binds, an iron ion in the heme is pulled into the plane of the heme. As the iron ion is coordinated to a histidine residue on one of the α helices of the hemoglobin, this chain is also moved. This causes other parts of the molecule to move and it opens other oxygen binding sites. Binding of the first oxygen makes it easier to bind other oxygens 4. Define active site, allosteric site, substrate, ligand. Active site: where the substrate binds to the enzyme. where the reaction takes place. allosteric site: a site other than the active site where regulatory molecules bind to the enzyme. substrate: the reactant of the enzyme reaction. binds to the active site. ligand: the molecule that binds to a receptor. 5. Show an energy diagram (energy vs time) for an exergonic reaction that has an activation energy. Show another one with a catalyst. Catalysis Uncatalyzed Catalyzed Transition G EA R G R P time P time 6. There are several ways enzymes lower activation energy. List three. Supply energy by binding….entropy increase of solvent upon binding releases energy and this is used for catalysis. Binding forces substrate into unstable transition state. Brings reactive groups together 7. How do enzymes work? Draw a diagram of an enzyme catalyzing a reaction. enzyme binds to substrate then product forms and diffuses out. E + S ---> ES---> EP ---> E + P S E S E P E E 8. To what does an enzyme bind? (Hint: it is not the substrate.) a transition state between the substrate and the product 9. Show competitive inhibition in an enzyme. competitive inhibitor competes with the substrate for the active site. I E S I E S 10. Show a Michaelis-Menton plot of an enzyme. Indicate the Km and Vmax on the graph. Also show both competitive and noncompetitive inhibition on M-M plots. V Vmax S Km V Vmax uninhib S Km Book Questions: Pp. 110 – 111:,49,55 Pp, 152 154: 9,39,40 Pg. 182: 19,20 inhib.lower Vmax